ORIGINAL REPORT
Pilonidal Sinus Disease is Associated with Severe Hidradenitis Suppurativa in a Spanish Cohort
Clara UREÑA-PANIEGO1, Marta GAMISSANS-CAÑADA2, Alejandro MOLINA-LEYVA1,3 and Jorge ROMANÍ2
1Hidradenitis Suppurativa Clinic, Dermatology Department, Hospital Universitario Virgen de las Nieves, Granada, 2Hidradenitis suppurativa Clinic, Hospital Universitari Parc Taulí, Autonomous University of Barcelona, Sabadell, Barcelona, Spain, and 3European Hidradenitis Suppurativa Foundation (EHSF). Dessau-Roßlau, Germany
Hidradenitis suppurativa is a chronic inflammatory disorder of the hair follicle with a high level of morbidity. Pilonidal sinus disease is a comorbid disorder and may be the reason for first contact with the healthcare system of patients with hidradenitis suppurativa. The aim of this study was to evaluate the frequency of association of pilonidal sinus disease and hidradenitis suppurativa and to explore whether pilonidal sinus disease defines a different clinical profile for patients with hidradenitis suppurativa. A cross-sectional study in which data regarding past history of pilonidal sinus disease, clinical and sociodemographic information were recorded during the first visit to the Hidradenitis Suppurativa Clinic of 2 tertiary hospitals. A total of 839 patients were included in the study. Of these, 51.7% (434/839) were male and mean age was 37.3 ± 13.6 years. Pilonidal sinus disease was present in 32.6% (269/839) of the patients and was associated with an early debut of hidradenitis suppurativa, a higher Hurley stage, inflammatory phenotype and a greater number of fistulas and perianal involvement. Elapsed time between pilonidal sinus disease and diagnosis of hidradenitis suppurativa was associated with higher disease severity. Pilonidal sinus disease is a frequent comorbidity and risk marker for hidradenitis suppurativa disease severity. Pilonidal sinus disease could be a sentinel event to identify patients who would benefit from close treatment and follow-up.
Key words: epidemiology; hidradenitis suppurativa; pilonidal sinus disease.
SIGNIFICANCE
Pilonidal sinus disease is the most frequent comorbidity in patients with hidradenitis suppurativa. However, little is known about its prognostic significance. The aim of this study was to explore, in a Spanish cohort, how pilonidal sinus disease can impact the course and severity of hidradenitis suppurativa. The results suggest that pilonidal sinus disease and elapsed time between pilonidal sinus disease and hidradenitis suppurativa diagnosis could be risk markers for hidradenitis suppurativa severity.
Citation: Acta Derm Venereol 2023; 103: adv6569. DOI https://doi.org/10.2340/actadv.v103.6569.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Apr 19, 2023; Published: Sep 27, 2023
Corr: Alejandro Molina-Leyva, Hidradenitis Suppurativa Clinic, Dermatology Department, Hospital Universitario Virgen de las Nieves, Granada, Spain. E-mail: Alejandromolinaleyva@gmail.com
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Hidradenitis suppurativa (HS) is an inflammatory dermatosis with a chronic and relapsing course. It presents as painful nodules and abscesses that may develop into fistulous tracts and retracting scars. HS affects intertriginous regions, such as armpits, breasts, inguinal, perianal and perineal areas, where the density of pilosebaceous units is high. A prevalence of HS of 1% is estimated in the general population (1). Its course and nature entail great morbidity and impact on quality of life (2–4). The pathogenesis of HS is multifactorial and implies the alteration of skin microbioma, abnormal activation of innate immunity and over-expression of inflammatory cytokines (5). These mechanisms converge in the pilosebaceous unit, prompting its occlusion and subsequent cysts and abscess formation (6). Classically, this phenomenon was considered a meeting point disorder, in which follicular occlusion leads to keratin retention and secondary bacterial colonization. Initially, the triad of HS, acne conglobata and dissecting cellulitis of the scalp was considered (7). Later in 1975 pilonidal sinus disease (PSD) was added, leading to the term follicular occlusion tetrad (8).
On the other hand, PSD presents as a frequently solitary cystic lesion in the intergluteal fold, which contains hair and tends to inflammation. In the past, it was thought its aetiology was purely congenital, and that it derived from remnants of the embryonic appendage. Currently, it is widely considered an acquired disease, since pilonidal cysts have been observed in areas such as the scalp or interdigital spaces in barbers and shearers (9, 10). Its pathogenesis seems to lie in the production of negative pressure within certain areas, which prompts the suction of hair nearby, and when chronically inflamed, can lead to a foreign body granulomatous reaction (11).
