ORIGINAL REPORT
Melanoma Prognosis and Associated Risk Factors: A Retrospective Cohort Study Using Semantic Map Analysis
Simone CAZZANIGA1,2#, Carole Anouk ZAHN1#, Seyed Morteza SEYED JAFARI1 and Robert Emil HUNGER1
1Department of Dermatology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland and 2Centro Studi GISED, Bergamo, Italy
#These authors contributed equally to this work and share first authorship.
The overall patterns of correlations among various melanoma risk factors have not yet been examined. The aim of this study was to assess the impact of different parameters on disease-free and melanoma-related overall survival. A retrospective cohort study was conducted encompassing all patients with a primary cutaneous melanoma diagnosed in a university referral centre. Associations were explored using semantic map analysis, which uses graph theory to find the strongest path of connections between variables. A total of 1,110 melanoma patients (median follow-up 10.6 years) were included. The analysis revealed a clustering of variables around 2 main hubs: Breslow thickness < 1 mm and ≥ 4 mm. Factors connected with high melanoma thickness were: older age, positive sentinel lymph node biopsy findings, presence of ulceration, nodular melanoma type, and light skin phototype. Both disease-free and melanoma-related overall survival were in this cluster and connected with positive sentinel lymph node biopsy and Breslow ≥ 4 mm. Patients with Breslow between 1 and 3.9 mm were also in this cluster and linked with negative sentinel lymph node biopsy, nodular melanoma and safety distance > 10 mm. This semantic analysis confirmed the close link between Breslow thickness, age, sentinel lymph node biopsy findings, skin type, melanoma subtype and prognosis, and provides prognostic information useful for the further stratification and management of patients with melanoma.
Key words: melanoma; outcome; prognosis; risk factors; semantic map analysis; survival.
SIGNIFICANCE
Melanoma may recur, and its metastasis may occur, many years after its first diagnosis. Therefore, it is crucial to understand the prognosis of this tumour and its association with other clinical characteristics. This study of 1,110 patients used an innovative approach that can provide an overall picture of the most important associations. The results show a clustering of patient’s and melanoma features around low and high tumour thickness. Specifically, older age, light skin type, lymph node biopsy results, melanoma subtype and its prognosis were all associated with thick melanoma. This information can be useful to predict patients’ course and plan their treatment.
Citation: Acta Derm Venereol 2023; 103: adv9591. DOI: https://doi.org/10.2340/actadv.v103.9591.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Apr 21, 2023; Published: May 29, 2023
Corr: Simone Cazzaniga, Department of Dermatology, Inselspital University Hospital, Freiburgstrasse 34, CH-3010 Bern, Switzerland. E-mail: simone.cazzaniga@insel.ch
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Cutaneous malignant melanoma (MM) may recur, and its metastasis may occur, many years after its first diagnosis. Therefore, it is crucial to analyse the course of cutaneous MM and follow patients over a long period. Several studies have independently identified and confirmed that tumour thickness, ulceration, presence of locoregional disease and micrometastasis in the sentinel lymph node represent useful and important prognostic risk factors for the stratification and management of patients with MM (1–3). Nonetheless, the overall patterns of correlations among various possible risk factors, together with the characteristics of tumours and patients have not yet been shown.
Different algorithms have been developed with the aim of exploring the multidimensional complex interactions among variables in databases. Among these, a data-mining algorithm, called semantic map analysis, which is able to show the most important connections between variables in a dataset, has been used in the context of different skin conditions, including chronic hand eczema, psoriasis, hidradenitis suppurativa and associations of vascular endothelial growth factor with MM patients’ characteristics and survival (4–7).
By using semantic map analysis, this study aimed to visualize a more comprehensive understanding of the associations among epidemiological and clinicopathological characteristics of patients with primary cutaneous MM, which critically affect disease-free survival (DFS) and melanoma-related overall survival (MROS).
MATERIALS AND METHODS
This single-centre retrospective study included all consecutive cases of single, primary, localized, histopathological confirmed cutaneous MM tumours that were diagnosed between February 1990 and July 2014 and followed up until July 2021 at the Department of Dermatology, University Hospital of Bern, Switzerland.
The following patients’ characteristics were systematically collected during the first evaluation and first staging: sex, age, Fitzpatrick’s skin type, tumour location and type, Breslow thickness, presence of ulceration, fascia excision during surgery, safety distance and sentinel lymph node biopsy (SLNB) findings. Furthermore, information on patients’ follow-up length, occurrence of distant, local and locoregional metastases, death and death related to MM were gathered from the electronic medical records.
