ORIGINAL REPORT
Prevalence of Isotretinoin Therapy in Adolescents and Young Adults With and Without Atopic Dermatitis: A Nationwide Prescription-based Population Study
Cathrine H. MOHN1,2, Hege S. BLIX3,4, Anja Maria BRÆND1, Per NAFSTAD1,5 and Jon Anders HALVORSEN1,2
1Department of General Practice, Institute of Health and Society, University of Oslo, 2Department of Dermatology, Institute of Clinical Medicine, Oslo University Hospital, University of Oslo, 3Department of Antibiotic Resistance and Infection Prevention, Norwegian Institute of Public Health, 4Department of Pharmacy, Division for Infection Control and Environmental Health Control, University of Oslo, and 5Department of Community Medicine and Global Health, Institute of Health and Society, University of Oslo, Oslo, Norway
Although isotretinoin has anti-inflammatory and immunomodulatory properties, it can exacerbate atopic dermatitis. National estimates of the extent to which patients with atopic dermatitis are affected by severe acne and isotretinoin tolerability are lacking. The aim of this study is to investigate isotretinoin therapy in patients with atopic dermatitis and to compare the nationwide prevalence with individuals without atopic dermatitis. All Norwegian residents were followed for 17 years until age 20–22 years in 2020. Approximately 28% of patients with atopic dermatitis had been treated for acne, and 8% had received isotretinoin before age 23 years. In those over 17 years old, significantly more patients with atopic dermatitis were treated with isotretinoin than those without. At age 22 years, 2.21% (95% confidence interval 1.92–2.49) of patients with atopic dermatitis were treated with isotretinoin, compared with 1.55% (95% confidence interval 1.44–1.65) of those without, representing 42.8% (1.43; 95% confidence interval 1.24–1.65) higher use in patients with atopic dermatitis. Patients who received long-term treatment (probable severe atopic dermatitis) tolerated isotretinoin similarly to patients who received short-term treatment (probable mild atopic dermatitis). There was significantly higher use of topical corticosteroids during isotretinoin therapy in patients with atopic dermatitis. Conclusively, severe acne (isotretinoin therapy) was associated with atopic dermatitis at the population level in young adults.
Key words: acne; dermatitis; atopic; dermatological agents; eczema; isotretinoin; pharmacoepidemiology; topical acne treatment; topical calcineurin inhibitor; topical corticosteroids.
SIGNIFICANCE
There are no nationwide studies on the estimated prevalence and tolerability of isotretinoin treatment in patients with atopic dermatitis (AD). Although isotretinoin has anti-inflammatory effects, isotretinoin therapy causes dryness in patients with AD who have an already impaired skin barrier. The results suggest that patients with AD, who had long-term (probable severe) disease courses, tolerated isotretinoin similarly to patients with AD with brief disease (probable mild) courses. At age 18–22 years, the frequency of severe acne was significantly higher in the population with AD than in those without. Contrary to previous assumptions, the results suggest that severe acne is a comorbidity in young adults with AD.
Citation: Acta Derm Venereol 2023; 103: adv9424. DOI: https://doi.org/10.2340/actadv.v103.9424.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Apr 27, 2023; Published: Jun 13, 2023
Corr: Cathrine Helene Mohn, Department of Dermatology, Oslo University Hospital, Postboks 4950 Nydalen, NO-0424 Oslo, Norway. E-mail: cathrinehmohn@outlook.com
Competing interests and funding: JAH has financial/personal connections to AbbVie and Celgene. CHM received a research grant from Sanofi Genzyme in 2021. The remaining authors have no conflicts/competing interest to declare.
INTRODUCTION
Atopic dermatitis (AD) affects up to 20% of children and 10% of adults in high-income countries (1). Another prevalent inflammatory (sometimes chronic) skin disease is acne vulgaris. Both conditions exhibit ceramide deficiency, which contributes to skin barrier dysfunction related to follicular hyperkeratinization and promotes comedone formation (2–8). Lipids, the key components of the skin barrier, are reduced in patients with AD (4, 9–11). Isotretinoin has extensive anti-inflammatory and immunomodulatory properties (12). However, isotretinoin drastically reduces sebum secretion in the stratum corneum, which can lead to excessive dryness and exacerbate AD (13). The diminished skin barrier function, inflammatory component and increased risk of infections in patients with AD raise the questions as to what extent patients are affected by acne and how tolerable is isotretinoin treatment.
