ORIGINAL REPORT
Ablative Fractional Laser Enhances Artificial or Natural Daylight Photodynamic Therapy of Actinic Field Cancerization: A Randomized and Investigator-initiated Half-side Comparative Study
Vivian LINDHOLM, Mari SALMIVUORI, Sonja HAHTOLA, Kerttu MÄKELÄ, Sari PITKÄNEN# and Kirsi ISOHERRANEN#
Department of Dermatology, Allergology and Venereology, Helsinki University Hospital and University of Helsinki, Helsinki, Finland
#These authors contributed equally.
Artificial daylight photodynamic therapy is a near-painless treatment for actinic keratoses, which can be performed indoors using a controlled light dose. Daylight photodynamic therapy is approved only for treatment of grade I–II actinic keratoses. The aim of this study was to evaluate whether fractional laser pre-treatment improves the outcomes of daylight photo-dynamic therapy for actinic keratoses of all grades. In addition, the study compared the outcomes of artificial and natural daylight photodynamic therapy. This randomized single-blinded split-side comparative study included 60 patients with ≥ 2 actinic keratoses of the head. Fractional laser pre-treatment was assigned randomly for actinic keratoses on 1 side of the head and, subsequently, the entire treatment area was treated with artificial or natural daylight photodynamic therapy. Fractional laser-mediated daylight photodynamic therapy achieved significantly higher complete clearance (50.0% vs 30.3%, p = 0.04), partial clearance (78.6% vs 50.0%, p < 0.01) and lesion-specific clearance (86.2% vs 70.2%, p < 0.01) than daylight photodynamic therapy alone at the 6-month follow-up. No significant differences were found in the outcomes of artificial vs natural daylight photodynamic therapy or grade I lesions vs grade II–III lesions. Thus, fractional laser pre-treatment appears to significantly increase the efficacy of artificial and natural daylight photodynamic therapy, and to be suitable for treatment of actinic keratoses of all grades.
Key words: actinic keratosis; laser therapy; photochemotherapy.
SIGNIFICANCE
Actinic keratoses are common precancerous skin lesions caused by chronic sun exposure, which can evolve into squamous cell carcinoma. Treatment of actinic keratoses is non-surgical, and daylight photodynamic therapy is an option. This study of 60 patients with actinic keratoses investigated whether ablative fractional laser pre-treatment increases the efficacy of daylight photodynamic therapy. At the 6-month follow-up visit, laser pre-treated lesions showed significantly higher complete clearance rates (50%) compared with daylight photodynamic therapy alone (30%). Laser pre-treatment was effective in the treatment of actinic keratoses of all thicknesses (p > 0.1). In addition, the treatment was mostly well tolerated (35/37 patients) and the cosmetic result good (36/39 patients).
Citation: Acta Derm Venereol 2023; 103: adv6579. DOI https://doi.org/10.2340/actadv.v103.6579.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: May 31, 2023; Published: Aug 16, 2023
Corr: Vivian Lindholm, Helsinki University Hospital, Skin and Allergy Hospital, Meilahdentie 2, 00250 Helsinki, Finland. E-mail: vivian.lindholm@helsinki.fi
Competing interests and funding: No medical companies participated in funding this study. VL has received support for congress fees from Pfizer and UCB Pharma, and a travel grant and speaker honoraria from Galderma. SH and MS have received support for a congress fee from Leo Pharma and MS support for a congress fee from Galderma.
This non-sponsored investigator-initiated study was supported by the Clinical Research Institute HUCH, Helsinki University Hospital’s Inflammation Centre and Emil Aaltonen Foundation. The funders did not have any impact on study design, data collection, data analysis, manuscript preparation and/or publication decisions.
INTRODUCTION
Photodynamic therapy (PDT) is a first-line treatment option for actinic field cancerization (1). The efficacy and cosmetic outcomes of PDT are adequate; however, conventional PDT (cPDT) can cause extensive pain during treatment (1).