The patients’ management (including: SLNB) and follow-up were scheduled according to the Swiss guidelines for the treatment and follow-up of cutaneous MM, in adherence with the international guidelines, depending on the tumour stage and time span after the initial diagnosis. Patients without regular follow-up visits were excluded from the study.
Informed consent was obtained from the patients and the study was conducted in accordance with the standards of the ethics committee of the Canton of Bern on human experimentation and with the Declaration of Helsinki 1975, revised 1983.
Statistical analysis
For descriptive purposes, continuous data were presented as medians with ranges, while categorical data were presented as absolute numbers with percentages. For analysis purposes, continuous data were also categorized using clinically relevant cut-off points. Kaplan–Meier method was used to produce survival estimates along with their 95% confidence intervals (95% CI).
Associations among patients’ demographics, clinical characteristics and survival were explored using semantic map analysis (4–7). This is a data-mining algorithm that is able to find and display the most important associations between different variables, taking into account the effect of other covariates in the system. The algorithm uses graph theory and maximum spanning tree (MST) in order to find and show the strongest path of connections between variables based on normalized coefficients from multivariable regression models (4, 7, 8). In order to avoid unstable associations, only connections with a p-value < 0.15 were considered by the algorithm. In the resulting map variables with many connections (hubs) are the most important for the system, while those with fewer connections or at the periphery (leaves) are the least important. Moreover, variables in the same area form clusters with straight lines showing the strongest associations. The strength of correlations found by the algorithm can be interpreted as mild, moderate or strong for values < 0.6, 0.6–0.79 and ≥ 0.8, respectively (4). Analyses were performed with SPSS v.26.0 (IBM Corp., Armonk, NY, USA) and MATLAB v.9.1 software (The MathWorks, Natick, MA, USA).
RESULTS
From the database, 1,110 patients (median age 56 years, 52.5% males) fulfilled the criteria for inclusion in the study (Table I). Most patients had Fitzpatrick’s skin type II (67.5%) or III (26.1%), while the most common MM locations were the trunk (40.0%) and lower extremities (28.5%). Nodular melanoma (NMM – 35.3%) and superficial spreading melanoma (SSM – 49.6%) were the most frequent types observed. Median Breslow thickness was 1.1 mm (range 0.1–45.0) with 47.1% of patients between 1–3.9 mm and 11.1% ≥ 4 mm. MM ulceration was histologically found in 16.1% of patients and 14.1% had positive SLNB findings.
| Characteristics | |
| Total | 1,110a |
| Sex, n (%) | |
| Female | 527 (47.5) |
| Male | 583 (52.5) |
| Age, years, median (range) | 56.0 (12.0–93.0) |
| < 40 years, n (%) | 211 (19.0) |
| 40–54 years, n (%) | 305 (27.5) |
| 55–69 years, n (%) | 357 (32.2) |
| ≥ 70 years, n (%) | 237 (21.4) |
| Skin type, n (%) | |
| I | 60 (5.5) |
| II | 734 (67.5) |
| III | 284 (26.1) |
| IV | 10 (0.9) |
| Primary tumour location, n (%) | |
| Head and neck | 158 (14.2) |
| Trunk | 444 (40.0) |
| Upper extremities | 192 (17.3) |
| Lower extremities | 316 (28.5) |
| Melanoma type, n (%) | |
| Nodular | 383 (35.3) |
| Superficial spreading melanoma | 539 (49.6) |
| Acral lentiginous melanoma | 34 (3.1) |
| Lentigo maligna melanoma | 53 (4.9) |
| Desmoplastic | 11 (1.0) |
| Amelanotic | 6 (0.6) |
| Undefined | 60 (5.5) |
| Presence of ulceration, n (%) | |
| No | 931 (83.9) |
| Yes | 179 (16.1) |
| Breslow thickness, mm, median, range | 1.1 (0.1–45.0) |
| < 1 mm, n (%) | 464 (41.8) |
| 1–3.9 mm, n (%) | 523 (47.1) |
| ≥ 4 mm, n (%) | 123 (11.1) |
| Fascia excision during surgery, n (%) | |
| No | 726 (65.4) |
| Yes | 384 (34.6) |
| Safety distance, mm, median, range | 10.0 (3.0–30.0) |
| Sentinel lymph node biopsy, safety distance, mm, n (%) | |
| Not performed | 218 (19.7) |
| – | 735 (66.3) |
| + | 156 (14.1) |
| aNumbers may not add up to the total due to missing data. | |
Melanoma survival
The median follow-up length of all included patient was 10.6 years, ranging from 15 days to 30.2 years. Table II reports estimated survival outcomes in the study population, overall and according to Breslow thickness groups.