The occurrence of acne has been associated with oral JAK inhibitors (a systemic immunosuppressant used to treat severe AD), particularly in the population with AD, compared with other indications (14). The association between AD and acne has been poorly studied at the population level. In a small Danish study with data from 1998, no association was found (15). In another Danish study of 6,600 patients with AD, the risk of acne was increased in patients over 30 years of age (16).
The authors are unaware of any up-to-date studies on the nationwide prevalence estimates for severe acne and the tolerability of acne treatment in patients with AD. Therefore, the objectives of this study were to: (i) compare the 12-month prevalence for severe acne (dispensed isotretinoin) in the population with or without AD; (ii) evaluate treatment days and discontinuation rates of isotretinoin treatment by severity (years of treatment) of AD; and (iii) examine whether topical corticosteroid (TCS) or topical calcineurin inhibitor (TCI) treatment increases during isotretinoin therapy in patients with AD.
METHODS
Study population
The study covered individuals born between 1998 and 2000 and residing in Norway. All children aged 4–6 years in 2004, when the Norwegian Prescription Database (NorPD) was established, were followed for 17 years to age 20–22 years in 2020.
In 2004, the Norwegian population included 60,316 4-year-olds, 59,667 5-year-olds, and 61,324 6-year-olds. In 2020, the population of 20-, 21-, and 22-year-olds was 66,813, 66,164, and 67,817, respectively.
Registers and coding classifications
The NorPD contains data on all prescription drugs dispensed by pharmacies, together with unique, encrypted personal identifiers that can be used to track medication dispensing at the individual level over time (17). All prescriptions dispensed for TCSs (Anatomical Therapeutic Chemical Classification (ATC) code D07) (18), tacrolimus (D11AH01), or pimecrolimus (D11AH02) were extracted from NorPD (using the 2021 ATC/DDD index). All other prescriptions dispensed for these patients were also extracted.
Patient characteristics catalogued included age, month and year of birth, sex, dispensing date, date of death, generic drug name and ATC codes. Reimbursable prescriptions were assigned codes from the International Statistical Classification of Diseases, Tenth Revision (ICD-10), and the International Classification of Primary Care, Version Two (ICPC-2) (17). Population statistics were obtained from Statistics Norway (19).
Algorithm for identifying patients with atopic dermatitis
Patients were considered to have AD if they met at least 1 requirement for either Criterion 1 or Criterion 2.
- Criterion 1 (based on medical diagnoses): patients with recorded reimbursement prescriptions, including associated disease-specific diagnoses of “atopic dermatitis/eczema”, recorded as ICD-10 (L20) or ICPC-2 (S87).
- Criterion 2 (based on disease-specific dispensed medication): patients with non-reimbursable prescriptions were considered to have AD if, within 1 year, the patients had either:
- ≥ 2 prescriptions for TCSs
- ≥ 2 prescription for TCI
Appendix S1 provides further explanation of the algorithm employed.
Because AD is considered a chronic disease, patients identified by the algorithm were defined as “having AD”. Patients with AD treated topically for more than 9 years were defined as “having a long-term disease course”. Patients with AD treated topically for less than 5 years were defined as “having a short-term disease course”.
Identifying individuals with acne
Topical retinoids (D10AD), anti-infectives (D10AF), and azelaic acid (D10AX) were defined as topical acne preparations.
Doxycycline (J01AA02), lymecycline (J01AA04), oxytetracycline (J01AA06), and tetracycline (J01AA07) were defined as tetracyclines. A cut-off value of 50 tablets of tetracyclines was chosen to exclude prescriptions for respiratory tract infections and sexually transmitted diseases. Isotretinoin (D10BA01), cyproterone and oestrogen (G03HB01) and tetracyclines were defined as systemic acne preparations.