Daylight-PDT (dPDT) has been introduced as a near-painless treatment alternative to cPDT. It enables treatment of large areas and is as effective as cPDT for thin grade I–II actinic keratoses (AKs) (2). However, for thicker AKs, the efficacy of dPDT can decrease (3). According to European guidelines, natural dPDT (ndPDT) can be used to treat grade I–II AKs at temperatures above 10°C from March to October in Finland, but not during rain (2). In environments with variable and cold weather conditions, in particular, an option is artificial dPDT (adPDT) performed indoors, although this has not yet been extensively studied. There are several benefits of adPDT: the pain is as low as in ndPDT (4), it can be performed at any time in a controlled and standardized environment, and there is no ultraviolet radiation. There have been a few studies on adPDT, using various light sources (5–8). Only 1 of these studies is a randomized controlled trial (RCT), in which adPDT was compared split-side with ndPDT on 22 patients, with comparable results (p = 0.21) (6).
Pre-treatment with ablative fractional laser (AFXL) facilitates absorption of the photosensitizer (9), and enhances the formation of the active substance, protoporphyrin IX (PpIX), compared with curettage and other pre-treatments (10). AFXL pre-treatment has proven effective without causing additional pain in cPDT (9, 11), and is thus a promising alternative to improve the outcome and possibly also extend the use of dPDT in the treatment of thicker AKs. To our knowledge, there are 5 studies on AFXL-mediated dPDT (AFXL-dPDT) (12–16). Four studies used natural daylight and 1 used a multi-light lamp as illumination source. All studies included AKs of all grades. There is a need for larger scale studies on the subject, as 4 of the studies included fewer than 20 patients.
MATERIALS AND METHODS
This study compared AFXL-dPDT with dPDT alone in a randomized controlled split-head and investigator-blinded study on 60 patients during the years 2018 to 2022 at the Dermatology Outpatient Clinic, Skin and Allergy Hospital, Helsinki University Hospital, Helsinki, Finland. All the recruited patient volunteers were informed about the study orally and in writing and provided written consent. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Helsinki University Hospital (HUS/137/13/03/01/16).
In randomization, the lesions on 1 side of the head were assigned to AFXL-dPDT and the other side, treated with dPDT alone, served as control. The method of randomization was a simple draw, in which patients blindly selected the side for AFXL. Patients with extensive field cancerization on the treatment area were assigned for AFXL field treatment (n = 9), in which the entire side of the treatment area, not only the individual AKs, was pre-treated with AFXL.
A sample size of 60 patients was estimated with power calculations, assuming that 70% of patients would receive complete clearance in AFXL-dPDT compared with 35% in dPDT. The recruited patients had ≥ 2 AKs in total, located on both sides of the head (scalp, forehead, temples, or cheeks). They were aged ≥ 18 years, with Fitzpatrick skin type I–III and were in follow-up at the hospital due to recurrent skin tumours or their precursors, or were referred to the clinic due to several AKs on the head. Patients with pigmented AKs, in situ-carcinomas, skin cancers, psoriasis or eczema on the treatment area were excluded. After exclusions and drop-outs, 56 patients were analysed in total. The study flow-chart is presented in Fig. 1. Immunosuppressed patients were included in the study, 5 patients were on immunosuppressive treatments (2 methotrexate, 1 sulfasalazine, 2 chemotherapy); however, none of the patients had recieved an organ transplant. A total of 18 patients underwent ndPDT and 38 patients adPDT, depending on the season. At the 6-month in-hospital control appointment, a blinded dermatologist (KI, SH, or KM) evaluated the lesion-specific clearance (LSC) for each lesion. Only persistent lesions were documented, not new untreated ones. Further treatment was planned for non-cleared lesions. Dyspigmentation after treatment was assessed and the patients were asked if they would agree to re-treatment with AFXL-dPDT.