HS and PSD are both associated with smoking, obesity and metabolic syndrome (12). Both disorders develop in areas subjected to great mechanical stress (13, 14). HS and PSD cysts additionally have in common histological and sonographic traits (12, 15). As a result of the aforementioned, some authors hypothesize that PSD may be a unilocalized form of HS (12). Nevertheless, other researchers suggest PSD to be an extension of the HS phenotype, in line with the theory of the tetrad of follicular occlusion.
The aims of this study were: (i) to evaluate the prevalence of PSD among patients with HS, (ii) to examine whether this subpopulation presents differential clinical characteristics, and (iii) to evaluate the elapsed time between PSD and HS diagnosis and to explore its prognostic implications.
MATERIALS AND METHODS
A cross-sectional study was performed, in which PSD diagnosis and elapsed time between PSD and HS were evaluated, with the aim of exploring the clinical profile of patients on the basis of this phenotypical characteristic.
Patients
All consecutive patients with HS attending the specialized unit for HS were recruited at their first visit to the Hospital Universitario Virgen de las Nieves (Granada, Spain) and Hospital Parc Taulí (Sabadell, Spain) between 2011 and 2021. All participants received all available information regarding the study and provided written informed consent.
Inclusion criteria
The inclusion criteria were as follows: (i) patients with clinical diagnosis of HS; (ii) informed consent from the patient or the legal representatives to be included in the study.
Exclusion criteria
The exclusion criteria were: (i) refusal by the patient or legal representative to participate in the study.
Variables of interest
Main variables.
- Diagnosis of PSD previous to the first visit of the patient to the HS unit. The diagnosis of PSD and age of presentation to a healthcare provider (be it general practitioner, general surgeon or other medical specialist) must be registered in the clinical record. This data was available for the cohort of patients of Hospital Universitario Virgen de las Nieves. In addition, all patients underwent complete physical examination to detect unnoticed PSD.
- Severity of hidradenitis suppurativa:
- The International HS severity scoring system (IHS4) was used to assess inflammatory activity. It was calculated using the following formula: (number of nodules ×1) + (number of abscesses ×2) + (number of fistulas ×4).
- The Hurley classification was used to assess structural damage. It consists of 3 stages (I, presence of abscesses without fistulous tracts or scars; II, recurrent abscesses and single or multiple fistulae and scars widely separated between them; III, abscesses and confluent fistulas with large areas of extensive scarring).
Other variables
- Socio-demographic, biometric and clinical variables, including age, sex, comorbidities (including body mass index and tobacco consumption) and previous treatments for HS, were recorded by clinical interview and physical examination.
Statistical analysis
Descriptive statistics were used to evaluate the characteristics of the sample. The Shapiro-Wilk test was used to assess the normality of the variables. Continuous variables were expressed as means and standard deviations (SD). Qualitative variables were expressed as relative and absolute frequency distributions. The χ2 test or Fisher’s exact test, as appropriate, were used to compare nominal variables, and the Student’s t-test or Wilcoxon-Mann-Whitney test were used to compare between nominal and continuous data. To explore possible associated factors, simple linear regression was used for continuous variables. The β coefficient and SD were used to predict the log odds of the dependent variable. Multivariate logistic regression analyses were performed to explore clinical and epidemiological variables associated with PSD. Epidemiological and statistical criteria were used to model variable selection. The effect of each exploratory variable in the model and its significance were studied. If the variable improved the model fit and adequacy (based on the likelihood ratio criteria and the significance of the parameter) it was kept; otherwise, the variable was excluded. Different models were fitted with respect to the factors related to PSD. The model was checked for pair-wise interaction between covariates. Potential confounding covariates were studied using a change of significance of the parameters in the model or a change of 30% of its value. Significance was set for all tests at 2 tails, p < 0.05. Statistical analyses were performed using JMP version 14.1.0 (SAS Institute, Cary, NC, USA).
RESULTS
Baseline characteristics
A total of 839 patients with HS were included in the study; 51.7% (434) of them were male. Smoking habit was present in 60.1% of patients and the inflammatory HS phenotype was the most common, affecting 47.3% (397) of the population. Of the subjects, 63% (530) presented Hurley stage II or III and a mean IHS4 of 8.5 ± 8.4 SD. PSD was present in 32.06% (269) of the patients. The main clinical and socio-demographic characteristics are summarized in Table I.