The defined daily dose (DDD) was used as the assumed mean maintenance dose per day (set at 30 mg per day by the World Health Organization (WHO) Collaborating Center for Drug Statistics Methodology) (17).
In Norway, isotretinoin is indicated for treating severe acne vulgaris (20). Therefore, this study defined individuals treated with isotretinoin as having severe acne. Individuals treated with topical retinoids, anti-infectives, azelaic acid, tetracycline, cyproterone and oestrogen, or isotretinoin were defined as having acne.
Persistence, attempts, and discontinuation of isotretinoin
Drug persistence was defined as the duration between the date of the first prescription and the presumed discontinuation date (days, according to DDDs, were added to the date of the last prescription). The discontinuation rate was referred to as a measure of isotretinoin tolerability. The number of days of drug supply was calculated by multiplying the number of dispensed packages by the number of DDDs per pack.
The recommended treatment interval between 2 isotretinoin treatments is at least 8 weeks, to which we added 1 month (since a patient might have accumulated a stockpile of medication). The date of the subsequent prescription was then considered the start of a new attempt if 3 months had elapsed since the presumed discontinuation date. A 16–24-week treatment duration is usually sufficient to achieve remission (21). A cut-off of 16 weeks was set to determine whether an individual had discontinued treatment. Individuals whose presumed end of treatment was in the last 3 months of the available data set were excluded from the persistence analysis (Table I).
Statistical analyses
The Poisson regression procedure was used to calculate 12-month prevalence (P) and prevalence ratio (PR) with 95% confidence intervals (95% CIs). Estimates were tested with χ2 tests. p-value < 0.05 (2-sided test) was considered statistically significant. Ps by age, sex, years of treatment (groups) and interactions were calculated.
Patients with AD were divided into 3 groups based on the number of years with TCS or TCI therapy. Patients with 1–4 years of treatment (group I) were set as the reference group to stratify and assess predictors and risk.
Descriptive statistics for continuous variables were reported as mean (SD), median (interquartile range; IQR), and frequency (percentage) for categorical variables. Population statistics from Statistics Norway (SSB) were presented by year of birth, sex, and age (based on the annual mid-year population in Norway). Data were analysed using Stata/MP software (version 17.0; StataCorp LLP; College Station, Texas, USA).
RESULTS
Patients with atopic dermatitis
The total number of patients with AD comprised 10,689 from the 1998 birth cohort, 10,697 from the 1999 birth cohort, and 11,025 from the 2000 birth cohort.
The follow-up period for all patients was 17 years. Most patients with AD received brief medical treatment. Thus, 22,968 patients (77.1%) were treated for 1–4 years (group I), 5,392 (17.7%) for 5–8 years (group II), and 2,010 (6.2%) for 9–17 years (group III). The proportion of females in the AD population was 54.4% (n = 17,593).
Prevalence of isotretinoin treatment
In over 17-year-olds, significantly more patients with AD than those without were treated with isotretinoin (increasing with age). This increased from 16.2% more (PR 1.16; 95% CI 1.07–1.26) at age 18 years to 42.8% (PR 1.43; 95% CI 1.24–1.65) at 22 years. Between the ages of 18 and 22 years, the prevalence of isotretinoin-treated individuals without AD decreased by 54.1% (PR 0.54; 95% CI 0.48–0.60), whereas it remained stable in patients with AD (Fig. 1 and Table SI).
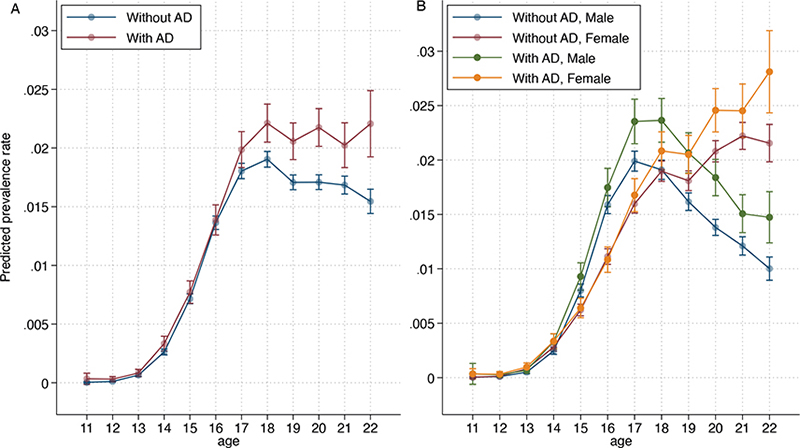
Fig. 1. Estimated 1-year prevalence in patients with atopic dermatitis (AD) and individuals without AD born in 1998, 1999, and 2000 receiving isotretinoin by (A) age and by (B) age and sex. Error bars indicate 95% confidence interval.
