SHORT COMMUNICATION
Trousseau Syndrome in a Case of Extramammary Paget’s Disease
Megumi FUJIMOTO1, Yosuke ISHITSUKA1, Atsushi TANEMURA1, Satoshi NOJIMA2 and Manabu FUJIMOTO1
1Department of Dermatology and 2Pathology, Osaka University Graduate School of Medicine, Osaka, Japan. *E-mail: ishitsuka@derma.med.osaka-u.ac.jp
Citation: Acta Derm Venereol 2023; 103: adv9405. DOI https://doi.org/10.2340/actadv.v103.9405.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Sep 6, 2023; Published: Sep 27, 2023
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
While mediating malignant growth through 3 separate processes (elimination, equilibrium, and escape phases), the immune system continuously scans for transformed cells emerging in the periphery (1). Extramammary Paget’s disease (EMPD) is a cutaneous adenocarcinoma (2), which is mostly considered intraepidermal and indolent (2). Based on the cancer immunoediting theory (1), in situ lesions represent a long-term cancer-immune equilibrium that keeps cancer cells within the epidermal niche, while immune escape brings about regional lymph node (LN) metastasis. However, this theory argues against the traditional view that some macule-forming, apparently non-invasive, and non-tumour-forming EMPDs progress rapidly and manifest as fatal pulmonary tumour thrombotic microangiopathy (PTTM) (3–5) or after years of postoperative follow-up (6). Since EMPD-associated PTTM has been reported to frequently precede diagnosis of occult EMPDs (3, 4), precise disease recognition and elucidation of the underlying pathomechanisms of EMPD are essential for establishing better management strategies (2). The description of coagulopathy associated with occult cancer dates back to 1865, when the physician Armand Trousseau described recurrent superficial migratory thrombophlebitis associated with mucin-rich adenocarcinomas (7). Chronic dissemination of intravascular coagulation in patients with Trousseau syndrome (TS) can result in cerebral infarction (8). We report here a case of EMPD-associated TS wherein, upon recurrence, Mucin-16 (MUC16, CA125)-positive tumour cells expanded locally.
CASE REPORT
A 72-year-old Japanese man presented with a 2-year history of perineal erosive erythema with depigmentation (Fig. 1a). However, lymphadenopathy was not clinically present. An elevated plasma D-dimer level (4.1 µg/mL [normal range: < 0.50 µg/mL]) led to identifying a thrombus in the ipsilateral soleal vein, of which patho-logical significance was not appreciated at that point. How-ever, the abnormality is a harbinger of poor prognosis in cancer. Extended resection with a horizontal margin of 2 cm was perform-ed. Histopathological evaluation revealed neither dermal/vasculature invasion nor residual tumour cells. However, 10 months after surgery, the patient developed indurative erythema surrounding the postoperative scar, and skin biopsy confirmed recurrence (Fig. 1b). Moreover, swollen bilateral inguinal LNs and an interstitial reticular pattern, suggestive of lymphangitis carcinonatosa, on the chest X-ray (Fig. 1c) indicated a poor prognosis (9). Therefore, docetaxel (DTX, 40 mg/m2) with concomitant oral S-1 (S1, 120 mg/day) was administered. However, 3 weeks after the first cycle, the patient experienced cramping pain and a swollen left leg, a typical sign of thrombophlebitis (7). Serum D-dimer (32.54 µg/mL) and lactate dehydrogenase (LDH; 1067 U/L [normal range: 124–222 U/L]) levels were elevated. The patient developed stupor. Despite detecting no metastasis, diffusion-weighted brain imaging revealed widespread cerebral infarctions (Fig. 1d). Although serum CA125 levels were within normal range at this stage, immunohistochemistry revealed noticeably higher levels of CA125 (Fig. 1b, e), along with lower inflammatory infiltrates locally positive for CD4 or CD8 (Fig. 1b, e). Two months after recurrence, the patient expired due to respiratory failure caused by steroid-refractory pulmonary fibrosis following the second cycle of the DTX/S1 regimen.