Clinical factors associated with pilonidal sinus disease in patients with hidradenitis suppurativa
Univariate analysis showed that PSD was more common among male patients (20.86% vs 11.20%; p < 0.001). Age of HS onset was lower in patients with PSD (R-squared: 0.02; p < 0.001). In addition, in patients with PSD, the severity of the disease was overall greater with a higher Hurley stage and IHS4 score. There were also a higher number of sinus tracts and regions involved in patients with PSD. Inflammatory phenotype and perianal involvement were more common in patients with PSD. These findings are summarized in Table I. Multivariate analysis showed that PSD was associated with male sex (p < 0.001), early HS onset (p < 0.001), higher Hurley stage (p < 0.01), number of sinus tracts (p < 0.001) and the presence of perianal involvement (p < 0.05).
For 106 patients, information regarding the elapsed time between PSD and HS diagnosis was available and was examined. No new diagnoses of PSD were made after starting follow-up at the specialized unit. A period of 11.05 ± 7.73 years elapsed between the diagnosis of PSD and that of HS. A lower educational level was associated with a longer time between PSD-HS diagnosis (R-squared: 0.4; p < 0.05). Longer time to HS diagnosis was also correlated with longer HS duration (R-squared 0.12; p < 0.001), increasing age (R-squared: 0.22; p < 0.001) and with a greater number of regions involved (R-squared: 0.03; p < 0.001). Although Hurley stage was not significantly associated with longer time to HS diagnosis, a trend could be observed (R-squared 0.03; p = 0.24) (shown in Fig. 1).
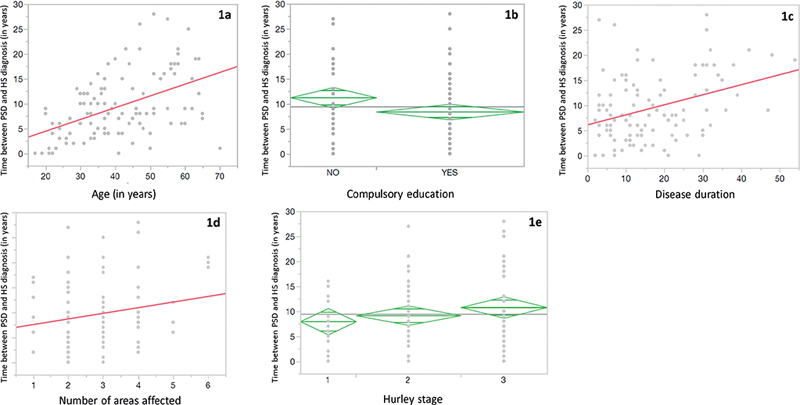
Fig. 1. For 106 patients for whom information regarding elapsed time between pilonidal sinus disease (PSD) and hidradenitis suppurativa (HS) diagnosis was available, this figure shows the relationships between elapsed time (in years) PSD and HS diagnosis as well as (a) patients’ age (in years), (b) educational level, (c) disease duration (in years), (d) number of areas affected, and (e) Hurley stage. Spanish compulsory education conventionally comprises 13 years.
DISCUSSION
This study found a high prevalence of PSD among patients with HS. Several comorbidities have been describ-ed for HS (18), but PSD is notably the disorder most frequently associated with HS (16–21). Worstman et al. (15) consider that PSD and HS share structural sonographic similarities, yet more fibrosis and scar tissue is found in intergluteal HS cysts compared with PSD. This could mean that PSD and HS are part of the same disease spectrum, but are detected at different time-points.Nevertheless, the importance of PSD as a prognostic factor for these patients has not been clearly outlined to date. This manifestation, as the current data shows, seems to be closely tied to a more severe course of HS.
In their series of 302 patients, Canoui-Poitrine et al. (18) described a trend towards greater HS severity in patients with PSD. In contrast with our result, no significant association was reported. A broader consensus exists regarding the role of PSD in HS group stratification. It is relatively established that, whereas female patients present with milder disease, affecting ventral regions (armpit, breasts and inguinal regions), males tend to display greater disease severity with atypical locations and more frequently related to others manifestations of follicular occlusion (22–26).
In 2019, Benhadou et al. conducted a cross-sectional study exploring the prevalence of inflammatory lesions in the intergluteal fold of patients with HS, in order to analyse the sociodemographic and clinical characteristics of this population. In concordance with this study, the current study showed that patients with a PSD record were more likely to be men, smokers, and have earlier onset of HS (24). It is worth noting the different approach to categorizing PSD and perianal involvement between the studies. While the current study did not consider perianal involvement and PSD as mutually exclusive categories, Benhadou et al. did. This would clarify the reason behind the disparity within the prevalence of perianal involvement in the aforementioned study (22%) in comparison with the current data (12.04%). In any case, the actual prevalence of perianal involvement in HS in the literature is unclear, with contradicting figures probably due to lack of standardization (16, 17, 20, 21, 28).
