SHORT COMMUNICATION
Atezolizumab Plus Bevacizumab-induced Recalcitrant Pyoderma Gangrenosum Treated with Baricitinib: A Case Report
Han Seul KIM1, Ji Eun KWON2 and Young Joon PARK1*
1Department of Dermatology and 2Department of Pathology, Ajou University School of Medicine, 164, World Cup-ro,, Suwon, Korea.
*E-mail: aro@ajou.ac.kr
Citation: Acta Derm Venereol 2023; 103: adv9646. DOI https://doi.org/10.2340/actadv.v103.9646.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jun 20, 2023; Published: Aug 1, 2023
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Pyoderma gangrenosum (PG) is a rare inflammatory skin disease that manifests as painful ulcers (1, 2). While the pathogenesis of PG is not fully understood, it is thought to be related to immune dysfunction, presenting as neutrophil dysfunction, keratinocyte apoptosis, and inappropriate inflammatory responses (3). PG has been reported in patients with a history of certain immune checkpoint inhibitors (4–6). We report here a case of PG that occurred following treatment with concomitant atezolizumab and bevacizumab and was stabilized with a Janus kinase (JAK) inhibitor.
CASE REPORT
A 53-year-old man presented to the department of dermatology with a crusted erythematous patch on his scrotum (Fig. 1A). He had undergone surgical excision of hepatocellular carcinoma (HCC) 1 year previously; he had no signs of hepatic impairment despite his history of chronic B-viral hepatitis and had been taking oral medication for diabetes mellitus. He started atezolizumab plus bevacizumab combination therapy as a part of a clinical trial 3 months prior to skin presentation. He reported a substantial amount of pain and itching in the affected area. A punch biopsy was performed at the border of the lesion, which showed diffuse lymphocytic infiltration, predominantly of plasma cells (Fig. 1A, B). Laboratory examinations including syphilis test did not show any signs of infection or systemic disease.
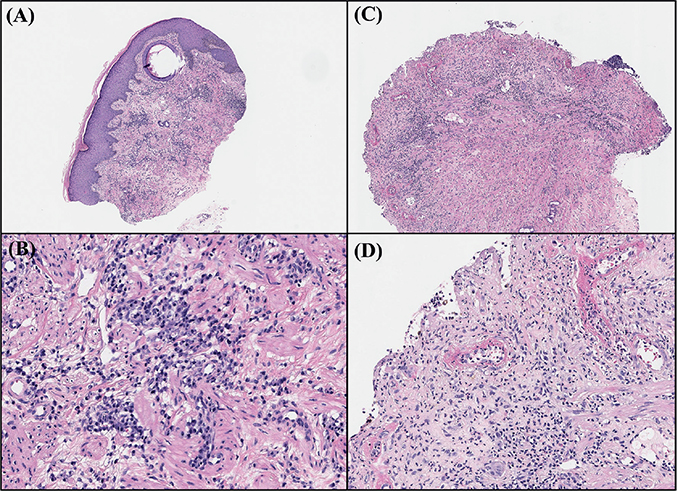
Fig. 1. Histological findings of current case. (A) Biopsy specimen from the edge of the scrotal lesion taken at the initial visit (haematoxylin and eosin (H&E), original magnification ×x40). (B) Diffuse lymphocytic infiltrate from upper to lower dermis characterized by extensive plasma cell infiltration (H&E, original magnification ×200). (C) Second biopsy specimen taken from of the developed scrotal ulcer (H&E, original magnification ×100). (D) Dense neutrophilic infiltrate with hyaline necrosis of blood vessels (H&E, original magnification ×200).
A systemic steroid treatment, equivalent to 8 mg methylprednisolone, was initiated with a clinical diagnosis of scrotal eczema. Due to his pre-existing diabetes and preference to avoid steroids, the patient preferred to initiate treatment with a lower steroid dose or explore alternative medication options. Despite the addition of cyclosporine and an increased steroid dose, the lesion increased in size and depth and was accompanied by continuous purulent discharge. Detailed information on the drugs administered during treatment is shown in Fig. 2. Written informed consent was obtained from the patient to publish images and data. As the patient’s lesion worsened, another skin biopsy was performed at the ulcerative lesion on the scrotum to exclude the possibility of other diseases. Histological examination exhibited dense neutrophilic infiltration in the dermis, adnexal structures, and blood vessels, suggestive of PG (Fig. 1C, D). Combination therapy for HCC was discontinued, but the painful ulcer persisted. Thus, the patient was admitted under the impression of PG and treated with intravenous methylprednisolone pulse and intravenous immunoglobulin (IVIG) therapy. Although the size and depth of the lesion stabilized after admission, the skin lesions showed an absence of epithelialization with continuous purulent discharge.