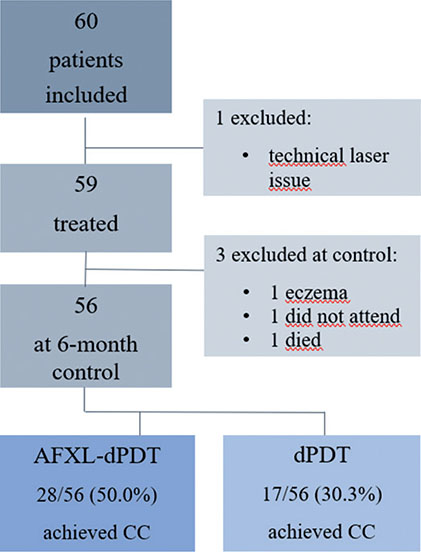
Fig. 1. Flow-chart of the study and patient complete clearance-results. AFXL-dPDT: ablative fractional laser-mediated daylight photo-dynamic therapy; CC: complete clearance; dPDT: daylight photodynamic therapy.
Treatment protocol
Before treatment, the lesions were documented with photographs and their location marked on a plastic sheet to enable precise follow-up of each lesion (Fig. 2). The thickness of the lesions was graded I–III by a dermatology resident (VL) or dermatologist (MS) (17). Daylight PDT was performed according to current guidelines (2). A chemical sun protection factor (SPF) 50 sunscreen was applied in a thin layer on all bare skin areas 15 min prior to ndPDT. The entire treatment area was curettaged, and, if necessary, liquid aluminium chloride was used for haemostasis. Then, a thin layer of lidocain anaesthetic spray (Xylocain®, Aspen Pharma, Ballerup, Denmark) was applied on the entire area, and if requied, additional lidocaine c. adrenalin 10mg/ml + 10 mikrog/ml injections were applied to areas recieving AFXL (n = 2). Lesions/fields assigned to AFXL treatment were treated with an ablative fractional CO2 laser (Candela® 19 W (Candela Medical®, Marlborough, US), Dot mode, spacing 1,000 µm, stack 3, scanning dwell time 1800 µs, repeat 0.5 s). Subsequently a thin layer of photosensitizer (methyl aminolaevulinate cream, Metvix®, Galderma, La Tour-de-Peilz, Switzerland) was applied in a thin layer on the treatment area. The patients were asked to move outdoors or to the adPDT room (IndoorLux®, Swiss Red AG, Murten, Switzerland, wavelength 350–750 nm, 15–25,000 lux) within 30 min for the 2-h illumination. NdPDT was performed only in June–August, not in the rain, at temperatures above 10°C and with at least a 10,000-lux illumination before starting ndPDT. Otherwise, the adPDT room was used.
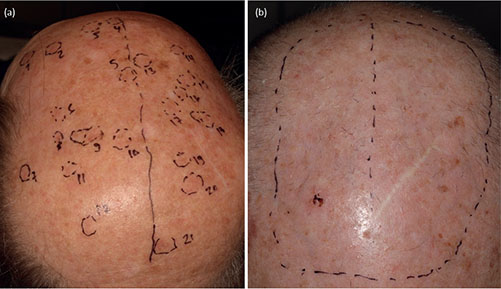
Fig. 2. (a) Forty-seven patients had laser treatment of lesions only, followed by daylight photodynamic therapy (dPDT) of the entire treatment area; and (b) 9 patients had laser treatment of the entire treatment area and subsequent dPDT, depending on the degree of field cancerization. One side of the treatment area recieved laser-treatment in addition to dPDT, and the other side dPDT alone. (a) Forty-seven patients had laser treatment of lesions only (lesions marked with number 1-21 in the picture), and (b) 9 patients had laser treatment of the entire treatment area (circumscribed with a dashed line in the picture), depending on the degree of field cancerization.
Outcome measures
Patient complete and partial clearance. The results are presented as patient complete clearance (CC), defined as all treated lesions of the patient in the corresponding treatment completely cleared, and partial clearance (PC) if, correspondingly, 75% of lesions were completely cleared at 6 months.
Lesion-specific clearance. LSC, the proportion of lesions that were completely, partially (somewhat cleared but still detectable), or not cleared (no signs of treatment response) at 6 months within the particular treatment, were additionally reported.
Secondary outcome measures
Illumination source. CC and PC of adPDT were compared with those of ndPDT at 6 months.
Lesion grade, treatment history. The study assessed if the lesion grade (grade I or grade II–III) or treatment history for AKs (no or previous treatment) affected the 6-month outcomes. Grades II–III were combined in the analyses due to the low number of grade III lesions (n = 26).
