SHORT COMMUNICATION
Possible Association of Interleukin-31/-31RA Signalling and Basophils with Itch in Porokeratosis
Satoshi OKUNO, Takashi HASHIMOTO, Riichiro SUGIURA and Takahiro SATOH
Department of Dermatology, National Defense Medical College, Tokorozawa, Saitama, 359-8513, Japan. E-mail: hashderm@ndmc.ac.jp
Citation: Acta Derm Venereol 2023; 103: adv6560. DOI: https://doi.org/10.2340/actadv.v103.6560.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Sep 6, 2023; Published: Oct 10, 2023
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Porokeratosis is a keratotic disorder involving genetically abnormal epidermal keratinocytes (1). Itch is not a common symptom of porokeratosis; however, a certain population of patients with porokeratosis experience moderate to severe itch. This itch in porokeratosis is often refractory to antihistamines (2), indicating a predominance of non-histaminergic itch. Interleukin (IL)-31, as a non-histaminergic itch mediator, is known to be a major pruritogen in atopic dermatitis. Th2 cells are considered a major cellular source of IL-31, exhibiting pruritogenic function through a heterodimeric receptor comprising IL-31 receptor A (IL-31RA) and oncostatin M receptor β (3–5). As a representative itchy variant of porokeratosis, eruptive pruritic papular porokeratosis (EPPP) is characterized by severe itch and, occasionally, lesional eosinophilic infiltration. Our prior study also demonstrated massive infiltration of basophils with upregulation of epidermal thymic stromal lymphopoietin (TSLP) and dermal periostin in EPPP (1, 4). Eosinophils, basophils, TSLP, and periostin are generally known to be capable of provoking itching sensation (6–8). The current study attempted to elucidate factors associated with itch in porokeratosis through immunofluorescence staining. This study was approved by the institutional review board of the National Defense Medical College (approval number #4477).
MATERIALS AND METHODS (SEE APPENDIX S1)
RESULTS
Skin biopsy specimens were collected from patients with porokeratosis (5 patients with itch symptoms designated as itchy porokeratosis (IP; 4 men and 1 woman; age range 56–75 years); 4 patients without itch symptoms as non-itchy porokeratosis (NIP; 3 men and 1 woman; age range 50–83 years); and 3 healthy individuals (HI; 3 women; age range 54–69 years)). Whether their lesions were itchy or non-itchy was based on the reported symptoms. Itch intensity scores (e.g. itch numerical rating scale scores) were not collected.
The number of dermal-infiltrating IL-31+ cells was increased significantly in IP compared with NIP (Mann–Whitney U test, p < 0.05), but no significant difference was seen between NIP and HI (Mann–Whitney U test, p > 0.05) (Fig. 1A). Dermal IL-31 was expressed mainly by CD3+ T cells (Fig. 1B and Fig. S1). Epidermal expression of IL-31 was enhanced in IP compared with NIP, while the difference was not significant (Mann–Whitney U test, p = 0.065) (Fig. 1A). Epidermal IL-31RA expression by keratinocytes was significantly enhanced in IP compared with NIP (Mann–Whitney U test, p < 0.05) (Fig. 1A), and the number of dermal IL-31RA-expressing cells also tended to increase in IP compared with NIP (Mann–Whitney U test, p = 0.065) (Fig. 1A). The expression of IL-31RA by dermal and epidermal nerve fibres that expressed a pan-axonal marker βIII-tubulin (Tuj1) was also confirmed (Fig. 1C).
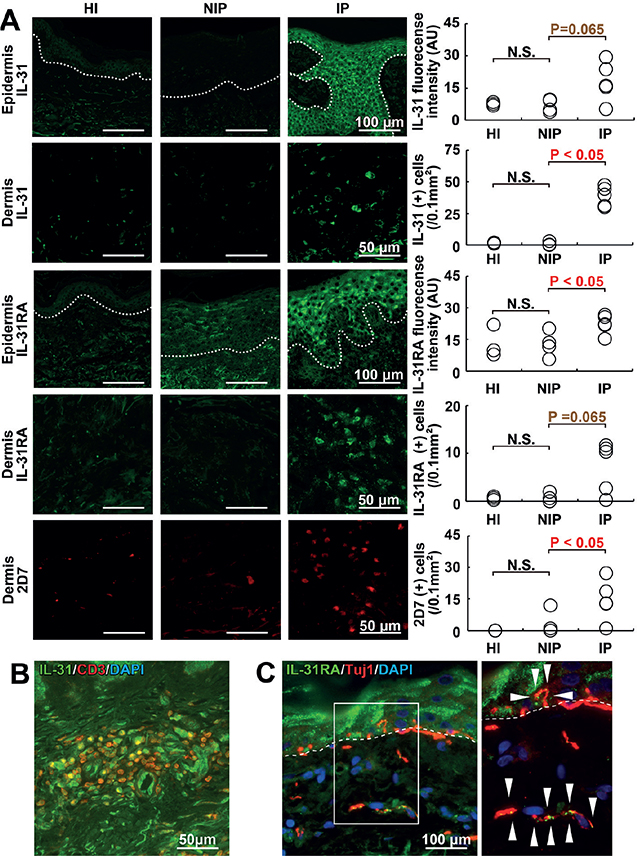
Fig. 1. Findings from immunofluorescence staining. Representative images of itchy porokeratosis (IP), non-itchy porokeratosis (NIP) and healthy individual (HI) skin with quantification of staining. (A) Epidermal expression of interleukin (IL)-31 (green), dermal infiltrating IL-31+ cells (green), epidermal expression of IL-31RA (green), dermal infiltrating IL-31RA+ cells (green), and dermal infiltrating 2D7+ basophils (red). White dotted lines indicate the dermo-epidermal junction. (B) IL-31+ dermal cells (green) express CD3 (red), a T cell marker. (C) Pan-neuronal marker β-III tubulin (Tuj1)+ sensory nerve fibres (red) express IL-31RA (green). The right panel is a white inset of the left panel. N.S: not significant; AU: arbitrary units.
