ORIGINAL REPORT
Prolonged Antipruritic Effect of Botulinum Toxin Type A on Cowhage-induced Itch: A Randomized, Single-blind, Placebo-controlled Trial
Leigh A NATTKEMPER1, Ashley VANDER DOES1, Carolyn M. STULL2, Michael J. LAVERY2, Rordigo VALDES-RODRIGUEZ2, Marlene MCGREGORY2, Yiong Huak CHEN3 and Gil YOSIPOVITCH1
1Department of Dermatology, Miami Itch Center, University of Miami Miller School of Medicine, Miami, FL, 2Department of Dermatology, Temple University Lewis Katz School of Medicine, Philadelphia, PA, USA and 3Biostatistics Unit, National University of Singapore Lon Lin School of Medicine, Singapore, Singapore
Botulinum toxin type A (Botox®) is thought to have antipruritic effects through inhibition of pruritic factors, including acetylcholine, substance P, and glutamate. The aim of this randomized, single-blind, placebo-controlled trial was to test the effect of botulinum toxin type A on cowhage, a non-histaminergic model for chronic itch. Botulinum toxin type A was injected into the arm of 35 healthy subjects, with a saline control injected into the contralateral arm. Thermal sensory parameters (warmth and heat thresholds and heat pain intensity) and itch intensity after cowhage application were examined on test areas. Botulinum toxin type A reduced itch intensity, overall perceived itch (area under the curve (AUC); percentage change from baseline), and peak itch intensity compared with the control at 1 week, 1 month, and 3 months. Botulinum toxin type A had no effect on thermal thresholds or heat pain intensity. In conclusion, botulinum toxin type A reduced cowhage itch for at least 3 months, which suggests that botulinum toxin type A is a potential long-lasting treatment for localized, non-histaminergic itch.
Key words: antipruritic; botulinum toxin; cowhage; itch.
SIGNIFICANCE
Severe, localized chronic itch can negatively impact quality of life and presents a therapeutic challenge. Botulinum toxin type A (BoNT/A; Botox®) may provide long-lasting, localized relief in certain itchy skin and neurological conditions; however, its effects in chronic (histamine-independent) itch pathways in healthy subjects has not been elucidated. This study utilized cowhage, a plant that induces non-histaminergic itch, to determine the effects of botulinum toxin type A on temperature and itch sensation in healthy subjects. It was demonstrated that botulinum toxin type A reduced itch for at least 3 months. These results suggest that botulinum toxin type A has potential for long-lasting itch relief in localized, chronic itch states.
Citation: Acta Derm Venereol 2023; 103: adv6581. DOI https://doi.org/10.2340/actadv.v103.6581.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: May 17, 2023; Published: Aug 16, 2023
Corr: Gil Yosipovitch, Department of Dermatology, Miami Itch Center, University of Miami Miller School of Medicine, 1600 NW 10th Ave, RMSB 2023, Miami, FL 33136, USA. E-mail: gyosipovitch@med.miami.edu
Competing interests and funding: GY is a consultant for Allergan.
INTRODUCTION
Severe, localized chronic pruritus is often challenging to manage, and available therapeutic options are limited. Topical treatments may provide transient relief, but must be reapplied frequently. Currently available systemic options include immunomodulatory and neuroactive agents, which may not be well tolerated by all patients. Antihistamines are largely ineffective for chronic itch, which is primarily mediated by histamine-independent pathways (1). Given the limitations of these therapies, novel approaches to the treatment of pruritus are needed.
Botulinum toxin type A (BoNT/A), a potent neurotoxin with an array of dermatological applications, has been reported to have antipruritic properties. Studies have shown that BoNT/A inhibits itch in localized inflammatory dermatoses, including dyshidrotic hand dermatitis, lichen simplex chronicus, inverse psoriasis, and atopic dermatitis (2–7). BoNT/A has also been used to relieve itch in neuropathic conditions including notalgia paraesthetica (6, 8, 9). In addition, in a double-blind, placebo-controlled study, subcutaneous BoNT/A significantly reduced histamine-induced itch intensity in healthy subjects (10). These findings, further summarized by Boozalis et al. (11) and Gazerani (12), suggest that BoNT/A may have the ability to reduce itch of varied aetiology.