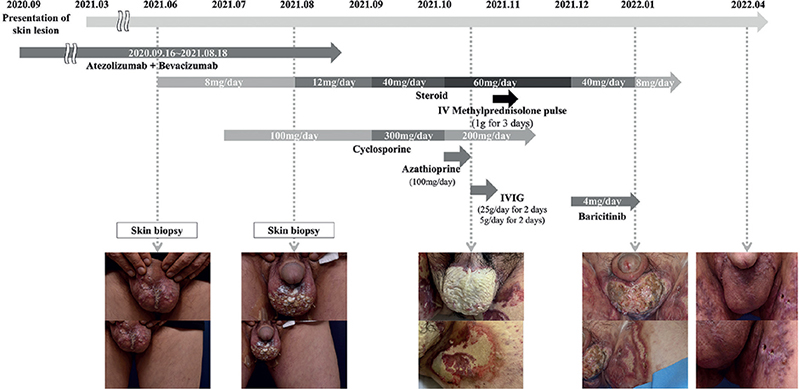
Fig. 2. Flowchart of patient history, and clinical photographs. Arrows indicate the duration of administration of each drug. IVIG: intravenous immunoglobulin therapy.
Three months after the initial visit, the patient reported diffuse oedema, especially on the face and legs. The peripheral oedema was thought to be related to chronic steroid treatment. Blood glucose levels were difficult to control and the HbA1c level increased significantly. Furthermore, the patient reported nausea and headaches. Consequently, the steroid dosage was reduced and cyclosporine was discontinued. The patient’s skin lesions deteriorated, and he reported increased pain coinciding with the medication tapering. As the patient experienced multiple side-effects with conventional therapy, baricitinib treatment was initiated with his consent. Within 2 weeks of baricitinib treatment, the lesion showed a significant reduction in purulent discharge and pain, along with an increased area of epithelialization. Two months later, split-skin grafting was performed on the remnant lesion, which resulted in near-full epithelialization. As of May 2023, the patient is under follow-up with no suspicious findings suggesting the reappearance of HCC or PG.
DISCUSSION
The clinical efficacy of atezolizumab plus bevacizumab combination therapy in unresectable HCC has recently been proven in large clinical trials (7). There have been reports on PG being associated with other immune checkpoint inhibitors, or bevacizumab (Table I) (4, 5, 8, 9). However, to the best of our knowledge, this is the first case of PG occurring after receiving atezolizumab plus bevacizumab combination therapy. It has been suggested that alterations in T-cell immune tolerance induced by programmed death-1 inhibitors may trigger T-cell dysregulation and subsequent development of PG (4). Although the mechanism of action remains unclear, atezolizumab may play a similar role in triggering PG. As bevacizumab is a vascular endothelial growth factor (VEGF) inhibitor, the anti-angiogenic effects of VEGF inhibition may induce necrosis and subsequent invasion of neutrophils, as reported previously (10). The combination of the 2 drugs may lead to extensive or recalcitrant PG, as in the current case.
| Drug (mechanism of action) | Patient, sex, age, years | Underlying malignancy | Onset | Location of lesions | Treatment | Clinical outcome | Cessation of causative drug |
| Pembrolizumab (PD-1 inhibitor) (4) | Man, 78 | Cutaneous squamous cell carcinoma | 2 months after pembrolizumab initiation | Trunk | Wound dressing Intralesional triamcinolone Oral prednisone |
Complete healing after 2 months from stopping of pembrolizumab | Yes |
| Ipilimumab (CTLA4 inhibitor) (5) | Woman, 56 | Metastatic melanoma | 4 months after first chemotherapy cycle | Unknown | Oral prednisolone Infliximab |
Complete healing after infliximab therapy | Unknown |
| Ipilimumab + Nivolumab (CTLA-4 inhibitor + PD-1inhibitor) (6) | Man 75 | Myelodysplastic syndrome | 2 weeks after ipilimumab and nivolumab initiation | Lower extremities | Intralesional triamcinolone Oral prednisone |
Slowly improving | Yes |
| Bevacizumab (VEGF inhibitor) (9) | Woman, 44 | Cervical adenocarcinoma | Days after first chemotherapy cycle | Trunk | Oral prednisone Oral cyclosporine |
Complete healing after systemic therapy | Yes |
| PD-1: programmed cell death protein 1; CTLA-4: cytotoxic T lymphocyte-associated antigen-4. | |||||||
We were unable to stabilize the disease using conventional medication. Addition of baricitinib resulted in a rapid decrease in discharge and subsequent epithelialization. Few studies have reported the effectiveness of baricitinib in patients with an inadequate response to steroid therapy (11, 12). A recent study showed that the JAK/STAT pathway is upregulated in patients with PG (13), which may provide a possible explanation for the clinical efficacy of JAK inhibitors in treating PG.
