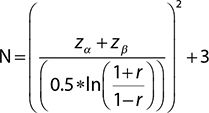RESEARCH ARTICLE
Correlational analysis between salivary and blood glucose levels in individuals with and without diabetes mellitus: a cross-sectional study
Laasya Shettigara#, Sanchita Sivaramanb, Rohini Raoc#, Sanjana Akhila Arunc, Aditi Chopraa, Shobha U Kamathd and Raju Ranad
aDepartment of Periodontology, Manipal College of Dental Sciences, Manipal, Manipal Academy of Higher Education, Manipal, India; bUBC School of Population and Public Health, British Columbia, Vancouver, Canada; cDepartment of Data Science and Computer Applications, Manipal Institute of Technology (MIT), Manipal, Manipal Academy of Higher Education, Manipal, India; dDepartment of Biochemistry, Kasturba Medical College and Hospital, Manipal, Manipal Academy of Higher Education, Manipal, India
ABSTRACT
Objective: To estimate the association of patient-related demographic, socioeconomic status, physical activity, stress, and dietary factors influencing the relationship between salivary and blood glucose levels in individuals with and without diabetes mellitus (DM).
Method: This cross-sectional study was conducted on 166 participants with and without DM. Saliva and blood were collected to estimate the glucose levels. Age, gender, occupation, socioeconomic and education level, BMI, hip to waist circumference, stress, dietary pattern, lifestyle, physical activity, family history of diabetes, and type of diabetes were recorded. The association of saliva to predict blood glucose levels was analysed using Spearman Rank Correlation and how these patient-related factors influence the correlation was estimated for future machine learning models. The difference in medians for various groups was calculated using the Mann-Whitney U Test or Kruskal Wallis Test.
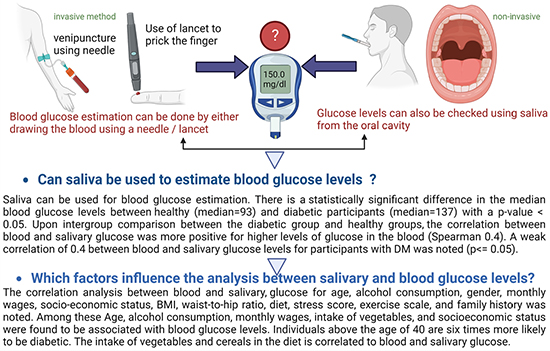
Results: Blood glucose level is not significantly correlated to salivary glucose level. However, a statistically significant difference in the median blood glucose levels for diabetic participants (median = 137) compared to healthy controls (p-value < .05) was noted. The correlation between blood and salivary glucose was more positive for higher levels of glucose (Spearman 0.4). Age, alcohol consumption, monthly wages, intake of vegetables, and socioeconomic status affect blood glucose levels.
Conclusion: A correlation between saliva and blood glucose levels in healthy individuals was weak. Saliva should only be used as a monitoring tool rather than a diagnostic tool and is more reliable for patients with poorly controlled diabetes mellitus.
GRAPHICAL ABSTRACT
KEYWORDS: Diabetes mellitus; type 2 diabetes mellitus; non-insulin-dependent diabetes mellitus; saliva; blood; blood glucose
Citation: ACTA ODONTOLOGICA SCANDINAVICA 2024; VOL. 83: 101–111. DOI: https://doi.org/10.1080/00016357.2023.2267678.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Odontologica Scandinavica Society. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 6 July 2023; Revised: 18 September 2023; Accepted: 30 September 2023; Published: 26 March 2024.
CONTACT Aditi Chopra aditi.chopra@manipal.edu Department of Periodontology, Manipal College of Dental Sciences, Manipal, Manipal Academy of Higher Education, Manipal, India
Competing interests and funding: No potential conflict of interest was reported by the author(s).
‘No funding was obtained for this study’.
#Both authors contributed equally and share the first authorship
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00016357.2023.2267678
Introduction
Diabetes mellitus (DM) is a group of metabolic diseases characterized by hyperglycaemia (increased blood glucose levels) either due to defects in insulin secretion, insulin action, insulin resistance, or both [1]. There are various forms of DM such as Type 1, Type 2, and gestational DM [2]. Type 2 DM is the most common form of DM. It occurs when cells do not respond to insulin (insulin resistance), and in turn, it reduces or inhibits the ingress of glucose into the cell. The glucose that is not utilized by the cells enters the blood circulation (free glucose) and results in hyperglycaemia. An individual with a fasting blood glucose level of more than 110 mg/dl or a glycated haemoglobin test (HbA1c > 6.5) is confirmed to have DM [3–4].