The antipruritic mechanism of BoNT/A has not been fully elucidated and is likely to be multifactorial. BoNT/A induces cleavage of SNAP-25 (synaptosomal-associated proteins of 25 kDa), which prevents the release of neurotransmitters from presynaptic vesicles (13). This results in inhibition of acetylcholine, which has been shown to induce itch in atopic dermatitis (14–16). In addition, BoNT/A inhibits the release of several neuropeptides with implicated roles in neuropathic pruritus and inflammation. These include substance P (SP), glutamate, and calcitonin-gene related peptide (CGRP) (17–21). Furthermore, downregulation of transient receptor potential cation channel, subfamily A, member 1 (TRPA1) and transient receptor potential cation channel, subfamily V, member 1 (TRPV1) ion channels, which mediate itch induction, has also been demonstrated following BoNT/A administration (22, 23).
In chronic pruritic diseases, the histamine-independent protease-activated receptor 2 (PAR-2) pathway is thought to serve as the predominant route of itch transmission. Endogenous PAR-2 agonists, such as tryptase, have been shown to induce itch in patients with atopic dermatitis (24). Exogenous activation of this pathway can be achieved by cutaneous application of spicules of the cowhage plant (Mucuna pruriens var. pruriens) (25). Cowhage contains mucunian, a cysteine protease that binds PAR-2 receptors and induces an intense itch sensation. Thus, cowhage-induced itch has been frequently implemented as an experimental model for chronic itch conditions and used for evaluation of antipruritic therapies (26–28).
The current study showed the antipruritic effect of BoNT/A in healthy human subjects using a non-histaminergic, cowhage-induced itch model. These findings suggest that a single injection of BoNT/A may be an effective treatment with a prolonged duration for localized chronic pruritic conditions.
MATERIALS AND METHODS
Study design
This was a single-centre, randomized, single-blind, placebo-controlled phase I trial (clinicaltrials.gov NCT02639052) in healthy adults. The trial was approved by the Temple University Institutional Review Board (Study ID: 126938 and performed according to Good Clinical Practice Guidelines. Written informed consent was obtained before any study-related procedures were performed.
After eligibility screening, 2 4×4 cm test sites, 1 on each arm, were designated on the subject’s volar forearms. The locations of these areas were recorded by photograph. Baseline sensory testing was conducted on both areas. Next, 1 test area was intradermally injected with Botox®, while the contralateral test area was intradermally injected with a placebo control (physiological saline). Subjects returned for follow-up sensory testing 1 week, 1 month, and 3 months after receiving the injections.
Participants
Healthy adult subjects (18–50 years old) with no history of BoNT/A use were included. Healthy volunteers were recruited by IRB approved flyers around the Temple University Medical School campus. The use of any oral or topical analgesics or antipruritics was not allowed 48 h before and during the trial. Subjects who were pregnant or breastfeeding were excluded. At baseline, demographic data were collected, and women of childbearing potential underwent a urine pregnancy test.
Interventions
The location (right or left arm) of the Botox® (Allergan, Dublin, Ireland) or saline placebo sites was randomized, and the subjects were blinded to the identity of the injections. Botox® was reconstituted with sterile, preservative-free 0.9% sodium chloride based on the dilution instructions on the label. Since Botox® was reported to diffuse within a 1–2 cm radius, a total of 10 units equally distributed among 5 injection sites (2 units per injection), within the 4×4 cm test area, were injected intradermally to ensure complete coverage (Fig. S1). The same volume of 0.9% saline (Henry Schein,Melville, NY) was injected intradermally into the contralateral test area.
Procedures
Sensory testing was performed at baseline (before treatment), and then 1 week, 1 month, and 3 months after treatment. Warmth thresholds, heat pain thresholds, heat pain intensity, and cowhage itch intensity were measured at each visit. These stimuli were always given in the same order and always started with the subject’s right arm. Thermal stimuli were delivered using the TSA-II Neurosensory Analyzer (Medoc Ltd, Ramat-Yishai, Israel) at each 4×4 cm area. The thermode warmed the skin surface at a linear rate of 0.4°C/s from a baseline of 32°C to a maximum of 50°C. At 50°C, the stimulus automatically terminated. Warmth sensation thresholds and then heat pain detection thresholds were determined 3 times by the ascending method of limits. The subjects were instructed to respond on detection of a thermal stimulus, and those values were used to compute the mean threshold. Heat pain was induced by the thermal stimulation delivered with rise and fall rates of 6°C/s, with a plateau duration of 5 s at 49°C and a minimum interval of 30 s between stimuli at baseline (32°C). Ratings of pain intensity were taken 3 times during the plateau temperature on a 0–10 visual analogue scale (VAS) anchored with “no sensation” on 0 and “the most intense, unbearable sensation imaginable” on 10.