Infiltration of a significant number of dermal 2D7+ basophils in IP was observed, but neither in NIP nor HI (Mann–Whitney U test, p < 0.05; IP vs NIP) (Fig. 1A). In contrast to basophils, the number of major basic protein (MBP)+ eosinophils in the dermis did not differ between IP and NIP (data not shown). Epidermal expression of TSLP and dermal deposition of periostin, both of which are potent pruritogens, were not significantly enhanced in IP compared with NIP or HI (Fig. 2A). No significant epidermal expression of periostin was detected in any of the groups, nor was any significant difference detected in dermal TSLP expression between the IP and NIP groups (data not shown).
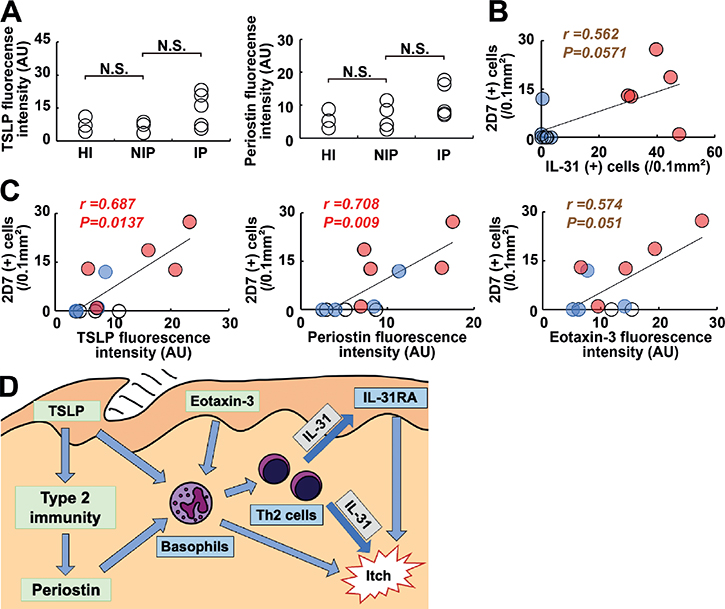
Fig. 2. Itch-associated factors in itchy porokeratosis. (A) Expression levels of epidermal thymic stromal lymphopoietin (TSLP) and dermal periostin semi-quantified with immunofluorescence intensities. (B, C) Correlation of interleukin (IL)-31+ cells with 2D7+ basophils (B) and correlations of 2D7+ basophils with epidermal TSLP, dermal periostin, and epidermal eotaxin-3 (C). White circles, healthy individuals (HI); pale blue circles, non-itchy porokeratosis (NIP); pale red circles, itchy porokeratosis (IP). (D) Proposed mechanism for itch in porokeratosis involving IL-31+ cells and basophils. Dermal IL-31, epidermal IL-31RA, and basophils are major players in itch in porokeratosis. Basophils can be attracted and/or activated by TSLP, periostin, and eotaxin-3. N.S.: not significant; AU: arbitrary units; r: Spearman’s rank correlation coefficient.
Next, the study investigated factors correlating with the number of dermal IL-31-expressing T cells. The number of 2D7+ basophils tended to correlate with the number of IL-31+ cells (Spearman’s correlation, r = 0.562 and p = 0.0571) (Fig. 2B). On the other hand, neither epidermal expression of TSLP nor dermal deposition of periostin correlated significantly with the number of IL-31+ T cells (data not shown; representative images in Fig. S2).