Around 463 million people between the age of 20–70 years are estimated to be living with DM, which represents 9.3% of the total world population. It is predicted that by 2030, 366 million individuals will suffer from DM, out of which 90% will suffer from Type 2 DM [5]. It is also estimated that 50.1% are undiagnosed cases and most of the population that are undiagnosed with DM (84.3%) belongs to low and middle-income countries [5]. The low diagnostic rate of DM is attributed to the high cost of testing DM, poor access to hematological laboratories, financial constraints, poor compliance and attitude of the patient towards regular health check-ups, especially in underdeveloped rural and sub-urban areas. The fear of needle pricks and withdrawing blood in a vacutainer using needles and syringes also preclude many from regular hematological testing [6].
To overcome the fear of needle pricking, currently hematological analysis for DM can be done either by using a lancet and glucometer [7]. Glucometers and the use of glucometer strips are less invasive and quicker methods of blood glucose estimation. However, the fear of needle prick and withdrawal of blood still exists among individuals using a glucometer, as it creates a sense of anxiety and stress among many individuals [8–11]. Studies have found that many individuals fear ‘self-testing’ at home and around 57% of individuals fear needle pricks even by a lancet [12–16]. Additionally, the armamentarium of using a glucometer is expensive for poor patients and some patients do not want to prick themselves regularly. Thus, glucometers are used mainly by people from the middle-higher socio-economic state, those with higher educational qualifications, and those who know how to use them [11]. A study by Farhan et al. (2017) found that the prevalence of home glucometer usage was only 59%. The cost of using a glucometer, refill lancets and strips are major factors limiting its use. The study also found that high socioeconomic status (p < .001), receiving care from private institutions (p < .001), higher education (p < .001), a family history of diabetes (p = .001), awareness regarding diabetes (p < .001), having diabetes for > five years (p < .001), and managing diabetes via pharmacological interventions (p < .001) (versus diet and exercise) were positive predictors of glucometer usage [14–16]. Additionally, the use of needles and lancets can be dangerous for individual with a bleeding disorders, those on anticoagulants and antiplatelet medications; as the pricked site may take a long time to clot [17]. Thus, the need for developing a viable and less invasive prick-free method for glucose estimation is always felt and researched [12–16].
Saliva has been tested as a potential biological fluid for estimating blood glucose levels [15–26]. Many studies have tested and compared the potential of using saliva compared to blood for glucose estimation and reported both positive and negative results [3,8,17–30]. A systematic review by Mascarenhas et al. (2014) evaluated the effectiveness of salivary glucose in estimating glycaemia and HbA1c and found that there is a large positive effect of Type 2 DM over salivary glucose (Hedge’s g = 1.37). The overall global correlation coefficient (r) between salivary glucose and glycaemia was large (r = 0.49). The strength of the correlation increased for higher glycaemia/HbA1c values [31–33]. Additionally, many studies have shown that the use of saliva for estimating blood glucose levels is influenced by many patient-related and environmental factors. Panchbhai et al. (2012) in their study concluded that ‘several factors such as age, stress, anxiety, diet, medical and drug history, smoking history, and nature of diabetes influence the salivary glucose levels and eventually its correlation to blood glucose level. Unless these factors are noted, salivary glucose cannot be employed to estimate blood glucose levels [19]. Although limited studies have previously discussed the role of these factors and how they affect the salivary and blood glucose levels, it is not clear how these factors would influence overall correlation if one has to utilize saliva for glucose estimation. To our knowledge, previous studies have not evaluated the correlation of these factors and how they influence the correlation between blood and salivary glucose levels. This evidence is vital for understanding how salivary glucose levels will change for a given individual and how one should use a multifactorial approach to estimate blood glucose levels using saliva more accurately. This is important as it would help data scientists, machine learning model developers, and clinicians before designing any app or algorithm that would utilize saliva for analyzing blood glucose levels. With this background, the study aims to estimate the association of various patient-related demographic, socioeconomic, stress, and dietary factors influencing the relationship between salivary and blood glucose levels in systematically healthy patients and patients with DM.
Methodology
The study is designed as a cross-sectional correlational study conducted from December 2020 to July 2021 and was conducted following the STROBE and SAGER guidelines. The study was conducted at the Department of Periodontology, Manipal College of Dental Sciences, Manipal, in collaboration with the Clinical Haematology Laboratory, Kasturba Medical Hospital, Manipal. The study was conducted in accordance with the tenets of the Helsinki Declaration (as revised in 2013) and has been approved by the Institutional Review Committee of Kasturba Medical College and Kasturba Hospital (IEC no:443/2020). This study was also registered at the clinical trial registry, in India with registry no (CTRI/2020/ 12/029832). The informed consent was obtained from all study participants in a written and verbal manner explaining the methodology and rationale of the study.