After a 10-min rest period, itch was induced by the application of cowhage (Mucuna pruriens var. pruriens) spicules on each test site, separately. A total of 40–45 cowhage spicules were applied by gentle rubbing into the skin in a circular motion for 30–45 s. Subjects were instructed to rate itch intensity on a 0–10 VAS scale anchored with “no sensation” on 0 and “the most intense, unbearable sensation imaginable” on 10, every 30 s until the itch subsided. Subjects were also instructed not to scratch the area. After itch subsided, the cowhage spicules were removed gently using adhesive tape.
Outcomes
The primary outcome was the change in itch intensity VAS ratings between the Botox® and placebo treatment at each visit for every 30-s report. This outcome included the change in the overall perceived itch VAS (area under the curve; AUC). Exploratory outcomes were the difference in peak itch intensity, peak itch time, and itch duration between treatments at each visit. Two secondary outcome measures were the change in thermal (warmth and heat pain) detection thresholds and in the pain intensity VAS between treatments at the follow-up periods.
Statistical analysis
All statistical analyses presented in this report were conducted using SPSS 20.0 (IBM Corp., Armonk, NY, USA). Statistical significance was defined as p < 0.05. Descriptive statistics for quantitative variables were presented as mean ± standard error of the mean (SEM). A mixed model analysis of variance (ANOVA) with least significance difference (LSD) post-hoc analysis was used to compare the main effects of the treatments (Botox® vs saline itch VAS) at each visit for every 30-s time-point. This analysis was also used to test percentage change differences. Otherwise, paired t-tests between the baseline and individual follow-up time-points were used for all other analysis. All reported p-values are 2-sided, where applicable.
RESULTS
Thirty-five healthy adults (mean ± SD age 26.8 ± 6.8 years) were enrolled and received treatment. One subject did not complete the 3-month follow-up visit. Subject demographics are described in Table I.
Itch intensity
At baseline, before any treatment was received, itch intensity VAS (p = 0.3), overall itch perception (AUC, p = 0.7), peak itch (p = 0.3), and itch duration (p = 0.3) did not differ between test sites.