Between the ages of 17 and 22 years, a significantly larger proportion of males with AD were treated with isotretinoin than were males without AD, ranging from 13.5% (PR 1.13; 95% CI, 1.00–1.28) more at age 17 years to 14.0% (PR 1.40; 95% CI 1.07–1.82) more at age 22 years. Between the ages of 20 and 22 years, more females with AD were treated with isotretinoin than were females without AD, ranging from 15.3% (PR 1.15; 95% CI 1.04–1.28) more at age 20 years to 33.5% more (PR 1.33; 95% CI, 1.12–1.59) at 22 years.
Acne treatment by group and discontinuation of isotretinoin
Of the 32,371 patients with AD, 28.2% (n = 9,114) were treated for acne, with 7.6% (n = 2,479) treated for severe acne (receiving isotretinoin), before the age of 22 years.
In group II, 14.3% (PR 0.86; 95% CI 0.77–0.96) fewer patients received isotretinoin than those in group I. Correspondingly, group III received 31.6% (PR 0.68; 95% CI 0.56–0.83) less isotretinoin treatment than group I. Group III received 30.3% (PR 0.70; 95% CI 0.51–0.95) less hormone therapy than group I (Table II). Discontinuation of isotretinoin ranged from 17.1% in group I to 22.3% in group III (statistically non-significant) (Table I).
Topical corticosteroids dispensed during isotretinoin therapy
Only 1,340 (54.1%) of the 2,479 patients with AD treated with isotretinoin received TCSs/TCIs during isotretinoin treatment. In groups I, II, and III, respectively, 48.2% (n = 964/1,999), 74.1% (n = 274/370), and 92.7% (n = 102/110) of patients received TCSs or TCIs during isotretinoin treatment. In groups II and III, 53.6% (PR 1.54; 95% CI 1.34–1.76) and 92.3% (PR 1.19; 95% CI 1.57–2.36) more patients received TCSs during isotretinoin treatment than in group I (Fig. 2 and Table SII).
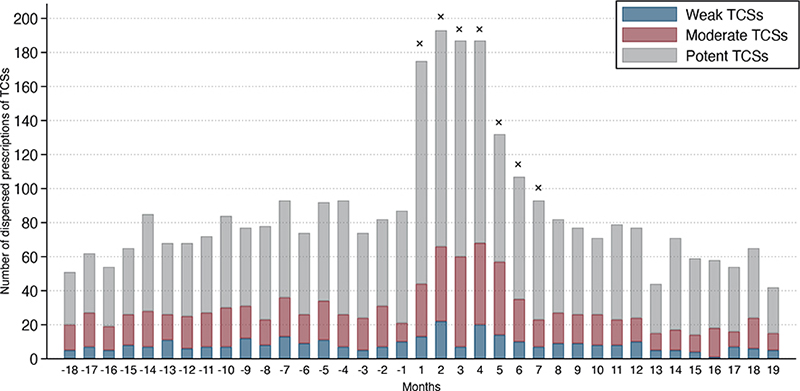
Fig. 2. Dispensed prescriptions to the 1,340 (of 2,479) patients with atopic dermatitis (AD) who received (weak, moderate, and potent) topical corticosteroids (TCSs) 18 months before and after initiation of isotretinoin treatment. Month 1 denotes the month in which the first isotretinoin was dispensed. × denotes months with a significantly higher number of total (all potencies) prescriptions dispensed compared with the median number of prescriptions 18 months before isotretinoin treatment. If patients had more than 1 treatment attempt with isotretinoin, only the first attempt was included in the graph. Table SII provides further details on monthly TCSs dispensed.