Treatment tolerability. Patient-estimated maximal pain during illumination was documented separately in AFXL-dPDT and dPDT treatments using a numerical rating scale (NRS) from 0 to 10. To further evaluate treatment tolerability, at the 6-month follow-up 37 patients were asked if they would agree to AFXL-dPDT treatment in the future. The first 19 patients were not asked this question, as it was added to the study protocol at a later stage.
Aesthetic outcome. Hypopigmentation or hyperpigmentation 6 months after treatment was documented for 39 patients (the first 17 patients were not assessed for this).
Statistical analysis
Cross-tabulation and McNemar’s test were used for CC and PC analyses, and cross-tabulation and Pearson’s χ2 for LSC analyses, adPDT-ndPDT comparison, and determination of confounding factors. The impacts of lesion grade or treatment history were determined using logistic regression. For pain calculations Wilcoxon signed-rank test was used. The analyses were performed by a trained statistician, with NCSS statistical software 12.0.17 (NCSS, Kaysville, UT, USA). Significance was set at p < 0.05.
Results
Main outcomes
Patient complete and partial clearance. AFXL-dPDT achieved statistically significantly higher (p = 0.04) CC (28/56 patients, 50.0%) compared with dPDT (17/56, 30.3%). PC was also significantly higher in AFXL-dPDT (44/56 patients, 78.6%; p < 0.01) (compared with dPDT (28/56, 50.0%). Detailed results are shown in Fig. 3. Mean lesion number of the patient in AFXL-dPDT and dPDT were comparable (p = 0.31, 4.6 in AFXL-dPDT and 4.4 in dPDT).
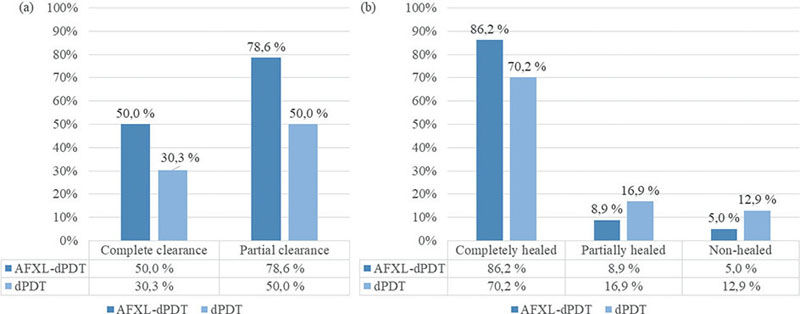
Fig. 3. Ablative fractional laser-mediated daylight photodynamic therapy (AFXL-dPDT) achieved significantly higher a) complete (p = 0.04), partial (p < 0.01) and b) lesion-specific (p < 0.01) clearance rates than did photodynamic therapy (dPDT).
Lesion-specific clearance. AFXL-dPDT reached significantly higher LSC than dPDT (p < 0.01). Of 260 lesions treated with AFXL-dPDT, 224 cleared completely (86.2%), compared with 174 of 248 (70.2%) in dPDT. Detailed results are shown in Fig. 3. The lesions within both treatment groups were comparable regarding the patient’s age (p = 0.17), sex (p = 0.83), skin type (p = 0.92), immunosuppression (p = 0.43), number of residual lesions (p = 0.86, definition in Table I) or treatment area of the patient (p = 0.57).