For the primary outcome, itch intensity VAS in the Botox®-injected area was significantly lower than in the saline-injected area at the 1-week, 1-month, and 3-month follow-up visits (Fig. 1; Table II). In addition, the overall perceived itch intensity (AUC) after Botox® treatment was significantly reduced from baseline at all visits (1 week, p = 0.006; 1 month, p < 0.0001; 3 months, p < 0.0001; Fig. 2a). The overall itch (AUC) after Botox® treatment also showed a decreased percentage change from baseline compared with saline treatment at 1 week (Botox –23.0%, saline –2.2%; p < 0.0001), 1 month (Botox –34.7%, saline –15.8%; p < 0.0001), and 3 months (Botox –29.3%, saline –13,9%; p < 0.0001; Fig. 2b).
| Time-point (s) | Baseline | 1 week | 1 month | 3 months |
| 0 | n.s. | p = 0.041* | p = 0.019* | p = 0.001* |
| 30 | n.s. | p < 0.0001* | p < 0.0001* | p = 0.006* |
| 60 | n.s. | p = 0.002* | p < 0.0001* | p = 0.001* |
| 90 | n.s. | p < 0.0001* | p < 0.0001* | p = 0.035* |
| 120 | n.s. | p = 0.001* | p = 0.010* | p = 0.004* |
| 150 | n.s. | p = 0.001* | p = 0.001* | p = 0.044* |
| 180 | n.s. | p = 0.011* | p = 0.020* | p = 0.008* |
| 210 | n.s. | p = 0.037* | p = 0.056 | p = 0.084 |
| 240 | n.s. | p = 0.053 | n.s. | p = 0.077 |
| 270 | n.s. | n.s. | n.s. | p = 0.085 |
| 300 | n.s. | n.s. | n.s. | p = 0.025* |
| *Timepoints after 300 s were not significantly different. | ||||
| n.s.: nonsignificant. | ||||
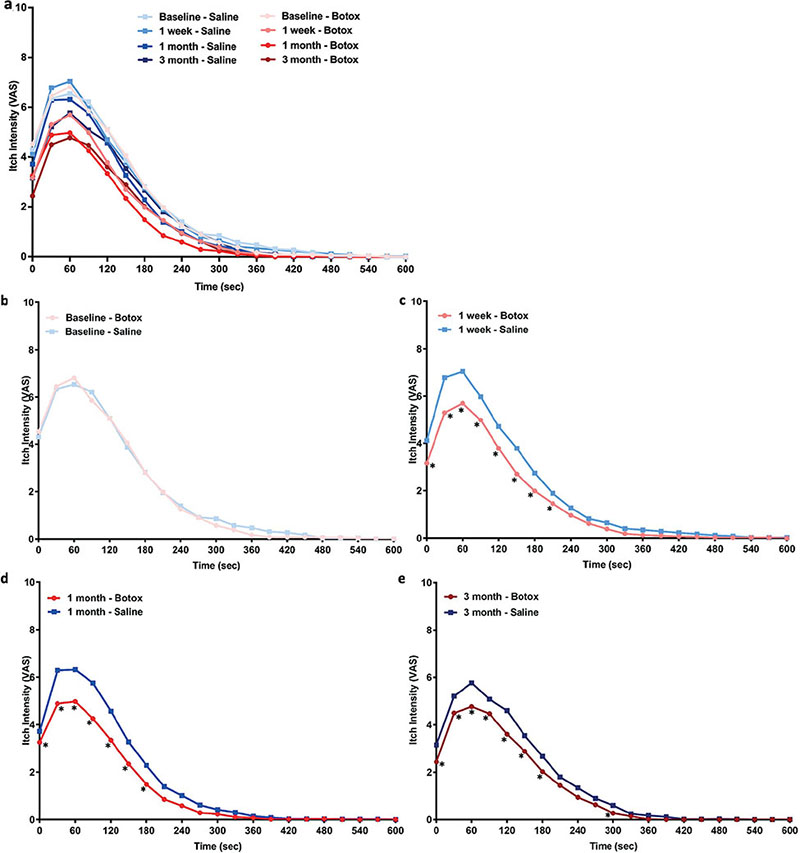
Fig. 1. Time courses (a: all combined) of cowhage-induced itch intensity (visual analogue scale; VAS 0–10) at (b) baseline, (c) 1-week, (d) 1-month, and (e) 3-months following Botox® treatment or saline control. At baseline, before any treatment was administered, the itch intensity time course did not significantly differ between test sites. At 1 week, 1 month and 3 months after treatment, Botox significantly reduced itch intensity compared with the saline control. *p < 0.05 at that specific time-point based on a mixed model analysis of variance (ANOVA) with least significance difference (LSD) post-hoc. A complete list of significantly different time-points and their p-values are listed in Table II.
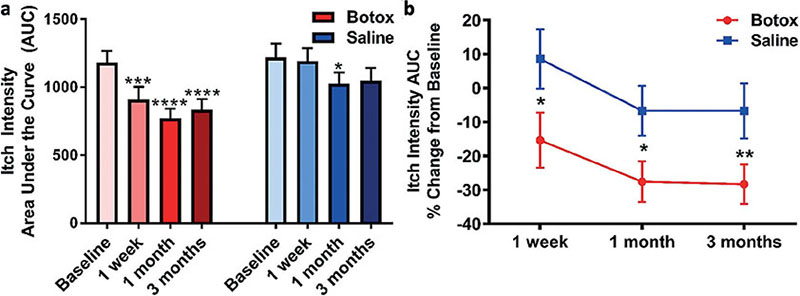
Fig. 2. Area under the curve (AUC) of the cowhage itch intensity time course, representing total itch perceived. (a) Compared with baseline, the Botox® treatment significantly decreased the AUC at 1 week (p = 0.006), 1 month (p < 0.0001), and 3 months (p < 0.0001) after treatment. (b) The percentage change in AUC from baseline was significantly different between treatments at 1 week (p < 0.0001), 1 month (p < 0.0001), and 3 months (p < 0.0001). Data reported as mean ± standard error of the mean (SEM) and analysed using (a) paired t-tests to the baseline or (b) a mixed model analysis of variance (ANOVA) with LSD post-hoc; p < 0.05*, p < 0.001***, p < 0.0001****.