More prescriptions (TCSs or TCIs) were dispensed in the first month of isotretinoin treatment, ranging from 155.6% (PR 2.56; 95% CI, 1.96–3.34) more in the first month to 41.7% (PR 1.42; 95% CI 1.06–1.90) more in the seventh month. This was calculated using the median number of prescriptions 18 months prior to isotretinoin treatment as the reference group (75.5; IQR; 69.2–81.8).
During the first 6 months of isotretinoin treatment, dispensed prescriptions of moderate potent and potent TCSs were significantly increased. Table SII provides further details.
There was no significant increase in TCIs, weak TCSs, or very potent TCSs.
DISCUSSION
These results suggest that severe acne is a comorbidity in young adults with AD. In patients under 18 years of age, no significant differences were observed in the 1-year prevalence of isotretinoin therapy between the populations with or without AD. This was consistent with the Danish studies (15, 22). The prevalence of isotretinoin-treated individuals in the population without AD decreased between the ages of 18 and 22 years. In contrast, it remained stable in patients with AD. At age 22 years, isotretinoin therapy was 42% higher in patients with AD than in individuals without AD. Although it can be speculated that patients with AD are more likely to use topical comedogenic treatments to treat xerosis, which may contribute to acne, this is unlikely to be the sole explanation. The pathogenesis of AD involves a complex interplay of various factors (skin barrier defects, immune dysregulation, and skin microbiome) that may predispose patients with AD to cutaneous inflammatory alterations and infections such as acne (23, 24).
In the Danish study, the 12-month prevalence of acne was 3.7% in the general population and 3.9% among patients with AD (16). The risk of severe acne in patients with AD increased with age, which is consistent with the current findings. However, in contrast to the current study, the risk of being treated for severe acne was lower in patients with AD who were younger than 30 years.
Patients with 9 or more years of AD therapy had a 30% lower risk of being treated with isotretinoin or hormone therapy than patients treated for only a few years. Patients with long-term disease course (and severe AD) are likely to have easier access to a dermatologist, which is the specialty that prescribes most isotretinoin in Norway. Consequently, the significantly lower use of isotretinoin in patients with long-term disease course is a robust finding. The explanation could be that some dermatologists are hesitant to prescribe isotretinoin to patients with severe and long-term disease course due to its side-effects (xerosis) or the higher prevalence of psychological problems in patients with AD (25).
Discontinuation of isotretinoin may be caused by various factors, including time-consuming/prolonged follow-up and side-effects, such as xerosis, retinoid dermatitis, pruritus, which are more common in patients with AD (26). No significant differences in the number of patients with AD who discontinued isotretinoin treatment by group were observed, suggesting that patients with nearly a decade or more of AD therapy, tolerated isotretinoin as well as patients with a shorter history. A study from 2020 found that 17% of the subjects with acne discontinued isotretinoin treatment due to side-effects (27), which is consistent with the results of the current study and, furthermore, signifies that patients with AD as well as individuals without AD tolerate isotretinoin.
Retinoid-induced skin irritation may be alleviated by moisturizer (28). Patients with severe AD may be accustomed to applying moisturizers regularly, which could prevent AD flare-ups and irritation, and thereby increase tolerance of isotretinoin. Moreover, alitretinoin (systemic retinoid), indicated for the treatment of chronic hand eczema (CHE), binds to the intracellular retinoic acid receptors A (RAR) and X (RXR) (29). In contrast, isotretinoin binds selectively to RAR (29). Although binding to both receptors is thought to be required for the control of CHE, isotretinoin has been shown to have extensive anti-inflammatory and immunomodulatory properties that may affect treatment tolerability in patients with AD (12).
According to a Belgian study (using similar methods to the current study) (30), the median duration of isotretinoin treatment in the study population was 19.9 weeks, which is shorter than the results of the current study (28 weeks). Treatment guidelines are the same in Belgium and Norway, and the study period partially overlapped (2012–2015). Some of the patients with AD in the current study may have received “lower-dose” isotretinoin treatment for an extended period to reduce side-effects (31). Therefore, the final period (from the last dispensing to treatment discontinuation) may be longer than estimated.