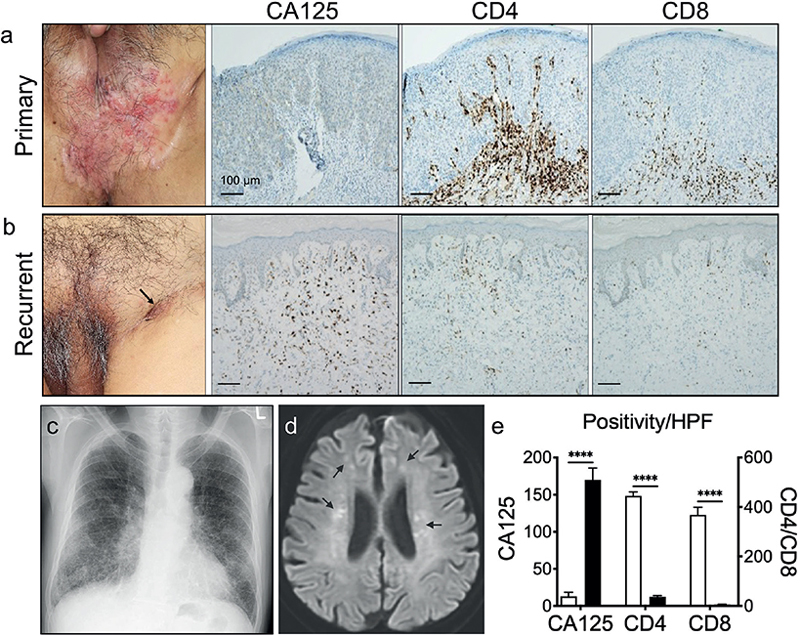
Fig. 1. (a) Primary lesion. (b) Recurrent lesion on the postoperative scar (arrow). (a) and (b) Corresponding immunohistochemical images of CA125/CD4/CD8. (c) Chest X-ray indicating lymphangitis carcinomatosa. (d) Magnetic resonance imaging showing multiple foci of cerebral infarc-tion (arrows). (e) Immuno-staining levels in the primary (white bar) and the recurrent (black bar) lesions quantified by use of the ImageJ analysis programme. ****p < 0.001, 2-way analysis of variance.
DISCUSSION
EMPD is a cutaneous adenocarcinoma in which malignant clinical course has been suggested to correlate with high mucin expression levels locally (10, 11) or systemically (peripheral blood) (12). Unlike the physio-logical functions of mucin, transformed cells exploit their molecular properties for invasion (7, 13). In the tumour microenvironment, tumour-derived mucin interacts with selectins expressed in platelets, leukocytes, and vascular endothelial cells (7, 13). This interaction promotes thromboembolism, immunosuppression, and metastatic spread (7), indicating that tumour-derived mucin is the cause of EMPD-associated TS. Since in situ EMPDs are often bland-looking and potentially overlooked, thrombotic complications can precede definitive diagnosis, sometimes manifesting as lethal PTTM (3–6). Mucin with the highest molecular weight, MUC16, is a membrane-bound, non-secretory form that may be responsible for inhibiting natural killer cell-mediated immune surveillance (13). Therefore, MUC16 may endow EMPD cells with a selective advantage (13) by promoting local expansion of MUC16-positive cells (1). Sawamura et al. recently performed a retrospective single-institution analysis and suggested that MUC16 expression levels in primary lesion significantly correlated with developing distant metastasis, with no LN metastasis (10). A single-institution analysis by Kato et al. (12) indicated that serum CA125 levels, which also rose sharply before death, were significantly associated with distant metastasis. Therefore, high local MUC16 expression levels may indicate that EMPD cells directly invaded the systemic circulation due to failure of the lymphatic system to maintain immunosurveillance. Although EMPD cells appear to metastasize to draining LNs before colonizing distant organs, routine sentinel LN sampling is not recommended (2). Currently, robust clinical/biological parameters are sought. Kato et al. (12) indicated that primary erosive lesions, but not tumour formation, correlated with developing LN/distant metastases. This may be important given that chronic local inflammation could promote tumourigenesis and perturb epidermal differentiation, both of which precede immune evasion of EMPD cells (1). This notion may have been further corroborated by a recent retrospective analysis of stage I melanomas by Ellis et al. (14). In that study, differentiation status of the overlying epidermis was assessed by expression levels of loricrin/autophagy and beclin 1 regulator (AMBRA1), which successfully identified high-risk stage I diseases with low disease-free survival rates independent of tumour thickness (14). Although future studies are required, the current case suggests that: sentinel LN sampling and/or adjunct therapies that augment local immunity, such as radiotherapy (2) or immunotherapy (15), may need to be prioritized in patients with primary erosive lesions or coagulopathy indicated by high plasma D-dimer levels. Precise recognition of the clinical entity and in-depth biological understanding would allow for more efficient risk stratification of patients with EMPD.
