Gianotti-Crosti syndrome (GCS), also called papular acrodermatitis of childhood, is a self-limiting skin disease of early childhood (1, 2). GCS is clinically characterized by the acute onset of papular or papulovesicular eruptions, distributed symmetrically, mainly on the face, buttocks, and extremities, which may last up to 8 weeks. The histopathological findings of GCS are non-specific and include focal parakeratosis, mild spongiosis, superficial perivascular infiltrate, papillary dermal oedema, and extravasated erythrocytes (3). GCS is considered a non-specific cutaneous host response to a variety of infectious agents, particularly viruses, such as Epstein-Barr virus (EBV), hepatitis B virus (HBV), cytomegalovirus (CMV), coxsackie virus, and parvovirus B19.
Erythema multiforme (EM) is a self-limiting, immune-mediated reaction that involves the skin and sometimes the mucosa (4, 5). The characteristic lesion in EM is the target lesion, which measures < 3 cm in diameter, has a round and well-defined border, and comprises 3 distinct zones. Most cases of EM are caused by medications or infectious agents, such as herpes simplex virus (HSV) or Mycoplasma pneumoniae.
Both GCS (6–9) and EM (4, 5, 10–12) can be triggered by various vaccinations, although the incidence is very low. We report here, to our knowledge, a first case in which skin lesions of GCS and EM have developed simultaneously after a single vaccine.
CASE REPORT
The patient, a 6-year-old Japanese boy, developed a fever (38.3°C) 5 days after the second live-attenuated measles-rubella vaccination. He had received the first dose of the same vaccination at 12 months old. Although the fever normalized to below 37.0°C by the next day after taking acetaminophen, multiple pruritic skin lesions appeared on the lower extremities that same day, and similar skin lesions extended to the upper extremities and face by the next day. Two days after the onset of skin eruption, the patient was brought to a paediatric doctor and was prescribed topical corticosteroids for 3 weeks under a diagnosis of eczema. However, no improvement was obtained from this treatment and the patient was referred to the Department of Dermatology at Kita-Harima Medical Center. Physical examination revealed scattered 1- to 3-mm erythematous papules on his cheeks (Fig. 1A). In addition, skin-coloured to erythematous, flat-topped papules <1.0 cm in diameter were seen distributed symmetrically on the extremities (Fig. 1B, C). Over the knees, several lesions had coalesced into plaques (Fig. 1C). In addition to these papular lesions, typical target lesions, comprising central disks of erythema, raised oedematous intermediate rings, and red outer rings, < 2 cm in diameter, were scattered symmetrically over the dorsal aspects of the ankles and feet (Fig. 1D). The trunk, palms, and soles remained unaffected. No cervical or axillary lymphadenopathy was palpable.

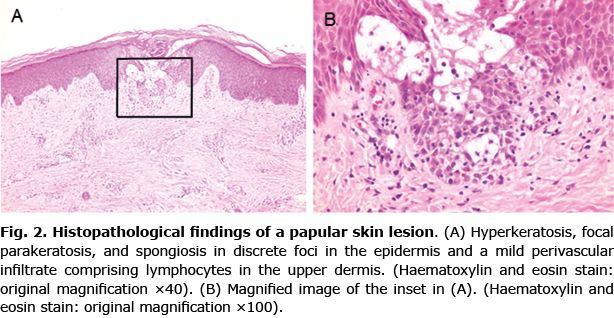
The patient had been taking montelukast sodium and ketotifen fumarate for bronchial asthma for 3 years. He had no past history of herpes simplex infection. He had not had serious respiratory symptoms recently. Routine blood tests, including full blood count and biochemical profile, showed no abnormalities. Serological tests for HBV, EBV, CMV, coxsackievirus-A16, and M. pneumoniae yielded negative results. Serum titers of antibodies against measles virus IgM and measles virus IgG, which were measured by enzyme immunoassay (EIA), were 0.19 (ref. < 0.8) and 26.6 (ref. < 2.0), respectively. Serum titers of antibodies against rubella virus IgM and rubella virus IgG, which were measured by EIA, were 0.08 (ref. < 0.8) and 5.6 (ref. < 2.0), respectively. Histopathological examination of a papule on the patients’ left thigh revealed hyperkeratosis, focal parakeratosis, and spongiosis in discrete foci in the epidermis, and a mild perivascular infiltrate comprising lymphocytes in the upper dermis (Fig. 2A, B). Based on the clinical and histopathological findings, the papular lesions were diagnosed as GCS. Target lesions were clinically diagnosed as EM. Both types of lesions were considered to have been triggered by the second measles-rubella vaccination and were treated with topical corticosteroid to alleviate itching. The skin lesions gradually improved and had almost cleared by 2 months after the first dermatological examination.