Elapsed time between PSD diagnosis and HS diagnosis has barely been studied in detail in the literature. The current results suggest that it may have an impact on HS progression. On the one hand, this may be due to the lack of knowledge and diagnostic difficulties that HS has presented to health professionals for many years. Currently, there seems to be greater awareness and knowledge of the disease among dermatologists, decreasing the time that elapses between the 2 diagnoses (29, 30). On the other hand, the educational level of patients could also play an important role in bringing forward or delaying the diagnosis of HS. This is especially relevant since the disease is more frequent in a population with a low sociocultural level. A longer time between both events hints at a trend towards the presence of more severe HS due to disease progression. HS is linked with long diagnostic delay, approximately 7 years from the debut of symptoms (4). Delayed HS diagnosis and treatment results in more extensive and severe disease, surgical management and impairment of patients’ career (31). PSD could precede full expression of HS and is more easily diagnosed by non-dermatologists (15, 32). Under these circumstances, the current data provides practical insight into the diagnosis and management of HS. PSD constitutes a prognostic factor for the severity of HS when presenting prior to the diagnosis of HS. Consequently, PSD could be used to identify higher-risk patients in an early fashion, acting as a sentinel event and prompting rapid diagnosis and close follow-up in this subgroup of patients. This strategy could possibly widen the window of opportunity for HS treatment.
Limitations
As a limitation of this study, causal inferences cannot be provided, given its cross-sectional character. Furthermore, the prevalence of PSD might be lower than the current data suggests due to the inherent selection bias. The patients all come from tertiary referral hospital, while mild disease is managed by primary care physicians and was not contemplated in our design. Male over-representation in the current population, given HS affects mostly women, may also respond to this cause, since males carry the burden of severe HS.
Future research could examine the aetiopathogenesis behind the observations presented in this paper or examine the prognostic weight of the remainder of the disorders within the follicular occlusion tetrad. In addition, implementing an educational programme for those patients undergoing PSD surgery, alerting them about symptoms related to HS in case they present in the future, would constitute a novel selective screening method for high-risk HS.
Conclusion
The presence of PSD prior to HS diagnosis could entail prognostic significance. It is associated with greater inflammatory activity and structural disease severity. It is a marker of patients with a higher risk of progression who could benefit from early detection, close follow-up and pro-active management. SPD is commonly diagnosed and treated by general practitioners and general surgeons and, therefore, could behave as a sentinel event to identify higher-risk patients with HS.
ACKNOWLEDGEMENTS
Reviewed and approved by Fundació de Recerca Parc Taulí; approval #2015520 and Hospital Universitario Virgen de las Nieves; approval #0105-N-20.
REFERENCES
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol 2009; 60: 539–561.
- Zouboulis CC, Bechara FG, Dickinson-Blok JL, Gulliver W, Horváth B, Hughes R, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization – systematic review and recommendations from the HS ALLIANCE working group. J Am Acad Dermatol 2019; 33: 19–31.
- Montero-Vilchez T, Diaz-Calvillo P, Rodriguez-Pozo J-A, Cuenca-Barrales C, Martinez-Lopez A, Arias-Santiago S, et al. The burden of hidradenitis suppurativa signs and symptoms in quality of life: systematic review and meta-analysis. Int J Environ Res Public Health 2021; 18: 6709.
- Saunte DM, Boer J, Stratigos A, Szepietowski JC, Hamzavi I, Kim KH, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol 2015; 173: 1546–1549.
- Jiang SW, Whitley MJ, Mariottoni P, Jaleel T, MacLeod AS. Hidradenitis suppurativa: host-microbe and immune pathogenesis underlie important future directions. J Invest Dermatol 2021; 1: 100001.
- Nazary M, van der Zee HH, Prens EP, Folkerts G, Boer J. Pathogenesis and pharmacotherapy of Hidradenitis suppurativa. Eur J Pharmacol 2011; 672: 1–8.
- Pillsbury DM, Shelley WB, Kligman AM. Bacterial infections of the skin. In: Pillsbury DM, editor. Dermatology. Philadelphia: WB Saunders; 1956, p. 482–484.
- Plewig G. Acne morphogenesis and treatment. S.l.: Berlin: Springer; 2012.
- Plewig G, Kligman AM. In: Plewig G, Kligman AM editors. Acne morphogenesis and treatment. Berlin: Springer; 1975, p. 190–192.
- Mohanna PN, Flemming AFS, Al-Sam SZ. Subungual pilonidal sinus of the hand in a dog groomer. Br J Plast Surg 2001; 54: 176–178.