The study further attempted to assess correlations between the number of basophils and TSLP, periostin, and eotaxin-3, as basophil-chemoattractants and/or activators. The number of dermal 2D7+ basophils correlated with epidermal expression of TSLP and dermal deposition of periostin (Spearman’s correlation, r = 0.687 and p = 0.0137; r = 0.708 and p = 0.009, respectively) (Fig. 2C). Epidermal expression of eotaxin-3 tended to correlate with the number of 2D7+ basophils (Spearman’s correlation, r = 0.574 and p = 0.051) (Fig. 2C), although no significant correlation was found, possibly due to the small sample size.
DISCUSSION
This study showed that dermal IL-31 and basophils were closely associated with itch in porokeratosis, while eosinophil infiltration did not appear to affect itch symptoms.
IL-31 can evoke itching sensation via directly stimulating peripheral nerve fibres through IL-31 receptors. In addition, IL-31 indirectly provokes itching sensation via the epidermal keratinocytes. IL-31 is reported to stimulate the epidermal keratinocytes to secrete some pruritogens, including leukotriene B4 and thromboxane A2 (5, 9). A recent study has suggested that IL-31/IL-31RA signalling in the epidermis contributes not only to itching but also to the abnormal epidermal differentiation process (10). Characteristic findings of the epidermis in porokeratosis may be associated with IL-31/IL-31RA signalling.
Basophils are known to directly or indirectly mediate itch in various ways, including secretion of pruritogens, induction of type 2 immune responses, and directly affecting sensory nerve fibres (11, 12). The mode of action by which basophils affect itch in porokeratosis remains uncertain. Of note, 2D7+ basophils expressed IL-31RA (Fig. S3), suggesting that basophils themselves can be affected by IL-31. Interplay between basophils and IL-31-secreting Th2 cells may contribute to itch in porokeratosis.
Epidermal expression of eotaxin-3, one of the chemoattractants for basophils, is closely associated with the number of dermal basophils, although no statistical significance was evident, possibly due to the limited sample numbers. Basophils also infiltrated into the epidermis expressing eotaxin-3 (Fig. S4). Likewise, the type 2 inflammation-associated proteins TSLP and periostin correlated significantly with the dermal basophil number. Infiltrating basophils expressed a basophil-activation marker CD203c (Fig. S5). These findings indicate that basophil infiltration and activation were principally mediated by eotaxin-3, TSLP and periostin, although the mechanism of upregulation of these proteins in IP is still to be elucidated.
Key limitations of this study were the small number of samples; hence accurate statistical analyses may not be possible in some targeted molecules. However, the study suggests the possible involvement of IL-31/IL-31RA signalling and basophils in porokeratosis-associated itch (Fig. 2D). These findings may be helpful in the management of itch in porokeratosis.
ACKNOWLEDGEMENTS
This study was partially supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (C) (# 19K08743 and 22K08395).
This study was approved by the IRB at the National Defense Medical College (# 4477).
REFERENCES
- Awatani K, Hashimoto T, Satoh T. Eruptive pruritic papular porokeratosis accompanied by eosinophilic and basophilic infiltrate with upregulation of epidermal CCL26/eotaxin-3 and thymic stromal lymphopoietin. J Dermatol 2021; 48: e382–383.
- Tee SI, Chong WS. Eruptive pruritic papular porokeratosis. Indian J Dermatol Venereol Leprol 2012; 78: 758–760.
- Datsi A, Steinhoff M, Ahmad F, Alam M, Buddenkotte J. Interleukin-31: the “itchy” cytokine in inflammation and therapy. Allergy 2021; 76: 2982–2997.
- Hashimoto T, Moriyama Y, Satoh T. Linear porokeratosis with severe itch accompanied by lesional upregulation of interleukin 31, thymic stromal lymphopoietin, and periostin. Eur J Dermatology 2021; 31: 570–572.
- Furue M, Furue M. Interleukin-31 and pruritic skin. J Clin Med 2021; 10: 1906.
- Hashimoto T, Yosipovitch G. Itchy body: topographical difference of itch and scratching and C nNerve fibres. Exp Dermatol 2019; 28: 1385–1389.
- Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013; 155: 285–295.
- Hashimoto T, Mishra SK, Olivry T, Yosipovitch G. Periostin, an emerging player in itch sensation. J Invest Dermatol 2021; 141: 2338–2343.
- Andoh T, Harada A, Kuraishi Y. Involvement of leukotriene B4 released from keratinocytes in itch-associated response to intradermal interleukin-31 in mice. Acta Derm Venereol 2017; 97: 922–927.
- Okuno S, Hashimoto T, Yamazaki Y, Okuzawa M, Satoh T. IL-31 and IL-31 receptor alpha in pemphigus: Contributors to more than just itch? J Dermatol 2023; 50: 927–930.
- Hashimoto T, Rosen JD, Sanders KM, Yosipovitch G. Possible roles of basophils in chronic itch. Exp Dermatol 2019; 28: 1373–1379.
- Wang F, Trier AM, Li F, Kim S, Chen Z, Chai JN, et al. A basophil-neuronal axis promotes itch. Cell 2021; 184: 422–440.