DISCUSSION
Vaccinations are important for preventing infectious disease by developing both individual and herd immunity. Although most vaccinations are considered relatively safe, adverse effects are associated with any vaccine, many of which are cutaneous (13). Many of these adverse effects on the skin are local reactions at the injection sites, such as pain, swelling, and redness, due to non-specific inflammatory and irritant reactions. In the case of measles-rubella vaccine, the frequency of these local reactions is ~10% according to the World Health Organization (WHO) (https://vaccine-safety-training.org/vaccine-reactions.html). However, it has been shown that vaccinations rarely induce new onset of dermatological conditions, such as GCS, lichen planus, and granuloma annulare (13). Furthermore, vaccinations have been associated in rare reports with generalized cutaneous hypersensitivity reactions, such as EM, Stevens-Johnson syndrome, and acute generalized exanthematous pustulosis (13).
In the case reported here, skin lesions of both GCS and EM developed simultaneously, 5 days after the second measles-rubella vaccination. Although other triggers of GCS and/or EM occurring shortly, but coincidentally, after the vaccination cannot be definitely excluded, measles-rubella vaccination seems reasonable to consider as the causative factor for the 2 skin diseases, in view of the temporal sequence of events and the absence of any other apparent precipitants. Although both GCS and EM are known to be able to be triggered by various vaccines (4–12), including measles-mumps-rubella vaccine (6, 10), this case represents the first description of simultaneous development of both GCS and EM after a single dose of this vaccine.
In the present case, neither of the 2 cutaneous diseases, GCS and EM, had developed after the first dose of measles-rubella vaccine, but they developed following the second dose of the same vaccine. To date, most cases of vaccine-induced cutaneous diseases have occurred at the first-dose vaccinations (6–10, 12), although several case reports have described patients who developed these diseases after the second-dose vaccinations (11, 14, 15). A possible explanation for the phenomenon that GCS and EM occurred at the second, but not the first, dose of vaccine in the present case is that both GCS and EM developed as a result of allergic reactions to vaccine components (e.g., adjuvants, stabilizers, and preservatives) that required sensitization by the first dose of vaccination. If GCS and EM developed as a result of a reaction caused by the antigen(s) in the measles and rubella vaccine, it is also possible that mother-derived antibodies against the antigen(s) remained in the patient when the first dose of measles-rubella vaccination was performed, and thus the reaction to the vaccine antigen(s) was reduced, inhibiting the development of the skin eruptions of GCS and EM at the first dose of vaccination.
The authors have no conflicts of interest to declare.
REFERENCES
- Chuh AA. Diagnostic criteria for Gianotti-Crosti syndrome: a prospective case-control study for validity assessment. Cutis 2001; 68: 207–213.
- Brandt O, Abeck D, Gianotti R, Burgdorf W. Gianotti-Crosti syndrome. J Am Acad Dermatol 2006; 54: 136–145.
- Stefanato CM, Goldberg LJ, Andersen WK, Bhawan J. Gianotti-Crosti syndrome presenting as lichenoid dermatitis. Am J Dermatopathol 2000; 22: 162–165.
- Zoghaib S, Kechichian E, Souaid K, Soutou B, Helou J, Tomb R. Triggers, clinical manifestations, and management of pediatric erythema multiforme: a systematic review. J Am Acad Dermatol 2019; 81: 813–822.
- Trayes KP, Love G, Studdiford JS. Erythema Multiforme: recognition and management. Am Fam Physician 2019; 100: 82–88.
- Velangi SS, Tidman MJ. Gianotti-Crosti syndrome after measles, mumps and rubella vaccination. Br J Dermatol 1998; 139: 1122–1123.
- Monastirli A, Varvarigou A, Pasmatzi E, Badavanis G, Georgiou S, Mantagos S, Tsambaos D. Gianotti-Crosti syndrome after hepatitis A vaccination. Acta Derm Venereol 2007; 87: 174–175.
- Kroeskop A, Lewis AB, Barril FA, Baribault KE. Gianotti-Crosti syndrome after H1N1-influenza vaccine. Pediatr Dermatol 2011; 28: 595–596.
- Retrouvey M, Koch LH, Williams JV. Gianotti-Crosti syndrome following childhood vaccinations. Pediatr Dermatol 2013; 30: 137–138.
- Bernardini ML, D’Angelo G, Oggiano N, Campanati A, Ficcadenti A, Coppa GV, Offidani A. Erythema multiforme following live attenuated trivalent measles-mumps-rubella vaccine. Acta Derm Venereol 2006; 86: 359–360.
- Katoulis AC, Liakou A, Bozi E, Theodorakis M, Alevizou A, Zafeiraki A, Mistidou M, Stavrianeas NG. Erythema multiforme following vaccination for human papillomavirus. Dermatology 2010; 220: 60–62.
- Monastirli A, Pasmatzi E, Badavanis G, Tsambaos D. Erythema multiforme following pneumococcal vaccination. Acta Dermatovenerol Alp Pannonica Adriat 2017; 26: 25–26.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol 2015; 33: 327–332.
- Saywell CA, Wittal RA, Kossard S. Lichenoid reaction to hepatitis B vaccination. Australas J Dermatol 1997; 38: 152–154.
- Stavrianeas NG, Katoulis AC, Kanelleas A, Hatziolou E, Georgala S. Papulonodular lichenoid and pseudolymphomatous reaction at the injection site of hepatitis B virus vaccination. Dermatology 2002; 205: 166–168.