Despite the use of dramatic stabilization, as in the current case, the use of baricitinib in recalcitrant PG should be considered with care, due to the limitations of the current report. First, baricitinib was not the only medication used: we concomitantly used a considerable amount of systemic steroids. Secondly, as the combination therapy of bevacizumab and atezolizumab was discontinued before the use of baricitinib, we cannot exclude the possibility of medication discontinuation affecting the outcome. However, this seems unlikely, as the lesions remained persistent despite 2 months of significant immunosuppressive treatments. Lastly, despite evidence of effective wound healing, the patient underwent a skin graft for the scrotum lesions. Therefore, the skin graft might have contributed to the recalcitrant PG resolution.
In summary, we report here a case of PG initiated after combined therapy with atezolizumab and bevacizumab, which was stabilized with an adjuvant JAK inhibitor. This rare case raises awareness of the cutaneous side-effects of immune checkpoint inhibitors and their combination with other therapeutic agents. We consider that the addition of JAK inhibitors might aid in the difficult treatment of recalcitrant PG. Further studies are needed to clarify the exact mechanism underlying the clinical efficacy of JAK inhibitors for treatment of PG.
REFERENCES
- Crowson AN, Mihm MC, Jr, Magro C. Pyoderma gangrenosum: a review. J Cutan Pathol 2003; 30: 97–107.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol 2018; 14: 225–233.
- Wu BC, Patel ED, Ortega-Loayza AG. Drug-induced pyoderma gangrenosum: a model to understand the pathogenesis of pyoderma gangrenosum. Br J Dermatol 2017; 177: 72–83.
- Tsibris H, Lian C, Ho A. Pembrolizumab-associated pyoderma gangrenosum in a patient with metastatic squamous cell carcinoma. Dermatol Online J 2021; 27.
- Rudolph BM, Staib F, Von Stebut E, Hainz M, Grabbe S, Loquai C. Neutrophilic disease of the skin and intestines after ipilimumab treatment for malignant melanoma – simultaneous occurrence of pyoderma gangrenosum and colitis. Eur J Dermatol 2014; 24: 268–269.
- Welborn ME, Kubicki SL, Patel AB. Pyoderma gangrenosum following initiation of immune checkpoint inhibitor therapy. J Immunother Precis Oncol 2018; 1: 82–84.
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020; 382: 1894–1905.
- Ellis SR, Vierra AT, Millsop JW, Lacouture ME, Kiuru M. Dermatologic toxicities to immune checkpoint inhibitor therapy: a review of histopathologic features. J Am Acad Dermatol 2020; 83: 1130–1143.
- Ellis C, M. M, LaFond A, Fivenson D. An interesting case of pyoderma gangrenosum. J Am Acad Dermatol 2016; 74.
- Akanay-Diesel S, Hoff NP, Kurle S, Haes J, Erhardt A, Haussinger D, et al. Sunitinib induced pyoderma gangrenosum-like ulcerations. Eur J Med Res 2011; 16: 491–494.
- Scheinberg M, Machado LA, LG MC, Ferreira SB, Michalany N. Successful treatment of ulcerated pyoderma gangrenosum with baricitinib, a novel JAK inhibitor. J Transl Autoimmun 2021; 4: 100099.
- Sitaru S, Biedermann T, Lauffer F. Successful treatment of pyoderma gangrenosum with Janus kinase 1/2 inhibition. JEADV Clinical Practice 2022; 1: 420–423.
- Ortega-Loayza AG, Friedman MA, Reese AM, Liu Y, Greiling TM, Cassidy PB, et al. Molecular and cellular characterization of pyoderma gangrenosum: implications for the use of gene expression. J Invest Dermatol 2022; 142: 1217–1220.e4.