A significant increase in the prescription of moderately potent and potent TCSs dispensed during the first seven months of isotretinoin therapy was observed. Only approximately half of patients with short-term disease course received TCSs or TCIs during isotretinoin treatment, compared with more than 9 in every 10 patients with long-term disease course. If the increase in TCSs was a preventive treatment strategy to avoid exacerbation, a similar increase would have been expected with all topical AD preparations. However, some patients may have collected TCS at the pharmacy, while simultaneously collecting their prescription for isotretinoin. Notwithstanding, there is no indication that patients would accumulate TCSs during their isotretinoin treatment when they do not need it, especially since those over 16 years of age must pay for some of the medication themselves. Therefore, it is reasonable to assume that the need for moderate potent and potent TCSs was high for at least 28 weeks, which is consistent with the mean duration of isotretinoin treatment in the current study (28 weeks).
Cyclosporine and JAK inhibitors have acne as a side-effect. None of the patients with AD were prescribed JAK inhibitors during the study period. Adjustments for cyclosporine yielded the same results. Other prescription drugs with acne side-effects were not adjusted for because they are assumed to be evenly distributed in the populations with and without AD.
Strengths and limitations
The Nordic countries have national health registries with validated, real-world epidemiological data (32). The large size of the novel longitudinal individual-based dataset and the complete coverage of all prescriptions dispensed by pharmacies to the entire Norwegian population ensures robustness with high significance and generalizability.
Overall, the prevalence of patients with severe acne is probably underestimated because the occurrence of untreated severe acne is unknown. Patients treated with isotretinoin are monitored regularly during the long follow-up period, which also reduces the risk of overestimating actual drug use.
Although an indication for isotretinoin treatment is severe acne (20), some individuals with moderate (and mild) disease may have been treated. However, in patients with AD, physicians are more likely to undertreat than overtreat acne for fear of aggravating the disease. In addition, Norway constantly had a positive net migration during the study period (19). Immigrants with unknown medical records may lead to artificially low prevalence estimates for isotretinoin-treated patients with AD. Overall, the prevalence of isotretinoin therapy in patients with AD is most likely underestimated and correspondingly robust.
The cost for an amount equivalent to 3 months of isotretinoin use is NOK 520.00 (October 2022). Moreover, social factors and the availability of a dermatologist may play a role in the accessibility of treatment.
Although more expensive, and sold in smaller packages than prescription medications, mild TCSs are available over the counter (OTC). However, the Norwegian social welfare system provides reimbursement for prescription drugs (free of charge until age 16 years/deductible fee after age 16 years) for chronic diseases such as AD. Consequently, an OTC purchase is expensive, and the analysed sample of patients with AD is probably representative (33).
TCSs are prescribed for a broad group of skin conditions, which may distort the accurate representation of AD drug treatment in the study population (34, 35). In addition, diagnoses recorded by physicians may be incorrect, leading to misclassification. A total of 28,612 (88.4%) of the 32,372 patients were diagnosed with AD (Criterion 1). According to a study, 2 or more annual TCS prescriptions yielded a sensitivity value of 40% and a positive predictive value (PPV) of 60% (34). Criterion 3 further increased the PPV.
Nearly 1.0% of prescriptions for TCSs or TCIs were excluded because of missing identification. AD, however, is defined as a chronic disease. Patients with AD would likely have received prior or subsequent medical treatment during the 17 years of follow-up. It is possible that most of the excluded prescriptions were among the included patients.
Conclusion
Treatment with isotretinoin was associated with AD at the population level after the age of 17 years. The number of TCSs/TCIs dispensed increased significantly during isotretinoin therapy, especially in patients with AD with a long-term disease course. There were no significant differences in discontinuation rates, the number of treatment attempts, suggesting that patients with a long-term disease course (probable severe AD) tolerated isotretinoin similarly to patients with a short-term disease course (probable mild AD).
ACKNOWLEDGEMENTS
The authors thank Harald Weedon-Fekjær for his contributions to the statistical analysis.
The datasets generated and/or analysed during this study are available from the corresponding author upon reasonable request. The observational study was analysed between January 2020 and October 2021.