As for the exploratory outcomes associated with itch intensity, Botox® treatment significantly affected the peak itch intensity and itch duration (Table III). The mean intensity of itch was decreased after Botox® treatment by 1.2 VAS units at 1 week (p = 0.001), 1.4 VAS units at 1 month (p < 0.0001), and 1.8 VAS units at 3 months (p < 0.0001) compared with baseline (Fig. 3a). In addition, the paired difference in peak itch intensity between the Botox® and saline treatments was significantly different at 1 week (p = 0.0002), 1 month (p = 0.0001) and 3 months (p = 0.006). The percentage change from baseline was also significantly lower for Botox® treatment vs saline treatment for all follow-up visits (1 week, p < 0.0001; 1 month, p < 0.0001; 3 months, p < 0.0001; Fig. 3b). The time when peak itch occurred was not affected. Compared with baseline, Botox® treatment decreased the duration of cowhage-induced itch at 1-week (p = 0.009), 1-month (p = 0.027), and 3-month (p = 0.015) follow-up visits (Fig. 3c). However, the percentage change from baseline was only different between the treatments at 1 week (Botox –15.6%, saline –6.1%; p < 0.0001; Fig. 3d).
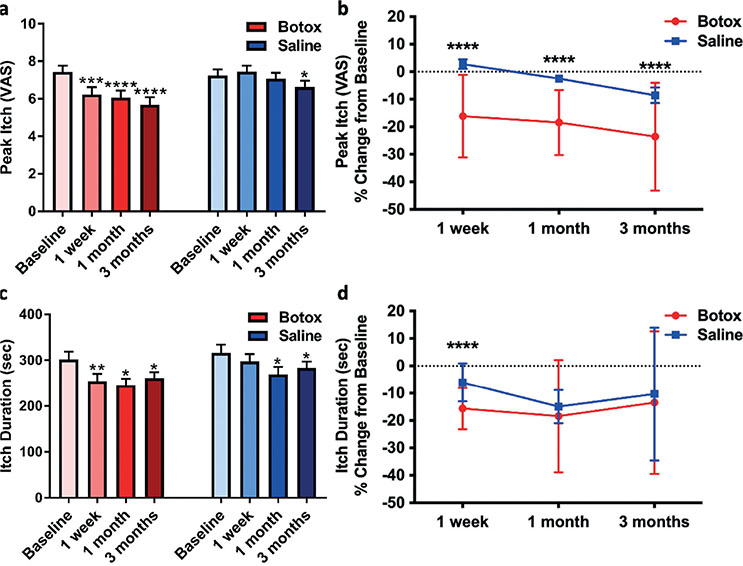
Fig. 3. Peak itch intensity and duration of cowhage itch. (a) Compared with baseline, the peak itch intensity was significantly reduced by the Botox® treatment after 1 week (p = 0.001), 1 month (p < 0.0001), and 3 months (p < 0.0001). (b) The percentage change from baseline was significantly different between treatments at 1 week (p < 0.0001), 1 month (p < 0.0001), and 3 months (p < 0.0001). (c) Compared with baseline, the duration of the itch was significantly shortened by Botox treatment after 1 week (p = 0.009), 1 month (p = 0.027), and 3 months (p = 0.015). (d) However, this reduction was only significantly different from the saline treatment at 1 week (p < 0.0001). Data reported as mean ± standard error of the mean (SEM) and analysed using paired t-test to (a and c) the baseline or (b and d) a mixed model analysis of variance (ANOVA) with least significance difference (LSD) post-hoc; p < 0.05*, p < 0.01**, p < 0.001***, p < 0.0001****.
Thermal thresholds
Botox® treatment did not statistically affect the warmth detection threshold, 1 of the secondary outcomes (Table IV; Fig. 4a). However, Botox® treatment slightly increased the heat pain detection threshold at 1 week (p = 0.034), 1 month (p = 0.074), and 3 months (p = 0.057). The saline treatment also caused a slight increase in the heat pain detection threshold for the follow-up visits (1 week, p = 0.046; 1 month, p = 0.06; 3 months, p = 0.081). As such, the heat pain threshold was not significantly different between the treatments (p = 0.98; Fig. 4b).