At 10 years, the overall DFS was 83.0% (95% CI 80.6–85.4), ranging from 95.3% (93.3–97.3) for Breslow < 1 mm to 79.2% (95% CI 75.4–82.9) for Breslow 1–3.9 mm, to 52.0% (95% CI 42.9–61.1) for Breslow ≥ 4 mm.
On the other side, MROS was 89.2% (95% CI 87.2–91.2), varying from 97.5% (95% CI 96.0–99.0) to 87.4% (95% CI 84.2–90.6) and to 63.1% (95% CI 53.6–72.6) for Breslow < 1, 1–3.9 and ≥ 4 mm, respectively (Fig. 1).
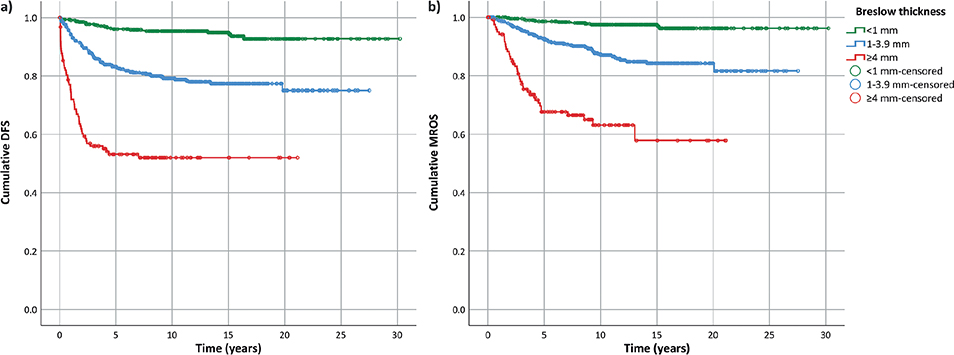
Fig. 1. Kaplan–Meier plot of (a) disease-free survival (DFS) and (b) melanoma-related overall survival (MROS) according to Breslow thickness groups.
Semantic map connections
The results of semantic map analysis of demographics, phenotypic, clinical characteristics and survival outcomes in the study population are shown in Fig. 2.
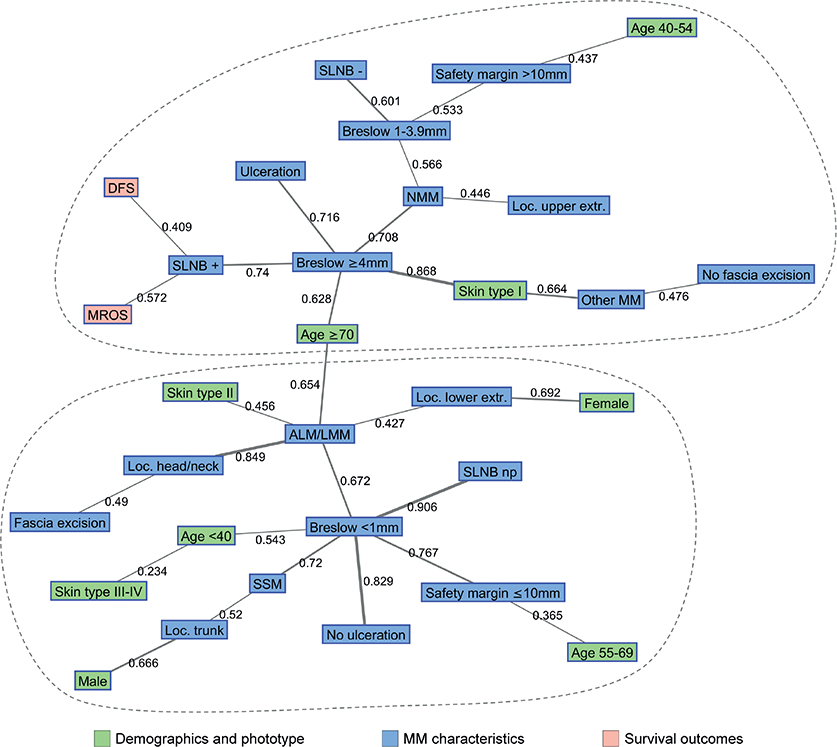
Fig. 2. Semantic map analysis of demographics, clinical characteristics and survival outcomes in the study population. ALM: acral lentiginous melanoma; DFS: disease-free survival; extr: extremities; loc: localization; LMM: lentigo maligna melanoma; NMM: nodular melanoma; MM: melanoma; MROS: melanoma-related overall survival; np: not performed; SLNB: sentinel lymph node biopsy; SSM: superficial spreading melanoma.