| Patient characteristics | |
| Patients in total, n | 56 |
| Age, years, mean | 76.6 |
| Sex, n (%) | |
| Male | 48 (86) |
| Female | 8 (14) |
| Fitzpatrick skin type, n (%) | |
| I | 7 (13) |
| II | 28 (50) |
| III | 21 (38) |
| Immunosuppression, n (%) | 5 (9) |
| Organ transplant recipients, n (%) | 0 (0) |
| Treatment history for actinic keratoses, n (%) | |
| No treatment | 8 (14) |
| Cryotherapy | 43 (77) |
| Topical treatment | 27 (48) |
| Photodynamic therapy | 14 (25) |
| Daylight treatment, n (%) | |
| Natural | 18 (32) |
| Artificial | 38 (68) |
| Treatment area, n (%) | |
| Scalp | 25 (45) |
| Forehead | 15 (27) |
| Temples | 4 (7) |
| Cheeks | 12 (21) |
| Lesion number, mean | 9.1 |
| Lesions in AFXL-dPDT | 4.6 |
| Lesions in daylight photodynamic therapy | 4.4 |
| Patients with field treatmenta, n (%) | 9 (16) |
| Lesion characteristics | |
| Lesions in total, n | 508 |
| Lesion grade, n (%) | |
| I | 361 (71) |
| II | 124 (24) |
| III | 23 (4) |
| Residual lesionb, n (%) | 115 (23) |
| Treatment group, n (%) | |
| AFXL-dPDT | 260 (51) |
| Daylight photodynamic therapy | 248 (49) |
| Daylight source, n (%) | |
| Natural | 144 (28) |
| Artificial | 364 (72) |
| aAFXL-field treatment in the ablative fractional laser-assisted daylight photodynamic therapy (AFXL-dPDT) study, conventional PDT (cPDT)-field treatment in cPDT study. bKnown residual tumour or previous field treatment on the treatment area within 1.5 years. | |
Secondary outcomes
Illumination source. AdPDT achieved similar efficacy as ndPDT. The outcomes (CC and PC) separately for AFXL-dPDT and dPDT and their p-values (p > 0.09) are shown in Table II. The groups receiving adPDT and ndPDT were comparable regarding age (p = 0.06), skin type (p = 0.41), previous treatment for AKs (p = 0.64), and lesion number (p = 0.26) before treatment.
Lesion grade. Grade I lesions cleared completely with a higher odds ratio (OR) of 1.42 compared with grade II–III lesions; however, this difference did not reach significance (p = 0.14). Significance was also not achieved even when assessing AFXL-dPDT (p = 0.84) and dPDT separately (p = 0.10) (Table III).
| AFXL-dPDT | dPDT | p-valuec | OR | 95% CI | |
| Lesion grade | |||||
| Grade I | 160/185 (86.5%) | 129/176 (73.3%) | 0.14 | 1.42 | 0.89–2.24 |
| Grade II–III | 64/75 (85.3%) | 45/72 (62.5%) | |||
| pa | 0.84 | 0.10 | |||
| Previous treatment | |||||
| No | 37/44 (84.1%) | 30/39 (76.9%) | 0.61 | 1.17 | 0.64–2.14 |
| Yes | 187/216 (86.6%) | 144/209 (68.9%) | |||
| p-valueb | 0.81 | 0.35 | |||
| ap-value for the impact of lesion grade for AFXL-dPDT or dPDT-treated lesions separately. bp-value for the impact of previous treatment for AFXL-dPDT or dPDT-treated lesions separately. cp-value for the overall impact of lesion grade/previous treatment for both treatments. | |||||
| AFXL-dPDT: ablative fractional laser-assisted daylight photodynamic therapy; 95% CI: 95% confidence interval; dPDT: daylight photodynamic therapy; OR: odds ratio. | |||||
Previous treatment. There was no significant difference (p = 0.61) in outcomes for patients with or without previous treatment for AKs (OR 1.17 if previous treatment), indicating a 17% higher treatment efficacy for patients with previous treatments. When assessing AFXL-dPDT (p = 0.81) and dPDT separately (p = 0.35), the outcomes for these groups also did not differ (Table III).
Treatment tolerability. Mean pain during dPDT illumination was 1.2 (NRS) in AFXL-dPDT treatment and comparable to dPDT (1.1, p = 0.71). Maximal pain during AFXL pre-treatment-pulses was a mean of 5.1. Thirty-five of the 37 patients (95%) stated that they would agree to re-perform AFXL-dPDT in the future.
Aesthetic outcome. When assessed for dyspigmentation, 3 of 39 (7.7%) patients had hypopigmentation in 1 of the treatment areas at the control. Of these patients, 1 had hypopigmentation from both AFXL-dPDT and dPDT, 1 only from dPDT treatment, and for 1 patient the side with hypopigmentation was not defined.