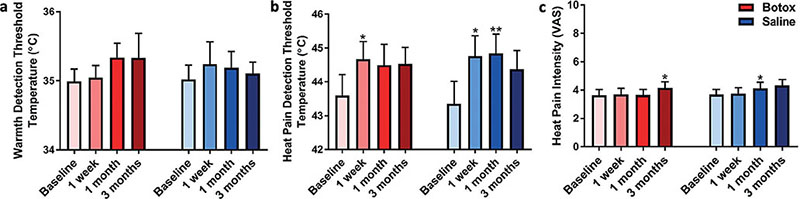
Fig. 4. Thermal thresholds and heat pain intensity. (a) The warmth detection threshold was not significantly affected by the Botox® treatment. (b) The heat pain detection threshold was slightly elevated by both the Botox and saline treatments, but these changes were not significantly different between treatments. (c) Nonetheless, the heat pain intensity remained relatively unaffected in both treatment groups. Data reported as mean ± standard error of the mean (SEM) and analysed using pair t-test to the baseline; p < 0.05*, p < 0.01**.
Pain intensity
For the final secondary outcome, the Botox® treatment did not affect heat pain intensity (Table IV). At the 3-month visit, the heat pain intensity VAS was increased from baseline (p = 0.02), but the heat pain intensity also increased for the saline treatment at 1 month (p = 0.02) and 3 months (p = 0.05). There was no significant difference in the heat pain intensity VAS between the Botox® and saline treatments (p = 0.13; Fig. 4c).
Safety
One subject reported development of a mild papular rash at the site of the baseline cowhage-itch induction. This adverse event resolved on its own a few hours after the application and did not occur with subsequent applications of cowhage.
DISCUSSION
This study found that BoNT/A significantly reduced the intensity and duration of cowhage-induced itch in healthy adults. The onset of antipruritic activity was observed as early as 1 week post treatment and was sustained over a 3-month period.
Notably, BoNT/A had a more profound effect on itch intensity and duration than on heat pain intensity and thermal thresholds. This outcome may have been influenced by a number of contributory factors. It is possible that some of the neurotransmitters inhibited by BoNT/A have dual roles in itch and pain sensation, while others may be more itch specific.
Several pruritogenic neurotransmitters inhibited by BoNT/A have complex relationships with itch and pain sensation. For example, acetylcholine is upregulated in patients with atopic dermatitis and has been shown to induce itch when injected into atopic skin. A similar effect has been demonstrated in some, but not all, psoriatic patients. However, when injected into the skin of healthy controls, acetylcholine induced burning pain instead of itch (14). Given the fact that some individuals with chronic itch conditions may be more sensitive to acetylcholine as a pruritogen at baseline, perhaps the antipruritic effect of BoNT/A would be enhanced in these individuals beyond what was observed in the healthy subjects in the current study. Indeed, a recent randomized, placebo-controlled trial by Khattab in 26 patients with atopic dermatitis showed statistically significant reductions in objective and subjective disease severity and quality of life measures after BoNT/A injection relative to placebo (p < 0.001), although itch was only a component of these assessments (7).
Another possible antipruritic mechanism of BoNT/A is inhibition of SP, CGRP, glutamate, and other pro-inflammatory peptides. These peptides are known to play a role in itch induction in a number of pruritic diseases. Increased concentration of SP- and CGRP-immunoreactive nerve fibres has been observed in lesional skin of patients with atopic dermatitis, nummular eczema, and prurigo nodularis (29, 30). Serum levels of SP have also been shown to be elevated in patients with atopic dermatitis and to correlate with itch intensity (31). It is thought that SP binds neurokinin-1 receptors to induce itch, and SP may also act through Mas-related G protein-coupled receptors on mast cells and sensory neurones (32). Therefore, inhibition of SP and other neuropeptides may significantly reduce itch in addition to inflammation.
BoNT/A has also been shown to affect expression of 2 ion channels that are involved in itch transmission. In a murine model, Cao et al. (22) demonstrated that a single injection of BoNT/A downregulated expression of TRPA1 in the dorsal root ganglia. TRPA1 is an essential component of the signalling pathways that promote histamine-independent itch and is thought to play a major role in the mediation of chronic pruritus (33, 34). In addition, downregulated expression of TRPV1, a component of histamine-sensitive signalling pathways, was also observed (22). Sustained downregulation of TRPA1 and TRPV1 may contribute to the versatility of BoNT/A in relieving cowhage-induced itch as well as acute histamine-induced itch. A recent study in murine models explored this relationship further (35). BoNT/A was found to significantly decrease scratching behaviour elicited via both histaminergic and non-histaminergic itch pathways (induced by the polymer 48/80 and chloroquine, respectively).