The study was approved by the Regional Committees for Medical and Health Research Ethics Southeast Norway (Ref, Norway (Ref, REK: 1927) in 2015, 2019, and 2021 and by the Norwegian Social Science Data Services (NSD) in December 2015. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines, the Consolidated Standards of Reporting Trials (CONSORT) statement and the ICMJE requirements on privacy and informed consent. The study was performed in accordance with the Declaration of Helsinki 1964, and its later amendments.
This study was performed in the Department of General Practice, Institute of Health and Society, University of Oslo and was funded by the Norwegian Research Fund for General Practice. The funders had no role in the study’s design and conduct; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
The study received a research grant from Sanofi Genzyme. Sanofi Genzyme had no role in the study’s design and conduct; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
REFERENCES
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020; 396: 345–360.
- Yamamoto A, Takenouchi K, Ito M. Impaired water barrier function in acne vulgaris. Arch Dermatol Res 1995; 287: 214–218.
- Stewart ME, Grahek MO, Cambier LS, Wertz PW, Downing DT. Dilutional effect of increased sebaceous gland activity on the proportion of linoleic acid in sebaceous wax esters and in epidermal acylceramides. J Invest Dermatol 1986; 87: 733–736.
- Agrawal K, Hassoun LA, Foolad N, Borkowski K, Pedersen TL, Sivamani RK, et al. Effects of atopic dermatitis and gender on sebum lipid mediator and fatty acid profiles. Prostaglandins Leukot Essent Fatty Acids 2018; 134: 7–16.
- Ottaviani M, Camera E, Picardo M. Lipid mediators in acne. Mediators Inflamm 2010; 2010.
- Choi CW, Choi JW, Park KC, Youn SW. Facial sebum affects the development of acne, especially the distribution of inflammatory acne. J Eur Acad Dermatol Venereol 2013; 27: 301–306.
- Danby SG, Andrew PV, Brown K, Chittock J, Kay LJ, Cork MJ. An investigation of the skin barrier restoring effects of a cream and lotion containing ceramides in a multivesicular emulsion in people with dry, eczema-prone, skin: the RESTORE study phase 1. Dermatol Ther (Heidelb) 2020; 10: 1031–1041.
- Li X, He C, Chen Z, Zhou C, Gan Y, Jia Y. A review of the role of sebum in the mechanism of acne pathogenesis. J Cosmet Dermatol 2017; 16: 168–173.
- Yin H, Qiu Z, Zhu R, Wang S, Gu C, Yao X, et al. Dysregulated lipidome of sebum in patients with atopic dermatitis. Allergy 2023; 78: 1524–1537.
- Firooz A, Gorouhi F, Davari P, Atarod M, Hekmat S, Rashighi-Firoozabadi M, et al. Comparison of hydration, sebum and pH values in clinically normal skin of patients with atopic dermatitis and healthy controls. Clin Exp Dermatol 2007; 32: 321–322.
- Wirth H, Gloor M, Stoika D. Sebaceous glands in uninvolved skin of patients suffering from atopic dermatitis. Arch Dermatol Res 1981; 270: 167–169.
- Abdelmaksoud A, Lotti T, Anadolu R, Goldust M, Ayhan E, Dave DD, et al. Low dose of isotretinoin: a comprehensive review. Dermatol Ther 2020; 33: e13251.
- Colgecen E, Ozyurt K, Ferahbas Kesikoglu A. The effect of systemic isotretinoin treatment on skin biophysicalparameters among patients with acne vulgaris. Turk J Med Sci 2016; 46: 1641–1644.
- Lee SD, Ahn HJ, Shin MK. A case series of acne following Janus kinase inhibitors in patients with atopic dermatitis. JAAD Case Rep 2022; 30: 11–16.
- Halling AS, Jemec GBE, Linneberg A, Thyssen JP. No association between atopic dermatitis and acne vulgaris in the general population. J Eur Acad Dermatol Venereol 2021; 35: e276–e278.