DISCUSSION
These results confirm the efficacy of AFXL-dPDT in a randomized setting with, to our knowledge, the highest number of patients published to date. Complete clearance at 6 months was achieved for 50% of patients in AFXL-dPDT, which was significantly higher than for dPDT alone (30%). Two previous studies on AFXL-dPDT reported CC values; in the first on 46 patients, 72% achieved CC in artificial AFXL-dPDT (13), and in the other on 12 patients 76% achieved CC in natural AFXL-dPDT, and 64% in ndPDT alone (15). The CC of the current patients was slightly lower compared with these studies; however, the current follow-up was longer (6 vs 3–4 months) (13–16). The patients recruited to the current study were mostly severely photodamaged and difficult-to-treat; 73% of the current patients had had previous field treatments for AKs (Table I) and 9% were immunosuppressed.
Regarding lesion-specific clearance of the current study, 86% of lesions in AFXL-dPDT, and 70% of lesions in dPDT cleared completely. In a previous study on 18 patients, the LSC-results were comparable to those of the current study; 81% of lesions receiving AFXL-ndPDT cleared completely (14). However, they did not present clinically relevant patient-specific clearance results.
To our knowledge, only 1 previous RCT on adPDT (6) has been published, in which adPDT with an operating room light-emitting diode (LED) light is compared with ndPDT. In the half-side comparative study on 22 patients, the amount of AKs reduced equally on both treatments (p = 0.21); by 62% in ndPDT and 68% in adPDT at 1 month. In the current study, the outcomes of adPDT and ndPDT were also similar (p < 0.09), although a trend towards superior CC rates for ndPDT was seen in AFXL-dPDT (Table II). Overall, adPDT appears to be as effective as ndPDT in the treatment of AKs.
AFXL creates microscopic channels in the tissue, which aids the absorption of photosensitizer and, in addition, has direct toxic effects on cells (11). AFXL increases treatment efficacy (11) and possibly also the treatment response for thicker AKs. In the current study, grade I lesions and grade II–III lesions achieved similar clearance in AFXL-dPDT (p = 0.84); however, these clearance rates were also similar in dPDT (p = 0.10). Although statistically insignificant, the proportion of completely cleared lesions of grade I (87%) compared with grade II–III (85%) were similar in AFXL-dPDT, whilst in dPDT a higher proportion of grade I lesions cleared completely (73%) compared with grade II–III lesions (63%).
To further increase the clinical relevance of the study, the outcomes of AFXL-dPDT were additionally compared with cPDT in our previous study branch, in which we compared cPDT with pulsed-dye laser-mediated PDT in a half-side comparative manner on 59 patients with an equal recruitment and inclusion process (18). The patient and lesion characteristics of both studies were similar regarding the patients’ age, skin type, treatment history and lesion grades; however, the mean lesion number per patient was significantly higher in the current study (p < 0.01). Promisingly, AFXL-dPDT achieved similar CC rates (50% vs 45%, p = 0.58) and even higher PC rates (79% vs 60%, p = 0.04) than cPDT. Also regarding LSC rates, AFXL-dPDT with 86% of lesions completely cleared, was superior to cPDT with 73% of lesions completely cleared (18). A previous study on 16 patients also achieved higher efficacy in AFXL-ndPDT compared with cPDT. They included organ transplant recipients and lesions of the trunk and extremities, both of which can be more difficult to treat (19). The results of the study were similar to those of the current study, with 74% of lesions cleared in AFXL-dPDT, 46% in ndPDT, 50% in cPDT, and 5% in AFXL alone at 3 months (16).
In the current study, the patient-reported pain values during the dPDT illumination were negligible, 1.2 and 1.1 in mean in AFXL-dPDT and dPDT, respectively. During the short-term AFXL-pre-treatment, mean maximal pain was 5.1, which is higher than the value (4.1) achieved during the illumination in cPDT in our previous study branch (18). However, the nature and duration of the treatments differ considerably; AFXL causes only short-term ( < 1 s pulses) pain on small ( < 1 cm2) areas at once, while the pain in cPDT is continuous on the entire treatment area during the 7–8-min illumination. Only 2 patients required local anaesthesia to complete AFXL. Of the assessed patients, 95% stated that they would agree to AFXL-dPDT in the future. Most previous studies on AFXL-dPDT showed similar pain values (14–16). Thus, when considering the current and the previous study branch’s results, AFXL-dPDT could be the choice of treatment instead of cPDT, with similar efficacy, but higher tolerance.