ACKNOWLEDGEMENTS
This research was supported in part by grants from the JSPS KAKENHI Grant, Grant-in-Aid for Scientific Research (C) (22K08404 to Y.I.), Lydia O’Leary Memorial Pias Dermatological Foundation to YI, and a Shiseido Basic Medical Research Grant from the Japanese Dermatological Association to YI.
REFERENCES
- O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 2019; 16: 151–167.
- Kibbi N, Owen JL, Worley B, Wang JX, Harikumar V, Downing MB, et al. Evidence-based clinical practice guidelines for extramammary Paget disease. JAMA Oncol 2022; 8: 618–628.
- Banno A, Chiba K, Kasai H, Nagami K. Ante-mortem diagnosis of pulmonary tumour thrombotic microangiopathy in a patient with unrecognised extramammary Paget’s disease. BMJ Case Rep 2016; 2016:bcr2016216666.
- Teruya H, Mukaigawara M, Hirata K. Progressive dyspnea in a woman with genital skin lesions. JAMA Oncol 2020; 6: 433–434.
- Kato A, Kato H, Komori S, Nakano S, Murase T, Nakamura M, et al. Pulmonary tumor thrombotic microangiopathy secondary to extramammary Paget’s disease: an autopsy case report and literature review. Case Rep Oncol 2021; 14: 1328–1332.
- Oyama Y, Nishida H, Kondo Y, Kusaba T, Kadowaki H, Harada T, et al. Pulmonary tumor thrombotic microangiopathy associated with extramammary Paget’s disease: An autopsy case report. Pathol Int 2020; 70: 680–685.
- Wahrenbrock M, Borsig L, Le D, Varki N, Varki A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest 2003; 112: 853–862.
- Ren R, Yan H, Gui Y, Zhao J, Wang H, Ji D, et al. Clinical features of trousseau syndrome with cerebral infarction as the initial manifestation. Neurologist 2020; 25: 117–121.
- Hatta N, Yamada M, Hirano T, Fujimoto A, Morita R. Extramammary Paget’s disease: treatment, prognostic factors and outcome in 76 patients. Br J Dermatol 2008; 158: 313–318.
- Sawamura S, Kajihara I, Tasaki Y, Otsuka-Maeda S, Sakamoto R, Kanazawa-Yamada S, et al. Overexpression of MUC16 (CA125) in extramammary Paget’s disease. Jpn J Clin Oncol 2020; 50: 1330–1332.
- Hata H, Abe R, Hoshina D, Saito N, Homma E, Aoyagi S, et al. MUC5AC expression correlates with invasiveness and progression of extramammary Paget’s disease. J Eur Acad Dermatol Venereol 2014; 28: 727–732.
- Kato H, Nakamura M, Watanabe S, Oda T, Morita A. Combined serum carcinoembryonic antigen and cytokeratin 19 fragment levels provide a sensitive biomarker for lymph node metastasis in extramammary Paget’s disease. J Dermatol 2020; 47: 763–769.
- Haridas D, Ponnusamy MP, Chugh S, Lakshmanan I, Seshacharyulu P, Batra SK. MUC16: molecular analysis and its functional implications in benign and malignant conditions. Faseb J 2014; 28: 4183–4199.
- Ellis R, Tang D, Nasr B, Greenwood A, McConnell A, Anagnostou ME, et al. Epidermal autophagy and beclin 1 regulator 1 and loricrin: a paradigm shift in the prognostication and stratification of the American Joint Committee on Cancer stage I melanomas. Br J Dermatol 2020; 182: 156–165.
- Guercio BJ, Iyer G, Kidwai WZ, Lacouture ME, Ghafoor S, Rossi AM, et al. Treatment of metastatic extramammary Paget disease with combination ipilimumab and nivolumab: a case report. Case Rep Oncol 2021; 14: 430–438.