- Patel MR, Bassini L, Nashad R, Anselmo MT. Barber’s interdigital pilonidal sinus of the hand: A foreign body hair granuloma. J Hand Surg Am 1990; 15: 652–655.
- Patey DH, Scarff RW. Pathology of postanal pilonidal sinus; its bearing on treatment. Lancet 1946; 2: 484–486.
- von Laffert M, Stadie V, Ulrich J, Marsch WC, Wohlrab J. Morphology of pilonidal sinus disease: some evidence of its being a unilocalized type of hidradenitis suppurativa. Dermatology 2011; 223: 349–355.
- Khanna A, Rombeau JL. Pilonidal disease. Clin Colon Rectal Surg 2011; 24: 46–53.
- Boer J, Nazary M, Riis PT. The role of mechanical stress in hidradenitis suppurativa. Dermatol Clin 2016; 34: 37–43.
- Wortsman X, Castro A, Morales C, Franco C, Figueroa A. Sonographic comparison of morphologic characteristics between pilonidal cysts and hidradenitis suppurativa: sonography of pilonidal cysts and hidradenitis. J Ultrasound Med 2017; 36: 2403–2418.
- Lee JH, Kwon HS, Jung HM, Kim GM, Bae JM. Prevalence and comorbidities associated with hidradenitis suppurativa in Korea: a nationwide population-based study. J Eur Acad Dermatol 2018; 32: 1784–1790.
- Ingram JR. The epidemiology of hidradenitis suppurativa. Br J Dermatol 2020; 183: 990–998.
- Canoui-Poitrine F, Revuz JE, Wolkenstein P, Viallette C, Gabison G, Pouget F, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol 2009; 61: 51–57.
- Hua VJ, Kilgour JM, Cho HG, Li S, Sarin KY. Characterization of comorbidity heterogeneity among 13,667 patients with hidradenitis suppurativa. JCI Insight 2021; 6: e151872.
- Schrader AMR, Deckers IE, van der Zee HH, Boer J Prens EP. Hidradenitis suppurativa: a retrospective study of 846 Dutch patients to identify factors associated with disease severity. J Am Acad Dermatol 2014; 71: 460–467.
- Vazquez BG, Alikhan A, Weaver AL, Wetter DA, Davis MD. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol 2013; 133: 97–103.
- Canoui-Poitrine F, Thuaut AL, Revuz JE, Viallette C, Gabison G, Poli F, et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol 2013; 133: 1506–1511.
- González-Manso A, Agut-Busquet E, Romaní J, Vilarrasa E, Bittencourt F, Mensa A, et al. Hidradenitis suppurativa: proposal of classification in two endotypes with two-step cluster analysis. Dermatology 2021; 237: 365–371.
- Benhadou F, Van der Zee HH, Pascual JC, Rigopoulos D, Katoulis A, Liakou AI, et al. Pilonidal sinus disease: an intergluteal localization of hidradenitis suppurativa/acne inversa: a cross-sectional study among 2465 patients. Br J Dermatol 2019; 181: 1198–1206.
- Cazzaniga S, Pezzolo E, Bettoli V, Abeni D, Marzano AV, Patrizi A, et al. Characterization of hidradenitis suppurativa phenotypes: a multidimensional latent class analysis of the National Italian Registry IRHIS. J Invest Dermatol 2021; 141: 1236–1242.
- Kimball AB, Sundaram M, Gauthier G, Guérin A, Pivneva I, Singh R, et al. The comorbidity burden of hidradenitis suppurativa in the United States: a claims data analysis. Dermatol Ther 2018; 8: 557–569.
- Yüksel M, Basım P. Demographic and clinical features of hidradenitis suppurativa in Turkey. J Cutan Med Surg 2020; 24: 55–59.
- McMillan K. Diagnoses of hidradenitis suppurativa in the United States, 1979–2012. Skin Appendage Disord 2015; 1: 117–122.
- Garg A, Lavian J, Lin G, Strunk A, Alloo A. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol 2017; 77: 118–122.
- Kokolakis G, Wolk K, Schneider-Burrus S, Kalus S, Barbus S, Gomis-Kleindienst S, et al. Delayed diagnosis of hidradenitis suppurativa and its effect on patients and healthcare system. Dermatology 2020; 236: 421–430.
- Lopes S, Vide J, Costa-Silva M, Azevedo F, Magina S. Awareness, knowledge, and practice patterns of general practitioner residents and specialists toward hidradenitis suppurativa: a survey study. Acta Dermatovenerol Alp Pannonica Adriat 2019; 28: 61–63.