Treatment of field cancerization is demanding, and studies comparing treatments head-to-head are lacking. An advantage of AFXL is that it can be used on large areas; the current study used it on areas > 290 cm2 with-out notable tolerability issues. In addition, the AFXL-dPDT-treatment does not require patient compliance to the same degree as, for example, topical treatments. In AFXL-dPDT the in-office time is short, consisting of only the rapid AFXL and subsequent application of photosensitizer. The downtime after the single AFXL-dPDT treatment is shorter, only a few weeks, compared with topical treatments that last from weeks up to months in addition to the subsequent weeks of downtime (20).
The disadvantages of AFXL-dPDT are the higher costs of the laser device and the photosensitizer cream, the higher pain values than in dPDT alone, the higher risk of skin reactions (21) and possibly, dyspigmentation post-treatment. However, the fairly high costs can be justified by a high efficacy and the possibility to treat large areas of field cancerization.
Artificial daylight PDT is not yet widely studied, and only 1 previous study has combined it with AFXL (13). A strength of this study is its intraindividual randomized controlled and investigator-blinded design. A limitation of the study is no histological verification of lesion diagnoses due to ethical reasons. Dyspigmentation or consent to retreatment were not documented for all study patients.
In conclusion, AFXL-dPDT appears to be effective and well-tolerated in the treatment of actinic field cancerization and reached significantly better outcomes than dPDT alone in this study. AFXL-dPDT was effective in the treatment of AKs of all grades, and compared with cPDT of our previous study branch, AFXL-dPDT was equally or even more effective. However, more large-sampled RCTs on the subject are required to certify these results. Based on the current results, we suggest that AFXL-dPDT is a valuable treatment option, especially for patients with multiple thicker or recurrent AKs or large areas of field cancerization.
ACKNOWLEDGEMENTS
The authors thank Timo Pessi, MSc, for performing the statistical analyses and the staff members of the Department of Dermatology, Helsinki University Hospital, for recruiting patients.
Institutional Review Board approval status: The study was conduct-ed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Helsinki University Hospital (protocol code HUS/137/13/03/01/16, date of approval 5 July 2016). The study is registered at clinicaltrials.gov (registration number NCT05456334).
REFERENCES
- Werner RN, Jacobs A, Rosumeck S, Erdmann R, Sporbeck B, Nast A. Methods and results report – evidence and consensus-based (s3) guidelines for the treatment of actinic keratosis – International League of Dermatological Societies in cooperation with the European Dermatology Forum. J Eur Acad Dermatol Venereol 2015; 29: 1–66.
- Morton CA, Wulf HC, Szeimies RM, Basset-Seguin N, Sotiriou E, Piaserico S, et al. Practical approach to the use of daylight photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: a European consensus. J Eur Acad Dermatol Venereol 2015; 29: 1718–1723.
- Wiegell SR, Fabricius S, Gniadecka M, Stender IM, Berne B, Kroon S, et al. Daylight-mediated photodynamic therapy of moderate to thick actinic keratoses of the face and scalp: a randomized multicentre study: daylight-mediated PDT of moderate to thick actinic keratoses. Br J Dermatol 2012; 166: 1327–1332.
- Mordon S, Vignion-Dewalle AS, Thecua E, Vicentini C, Maire C, Deleporte P, et al. Can daylight-PDT be performed indoor? G Ital Dermatol E Venereol Organo Uff Soc Ital Dermatol E Sifilogr 2018; 153: 811–816.
- Kellner C BS Hollstein S, Renhold U. Simulated-daylight photodynamic therapy with BF-200 aminolaevulinic acid for actinic keratosis: assessment of the efficacy and tolerability in a retrospective study. Br J Dermatol 2015; 172: 1132–1164.