Sample size calculation
The total sample size was calculated considering Alpha, with Significance as .05, and Beta, with the power of the study at 80% (1-Power = 0.20); the standard deviation between the outcomes: 0.1 using the following formula was found to be 83 in each group.
Inclusion and exclusion criteria
All individuals coming to the hematological laboratory at Kasturba Medical Hospital and the out-patient Department of Periodontology, Manipal were screened for the following inclusion and exclusion criteria:
Inclusion criteria:
- All systemically healthy participants and participants diagnosed with controlled and uncontrolled Type 2 DM in the age range of 20–75 years who have come for blood glucose estimation were included.
Exclusion criteria:
- Participants with any other systemic diseases other than DM.
- Participants taking any medication or drugs other than anti-diabetic medications.
- Participants taking any antibiotics or analgesics in the past month.
- Participants having any active infection that requires emergency treatment at the time of recruitment.
- Pregnant and lactating women to rule out gestational DM.
- Mentally and physically challenged individuals.
- Participants undergoing any radiation and chemotherapy.
After screening all the participants for the presence of DM and for healthy (non-diabetic individuals), oral and written informed consent was taken from all the participants. The following personal and demographic data was then collected on a printed data collection form by two investigators (LS and AC): age (in years), gender, address (district/place), occupation; gross income (in INR); no. of working hours; socioeconomic status (low/middle/high), education level; type of diabetes; family history of diabetes (father/mother/paternal/maternal grandmother/paternal/maternal grandmother); nature of diabetic medication and duration of DM. After recording the demographics, weight (in kg), height (in cm), hip circumference (in cm), and waist circumference (in cm) were recorded with a duly calibrated measuring tape. All participants were questioned about their lifestyle and levels of physical activity by using the International Physical Activity Questionnaires (IPAQ) index as described previously [34]. Based on the IPAQ, domain-specific scores for each type of physical activity were noted, and based on this the type of activities performed by each participant was divided into sedentary/low/moderate/ high activity. The participant’s diet history was recorded to note the type of diet, and the number of times the participant consumed fruits and vegetables (fibers), tanned food, hydrogenated and processed food, and alcohol. A standard diabetic and diet questionnaire as described previously was followed to note if the individual follows a healthy diet [35].
The individuals were also questioned regarding their stress levels by utilizing the Perceived Stress Scale (PSS)-10 [36]. A total score ranging from 0 to 40 was computed by reverse scoring the four positively worded items and then summing all the scale items. Higher scores indicated greater levels of perceived stress. Subscale scores were computed by summing the six negatively worded items (Items 1, 2, 3, 6, 9, and 10) for Factor 1 (‘Negative’) and the four positively worded items (Items 4, 5, 7, and 8) for Factor 2 (‘Positive’), with higher scores indicating greater negative distress/stress feelings and greater positive stress feelings and coping abilities, respectively. Following the collection of demographic data, physical activity score, diet chart, and stress score, all participants were requested to provide blood and salivary samples. The saliva and blood samples were collected by a trained technician as follows.
Collection of saliva for salivary glucose estimation
The unstimulated whole saliva was collected using the ‘spitting method’ [37]. The unstimulated saliva was preferred to stimulated saliva as alterations in the salivary composition have been noted with stimulated saliva. All participants were requested to sit comfortably in an upright position. Participants wearing any denture or removable prosthesis wearers were asked to remove the prosthesis before the saliva collection. The subjects were requested to spit out the saliva accumulated in the mouth into an Eppendorf vial. 2–3 ml of unstimulated saliva were collected and stored at minus 80 degrees Celsius until further analysis. The saliva samples were first centrifuged at 3000 rotations per minute for 20 min to obtain a clear supernatant. The supernatant was used for the estimation of salivary glucose using the glucose oxidase end-point assay as described previously [37].