Despite demonstrating a significant antipruritic effect, BoNT/A did not diminish the intensity of heat pain in the current subjects. Rather, modest increases in heat pain sensitivity were observed in both BoNT/A- and saline-treated areas 3 months after administration. The cause of this effect remains unclear, as BoNT/A is known to possess antinociceptive properties. This was demonstrated in a mouse model in which both acute and chronic pain were induced and the response to BoNT/A recorded (36). BoNT/A improved both acute (formalin-evoked) and chronic (neuropathic and inflammatory) pain in control mice, but no longer provided antinociceptive activity in mice lacking the SP and neurokinin 1 receptor encoding genes, thus showing that the SP-ergic system is necessary for BoNT/A’s pain modulation. In human studies, analgesic effects of BoNT/A have also been clearly demonstrated in chronic neuropathic pain conditions, including trigeminal neuralgia and postherpetic neuralgia (37). Thus, BoNT/A may selectively inhibit some forms of acute and chronic pain, while exerting less of an effect on heat pain sensation.
For patients who experience chronic, localized itch, BoNT/A has the potential to be a unique therapeutic option with minimal adverse effects and a long duration of action, with a limitation that the affected area would require injections to be dispersed every 1–2 cm to ensure treatment coverage. Indeed, the antipruritic effect of BoNT/A strengthened throughout the duration of the current study, with the greatest reduction in itch intensity occurring at the 3-month follow-up visit. Although this study did not assess the effect of BoNT/A beyond this time-point, it is possible that BoNT/A may continue to provide relief for a more extensive period of time. The longevity of this treatment option is unmatched by current therapies and could be instrumental in breaking the itch-scratch cycle that often perpetuates chronic itch conditions. Additional trials in patients with localized chronic itch are needed in order to further explore the itch-relieving effect of BoNT/A in a patient population.
ACKNOWLEDGEMENTS
The authors thank Allergan for funding this study.
REFERENCES
- Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med 2013; 368: 1625–1634.
- Swartling C, Naver H, Lindberg M, Anveden I. Treatment of dyshidrotic hand dermatitis with intradermal botulinum toxin. J Am Acad Dermatol 2002; 47: 667–671.
- Heckmann M, Heyer G, Brunner B, Plewig G. Botulinum toxin type A injection in the treatment of lichen simplex: an open pilot study. J Am Acad Dermatol 2002; 46: 617–619.
- Zanchi M, Favot F, Bizzarini M, Piai M, Donini M, Sedona P. Botulinum toxin type-A for the treatment of inverse psoriasis. J Eur Acad Dermatol Venereol 2008; 22: 431–436.
- Wollina U, Karamfilov T. Adjuvant botulinum toxin A in dyshidrotic hand eczema: a controlled prospective pilot study with left-right comparison. J Eur Acad Dermatol Venereol 2002; 16: 40–42.
- Gharib K, Mostafa A, Elsayed A. Evaluation of botulinum toxin type A injection in the treatment of localized chronic pruritus. J Clin Aesthet Dermatol 2020; 13: 12–17.
- Khattab FM. Evaluation of botulinum toxin A as an optional treatment for atopic dermatitis. J Clin Aesthet Dermatol 2020; 13: 32–35.
- Weinfeld PK. Successful treatment of notalgia paresthetica with botulinum toxin type A. Arch Dermatol 2007; 143: 980–982.
- Datta S, Mahal S, Bhagavan SM, Govindarajan R. Use of botulinum toxin type A in a patient with refractory itch from notalgia paresthetica. J Clin Neuromuscul Dis 2020; 21: 243–244.
- Gazerani P, Pedersen NS, Drewes AM, Arendt-Nielsen L. Botulinum toxin type A reduces histamine-induced itch and vasomotor responses in human skin. Br J Dermatol 2009; 161: 737–745.
- Boozalis E, Sheu M, Selph J, Kwatra SG. Botulinum toxin type A for the treatment of localized recalcitrant chronic pruritus. J Am Acad Dermatol 2018; 78: 192–194.
- Gazerani P. Antipruritic effects of botulinum neurotoxins. Toxins (Basel) 2018; 10: 143.
- Huang W, Foster JA, Rogachefsky AS. Pharmacology of botulinum toxin. J Am Acad Dermatol 2000; 43: 249–259.
- Heyer GR, Hornstein OP. Recent studies of cutaneous nociception in atopic and non-atopic subjects. J Dermatol 1999; 26: 77–86.