- Thyssen JP, Nymand LK, Maul JT, Schmid-Grendelmeier P, Wu JJ, Thomsen SF, et al. Incidence, prevalence and risk of acne in adolescent and adult patients with atopic dermatitis: a matched cohort study. J Eur Acad Dermatol Venereol 2022; 36: 890–896.
- WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC classification and DDD assignment 2022. [Accessed Mars 10, 2022] Available from: https://www.whocc.no/atc_ddd_index/
- World Health Organization (WHO) – I nternational Classification of Primary Care, Second edition (ICPC-2). [Accessed date Mars 10, 2022] Available from: https://www.whocc.no/atc_ddd_index/
- Norwegian Government. Statistics Norway (Statistisk Sentralbyrå). [Accessed April 4, 2022] Available from http://www.ssb.no/en.
- Helsedirektoratet. Isotretinoin (Vedlegg 1 til § 5–14). [Accessed date November 18, 2022] Available from https://www.helsedirektoratet.no/rundskriv/kapittel-5-stonad-ved-helsetjenester/vedlegg-1-til--5-14-legemiddellisten/virkestoffer/isotretinoin.
- Felleskatalogen. [Accessed September 5, 2022] Available from https://www.felleskatalogen.no/medisin/isotretinoin-orifarm-orifarm-generics-560319.
- Larsson PA, Lidén S. Prevalence of skin diseases among adolescents 12–16 years of age. Acta Derm Venereol 1980; 60: 415–423.
- Roh YS, Huang AH, Sutaria N, Choi U, Wongvibulsin S, Choi J, et al. Real-world comorbidities of atopic dermatitis in the US adult ambulatory population. J Am Acad Dermatol 2022; 86: 835–845.
- Sun D, Ong PY. Infectious complications in atopic dermatitis. Immunol Allergy Clin North Am 2017; 37: 75–93.
- Davis DMR, Drucker AM, Alikhan A, Bercovitch L, Cohen DE, Darr JM, et al. American Academy of Dermatology Guidelines: awareness of comorbidities associated with atopic dermatitis in adults. J Am Acad Dermatol 2022; 86: 1335–1336.e1318.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol 2013; 26: 290–293.
- Alshammari S, Alamari Y, Alazani A, Almuhanna S, Pinjabi L, Alsnadi N. Prevalence and associated risk factors of acne relapse among Saudi acne vulgaris patients using isotretinoin. Saudi Pharm J 2020; 28: 374–379.
- Laquieze S, Czernielewski J, Rueda M-J. Beneficial effect of a moisturizing cream as adjunctive treatment to oral isotretinoin or topical tretinoin in the management of acne. J Drugs Dermatol 2006; 5: 985–990.
- King T, McKenna J, Alexandroff AB. Alitretinoin for the treatment of severe chronic hand eczema. Patient Prefer Adherence 2014; 8: 1629–1634.
- Biset N, Lelubre M, Senterre C, Amighi K, Bugnon O, Schneider MP, et al. Assessment of medication adherence and responsible use of isotretinoin and contraception through Belgian community pharmacies by using pharmacy refill data. Patient Prefer Adherence 2018; 12: 153–161.
- Rademaker M. Isotretinoin: dose, duration and relapse. What does 30 years of usage tell us? Australas J Dermatol 2013; 54: 157–162.
- Smith Jervelund S, De Montgomery CJ. Nordic registry data: value, validity and future. Scand J Public Health 2020; 48: 1–4.
- Norrlid H, Hjalte F, Lundqvist A, Svensson A, Tennvall GR. Cost-effectiveness of maintenance treatment with a barrier-strengthening moisturizing cream in patients with atopic dermatitis in Finland, Norway and Sweden. Acta Derm Venereol 2016; 96: 173–176.
- Mulder B, Groenhof F, Kocabas LI, Bos HJ, De Vries TW, Hak E, et al. Identification of Dutch children diagnosed with atopic diseases using prescription data: a validation study. Eur J Clin Pharmacol 2016; 72: 73–82.
- Ortqvist AK, Lundholm C, Wettermark B, Ludvigsson JF, Ye W, Almqvist C. Validation of asthma and eczema in population-based Swedish drug and patient registers. Pharmacoepidemiol Drug Saf 2013; 22: 850–860.