There was a quite clear clustering of variables around the main hubs of the system, which were Breslow thickness < 1 and ≥ 4 mm. Factors connected to high MM thickness were older age (≥ 70 years), positive SLNB findings, presence of ulceration, NMM type and light skin phototype (type I). Both DFS and MROS were in this cluster and connected to positive SLNB and Breslow ≥ 4 mm. Patients with Breslow between 1 and 3.9 mm were also in this cluster and linked with negative SLNB results, NMM and safety distance > 10 mm.
In contrast, low MM thickness (< 1 mm) was associated with absence of ulceration, SSM, especially on the trunk and in males, acral lentiginous melanoma (ALM) or lentigo maligna melanoma (LMM), safety distance ≤ 10 mm, younger age (< 40 years) and medium or dark skin phototypes (type III/IV).
Finally, patients with ALM or LMM tend to form a specific subcluster connected to fair skin phototype (type II), older age (≥ 70 years) and localization on head/neck or lower extremities, especially in females.
DISCUSSION
To our knowledge, this is the first study to show a broad overview of factors associated with MM characteristics and survival by using semantic map analysis. The analysis confirmed the well-established strong associations between high tumour thickness and positive SLNB, as well as ulceration, NMM and fair skin type (phototype I).
Breslow thickness is one of the most important factors in the prognosis of MM patients (3, 9, 10). The semantic map analysis pointed out a clear clustering of variables around the main hubs of the system, which were represented by Breslow thickness groups. The current results show close linking between Breslow thickness, age, SLNB findings, skin type, MM subtype and prognosis in patients with primary cutaneous MM, with a median follow-up of > 10 years.
Concerning the other histopathological predictors, besides Breslow thickness in patients with MM, the current study could confirm the close linking between a low Breslow (< 1 mm) and the absence of ulceration, whereas a higher Breslow (≥ 4 mm) showed a close connection with ulceration (correlation = 0.716) (11, 12). However, this study did not include other specific prognostic predictors, such as the MM mitotic rate or BRAF, NRAS, HRAS oncogenes in the analysis.
Several prospective studies have shown and confirmed the positive association between SLNB results and DFS (3, 13, 14). One paper even stated the histopathology of SLNB as the second important significant prognostic factor of DFS (3). Similarly, in the current study, patients with positive SLNB findings were directly associated with thick MM (Breslow ≥ 4 mm) as well as with lower MROS and DFS.
It was also found that ALM and LMM localized on the lower extremities or the head/neck region, respectively, were linked to older patients with Fitzpatrick’s skin type II. A possible explanation could be the longer lasting potential of ultraviolet damage in sun-exposed areas in fair skin over time. A connection between ALM or LMM, lower extremities and female sex was also found, whereas males were more affected by SSM on the trunk, although it remains unclear why this category of patients is specifically affected.
Thin MM tends to correlate only mildly with age, in contrast to thick MM, showing a higher prevalence in older patients regardless of sex.
Furthermore, the current study showed that, especially younger patients between age 40 and 54 years with a higher Breslow thickness (1–3.9 mm), had a larger safety margin (> 10 mm) in the surgical procedure. In addition, SLNB removal was not performed in only 19.7% of all 1,110 patients, even if the Breslow thickness was less than 1 mm in many more patients (41.8%). It should be borne in mind that the guidelines and recommendations for safety distance and SLNB removal changed over the years and a younger age might also influence the radicality and complexity of the procedure (15).
In the current study the overall DFS and MROS were 83.0% and 89.2% at 10 years, respectively. These estimates are in line with the results obtained from other series (16–18), although pre-existing survival data at 15 and 20 years are missing due to the shorter follow-up period (mostly 5–10 years) considered in other studies. A possible limitation of this study was that the analysis was mainly exploratory and specific hypotheses emerging from the map should be better tested in further ad hoc studies. The novel treatment options, such as immune checkpoint inhibitors in advanced MM, have revolutionized the prognosis and survival rate of patients over time and could have influenced the current results regarding survival rate. Another possible limitation was the retrospective study design, which could have introduced biases in the associations found. In addition, we did not include comorbidities and level of lactate dehydrogenase (LDH) of each patient, which could have affected the course of the disease. Finally, this study was planned as a single-centre initiative. Future studies should focus on larger datasets from nationwide or population-based cohorts and could also evaluate the effect of treatment era and therapies prescribed using the same methodology.