- Wessler I, Reinheimer T, Kilbinger H, Bittinger F, Kirkpatrick CJ, Saloga J, Knop J. Increased acetylcholine levels in skin biopsies of patients with atopic dermatitis. Life Sci 2003; 72: 2169–2172.
- Heyer G, Vogelgsang M, Hornstein OP. Acetylcholine is an inducer of itching in patients with atopic eczema. J Dermatol 1997; 24: 621–625.
- Kim DW, Lee SK, Ahnn J. Botulinum toxin as a pain killer: players and actions in antinociception. Toxins (Basel) 2015; 7: 2435–2453.
- Welch MJ, Purkiss JR, Foster KA. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon 2000; 38: 245–258.
- Durham PL, Cady R, Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: implications for migraine therapy. Headache 2004; 44: 35–43.
- Ishikawa H, Mitsui Y, Yoshitomi T, Mashimo K, Aoki S, Mukuno K, Shimizu K. Presynaptic effects of botulinum toxin type A on the neuronally evoked response of albino and pigmented rabbit iris sphincter and dilator muscles. Jpn J Ophthalmol 2000; 44: 106–109.
- Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol 2005; 174: 977–983.
- Cao LF, Si M, Huang Y, Chen LH, Peng XY, Qin YQ, et al. Long-term anti-itch effect of botulinum neurotoxin A is associated with downregulation of TRPV1 and TRPA1 in the dorsal root ganglia in mice. Neuroreport 2017; 28: 518–526.
- Huang SH, Wu KW, Lo JJ, Wu SH. Synergic effect of botulinum toxin type A and triamcinolone alleviates scar pruritus by modulating epidermal hyperinnervation: a preliminary report. Aesthet Surg J 2021; 41: NP1721–NP1731.
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci 2003; 23: 6176–6180.
- Papoiu AD, Tey HL, Coghill RC, Wang H, Yosipovitch G. Cowhage-induced itch as an experimental model for pruritus. A comparative study with histamine-induced itch. PLoS One 2011; 6: e17786.
- Papoiu AD, Chaudhry H, Hayes EC, Chan YH, Herbst KD. TriCalm® hydrogel is significantly superior to 2% diphenhydramine and 1% hydrocortisone in reducing the peak intensity, duration, and overall magnitude of cowhage-induced itch. Clin Cosmet Investig Dermatol 2015; 8: 223–229.
- Papoiu AD, Kraft RA, Coghill RC, Yosipovitch G. Butorphanol suppression of histamine itch is mediated by nucleus accumbens and septal nuclei: a pharmacological fMRI study. J Invest Dermatol 2015; 135: 560–568.
- Nattkemper LA, Zhi K, Romero KE, Shah SM, Ju T, Fourzali K, et al. Antipruritic effect of topical acetaminophen gel in histaminergic and non-histaminergic itch provocation: a double-blind, vehicle-controlled pilot study. Acta Derm Venereol 2022; 102: adv00640.
- Abadía Molina F, Burrows NP, Jones RR, Terenghi G, Polak JM. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol 1992; 127: 344–351.
- Jarvikallio A, Harvima IT, Naukkarinen A. Mast cells, nerves and neuropeptides in atopic dermatitis and nummular eczema. Arch Dermatol Res 2003; 295: 2–7.
- Salomon J, Baran E. The role of selected neuropeptides in pathogenesis of atopic dermatitis. J Eur Acad Dermatol Venereol 2008; 22: 223–228.
- Azimi E, Reddy VB, Pereira PJS, Talbot S, Woolf CJ, Lerner EA. Substance P activates Mas-related G protein-coupled receptors to induce itch. J Allergy Clin Immunol 2017; 140: 447–453.e3.
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 2011; 14: 595–602.
- Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, et al. The ion channel TRPA1 is required for chronic itch. J Neurosci 2013; 33: 9283–9294.
- Ramachandran R, Marino MJ, Paul S, Wang Z, Mascarenhas NL, Pellett S, et al. A study and review of effects of botulinum toxins on mast cell dependent and independent pruritus. Toxins (Basel) 2018; 10: 134.
- Matak I, Tékus V, Bölcskei K, Lacković Z, Helyes Z. Involvement of substance P in the antinociceptive effect of botulinum toxin type A: Evidence from knockout mice. Neuroscience 2017; 358: 137–145.
- Oh HM, Chung ME. Botulinum toxin for neuropathic pain: a review of the literature. Toxins (Basel) 2015; 7: 3127–3154.