- O’Gorman SM, Clowry J, Manley M, McCavana J, Gray L, Kavanagh A, et al. Artificial white light vs daylight photodynamic therapy for actinic keratoses: a randomized clinical trial. JAMA Dermatol 2016; 152: 638.
- von Dobbeler C, Schmitz L, Dicke K, Szeimies RM, Dirschka T. PDT with PPIX absorption peaks adjusted wavelengths: safety and efficacy of a new irradiation procedure for actinic keratoses on the head. Photodiagnosis Photodyn Ther 2019; 27: 198–202.
- Maire C, Vignion-Dewalle AS, Cartier H, Mordon S. Artificial white light photodynamic therapy for actinic keratosis: a study of 38 patients in private office practice. J Eur Acad Dermatol Venereol 2020; 34: 165–167.
- Haedersdal M, Sakamoto FH, Farinelli WA, Doukas AG, Tam J, Anderson RR. Pretreatment with ablative fractional laser changes kinetics and biodistribution of topical 5-aminolevulinic acid (ALA) and methyl aminolevulinate (MAL). Lasers Surg Med 2014; 46: 462–469.
- Bay C, Lerche CM, Ferrick B, Philipsen PA, Togsverd-Bo K, Haedersdal M. Comparison of physical pretreatment regimens to enhance protoporphyrin ix uptake in photodynamic therapy: a randomized clinical trial. JAMA Dermatol 2017; 153: 270.
- Steeb T, Schlager JG, Kohl C, Ruzicka T, Heppt MV, Berking C. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol 2019; 80: 947–956.
- Yoon J, Kim YC. Daylight photodynamic therapy with ablative carbon dioxide fractional laser for treating actinic keratosis in Asians: a case series. Photodiagnosis Photodyn Ther 2020; 31: 101905.
- Paasch U, Said T. Treating field cancerization by ablative fractional laser and indoor daylight: assessment of efficacy and tolerability. J Drugs Dermatol 2020; 19: 425–427.
- Wenande E, Phothong W, Bay C, Karmisholt KE, Haedersdal M, Togsverd-Bo K. Efficacy and safety of daylight photodynamic therapy after tailored pretreatment with ablative fractional laser or microdermabrasion: a randomized, side-by-side, single-blind trial in patients with actinic keratosis and large-area field cancerization. Br J Dermatol 2019; 180: 756–764.
- Rizvi S, Veierød M, Mørk G, Helsing P, Gjersvik P. Ablative fractional laser-assisted daylight photodynamic therapy for actinic keratoses of the scalp and forehead in organ transplant recipients: a pilot study. Acta Derm Venereol 2019; 99: 1047–1048.
- Togsverd-Bo K, Lei U, Erlendsson AM, Taudorf EH, Philipsen PA, Wulf HC, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients – a randomized controlled trial. Br J Dermatol 2015; 172: 467–474.
- Lindholm V, Pitkänen S, Schröder M, Hahtola S, Sahi H, Halme H, et al. Pulsed dye laser-mediated photodynamic therapy is less effective than conventional photodynamic therapy for actinic field cancerization: a randomized half-side comparative study. Acta Derm Venereol 2021; 101: adv00404.
- Olsen EA, Lisa Abernethy M, Kulp-Shorten C, Callen JP, Glazer SD, Huntley A, et al. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J Am Acad Dermatol 1991; 24: 738–743.
- Braathen LR, Morton CA, Basset-Seguin N, Bissonnette R, Gerritsen MJP, Gilaberte Y, et al. Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology: PDT for skin field cancerization. J Eur Acad Dermatol Venereol 2012; 26: 1063–1066.
- Dragieva G, Hafner J, Dummer R, Schmid-Grendelmeier P, Roos M, Prinz BM, et al. Topical photodynamic therapy in the treatment of actinic keratoses and Bowen’s disease in transplant recipients. Transplantation 2004; 77: 115–21.
- Ibrahim O, Wenande E, Hogan S, Arndt KA, Haedersdal M, Dover JS. Challenges to laser-assisted drug delivery: applying theory to clinical practice. Lasers Surg Med 2018; 50: 20–27.