Overall, the current study presents a comprehensive picture of the most relevant associations between patients’ demographics, phenotypic features, MM characteristics and its prognosis in 1,110 patients followed up for more than 20 years. Based on semantic map analysis, this study identified the most relevant correlations for this potential aggressive tumour and provide prognostic information for the clinical management of patients in the future.
REFERENCES
- Balch CM, Gershenwald JE. Clinical value of the sentinel-node biopsy in primary cutaneous melanoma. N Engl J Med 2014; 370: 663–664.
- Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med 2014; 370: 599–609.
- Moehrle M, Schippert W, Rassner G, Garbe C, Breuninger H. Micrometastasis of a sentinel lymph node in cutaneous melanoma is a significant prognostic factor for disease-free survival, distant-metastasis-free survival, and overall survival. Dermatol Surg 2004; 30: 1319–1328.
- Cazzaniga S, Apfelbacher C, Diepgen T, Ofenloch RF, Weisshaar E, Molin S, et al. Patterns of chronic hand eczema: a semantic map analysis of the CARPE registry data. Br J Dermatol 2018; 178: 229–237.
- Cazzaniga S, Anzengruber F, Augustin M, Boehncke WH, Borradori L, Conrad C, et al. Linkage between patients’ characteristics and prescribed systemic treatments for psoriasis: a semantic connectivity map analysis of the Swiss Dermatology Network for Targeted Therapies registry. J Eur Acad Dermatol Venereol 2019; 33: 2313–2318.
- Thomi R, Cazzaniga S, Seyed Jafari SM, Schlapbach C, Hunger RE. Association of hidradenitis suppurativa with t helper 1/t helper 17 phenotypes: a semantic map analysis. JAMA Dermatol 2018; 154: 592–595.
- Cazzaniga S, Wiedmer C, Frangež Ž, Shafighi M, Beltraminelli H, Weber B, et al. Association of vascular endothelial growth factor subtypes with melanoma patients’ characteristics and survival: a semantic connectivity map analysis. Acta Derm Venereol 2020; 100: adv00019.
- Pemmaraju S, Skiena S. Combinatorics and graph theory in mathematica. In: Pemmaraju S, Skiena S, editors. Computational discrete mathematics. Cambridge: Cambridge University Press; 2003, p. 336–337.
- Abbas O, Miller DD, Bhawan J. Cutaneous malignant melanoma: update on diagnostic and prognostic biomarkers. Am J Dermatopathol 2014; 36: 363–379.
- Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001; 19: 3622–3634.
- Balch CM, Wilkerson JA, Murad TM, Soong SJ, Ingalls AL, Maddox WA. The prognostic significance of ulceration of cutaneous melanoma. Cancer 1980; 45: 3012–3017.
- Bønnelykke-Behrndtz ML, Schmidt H, Christensen IJ, Damsgaard TE, Møller HJ, Bastholt L, et al. Prognostic stratification of ulcerated melanoma: not only the extent matters. Am J Clin Pathol 2014; 142: 845–856.
- Seyed Jafari SM, Jäckle P, Michel A, Angermeier S, Hunger R, Shafighi M. Prognostic value of sentinel lymph node biopsy in melanomas of different Breslow’s thickness. Swiss Med Wkly 2016; 146: w14358.
- Stoffels I, Boy C, Pöppel T, Kuhn J, Klötgen K, Dissemond J, et al. Association between sentinel lymph node excision with or without preoperative SPECT/CT and metastatic node detection and disease-free survival in melanoma. JAMA 2012; 308: 1007–1014.
- Garbe C, Amaral T, Peris K, Hauschild A, Arenberger P, Bastholt L, et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment – update 2019. Eur J Cancer 2020; 126: 159–177.
- Lim Y, Lee J, Lee DY. Is the survival rate for acral melanoma actually worse than other cutaneous melanomas? J Dermatol 2020; 47: 251–256.
- Giblin AV, Thomas JM. Incidence, mortality and survival in cutaneous melanoma. J Plast Reconstr Aesthet Surg 2007; 60: 32–40.
- Sim FH, Nelson TE, Pritchard DJ. Malignant melanoma: Mayo Clinic experience. Mayo Clin Proc 1997; 72: 565–569.