Estimation of the blood glucose levels
2 ml of blood was collected. The patient was made to comfortably sit on a chair with arms extended straight from the shoulders onto a wooden plank. The antecubital fossa was exposed and a tourniquet was tied at 1.5–2 inches above the fossa. The area was cleaned and made sterile by using cotton wool soaked in methylated spirit. Using a BD vacutainer needle, the antecubital vein was punctured and 2 ml of whole blood was drawn. The hemostasis was achieved at the puncture site by pressing with a cotton plug soaked with spirit following which a bandage was applied. The blood was then centrifuged at 3000 rpm for about 5 min to separate the serum and evaluate the glucose level. One millilitre of glucose reagent was added to 10: l of the test sample and glucose standard. Both were incubated at 37 °C for about 10 min. The absorbance values were measured with a semi-automated analyzer [37–40].
Statistical analysis
After data collection, all the details were compiled into a Microsoft Excel (version. 2019) The programming language used for performing the statistical analysis is Python 3.8.16. The packages used for analysis are Pandas (Version 1.3.5) and Scipy (Version 1.7.3). The packages used for visualizing results are Seaborn (Version 0.11.2) and Matplotlib (Version 3.2.2). Kolmogorov-Smirnov test and Shapiro–Wilk test for various significance levels were performed and the blood and salivary glucose features were not following a normal distribution. Since Pearson correlation assumes normality in data, we analyzed results using the Spearman Rank Correlation and checked its statistical significance. The median is chosen as the measure of centrality, and the minimum and the maximum of all variables were calculated and used to describe the distribution. The Student’s T-test and Unadjusted Odds Ratio were calculated for further understanding of the underlying relationships. The difference in medians for various groups was calculated using the Mann-Whitney U Test or Kruskal Wallis Test. The results are then tabulated and visualized as follows.
Results
A total of 2,000 participants were screened and questioned to check the presence of Type 2 DM. Out of 2000, 166 participants (both healthy and diabetic participants) were recruited, of which 99 were females and 67 were males. The mean age ± SD for the females (n = 99) was 37.89 ± 18.52 and for males (n = 67) was 46.84 ± 17.673. The overall mean and standard deviation of age, among 166 samples were 41.5 ± .18.6; the Median [Min-Max] is 40 [20–78]. Based on the blood glucose level results, the participants were divided into the diabetic group and the healthy control. The results showed that there were 83 healthy controls (group A) (male:31; female:52) and 83 participants with DM (Group B) (male: 36; female:47). The mean age in the healthy control and diabetic individuals were respectively 33 and 49. The age and male-to-female distribution has been depicted in Table 1.
Taking the overall cohort of 166 participants, the mean blood glucose was found to be 127.34 ± 58.2, and the median [Min-Max] was found to be 110.5 [65–581]). The mean and standard deviation for salivary glucose distribution was 3.94 ± 4.59 with a median [Min-Max] 2.5 [0.317–27.759] (Figure 1).
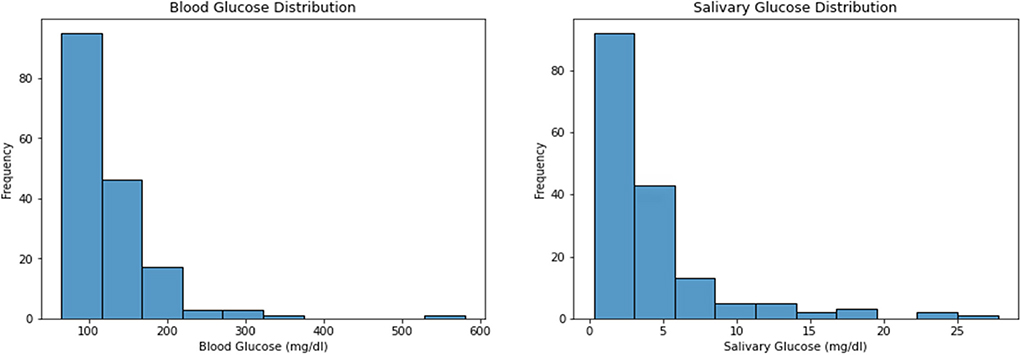
Figure 1. Describes the overall distribution of salivary and blood glucose levels.
As per Spearman rank correlation (0.15), we found no correlation between blood glucose and salivary glucose overall, however, a statistically significant difference was noted between salivary and blood glucose levels for those with high blood glucose levels. Using the Spearman correlation, we noted a weak correlation of 0.4 between the blood glucose and salivary glucose levels for Group B (Diabetic) considering the significance level of p< = .05. Upon intergroup comparison between the blood and salivary glucose levels using the Mann-Whitney U tests between group A and group B, a statistically significant difference in the distribution of
blood glucose levels between group A and group B (p-value< .05) was noted, but the same could not be represented in the salivary glucose levels between group A and group B. (Table 2 and Figure 2)
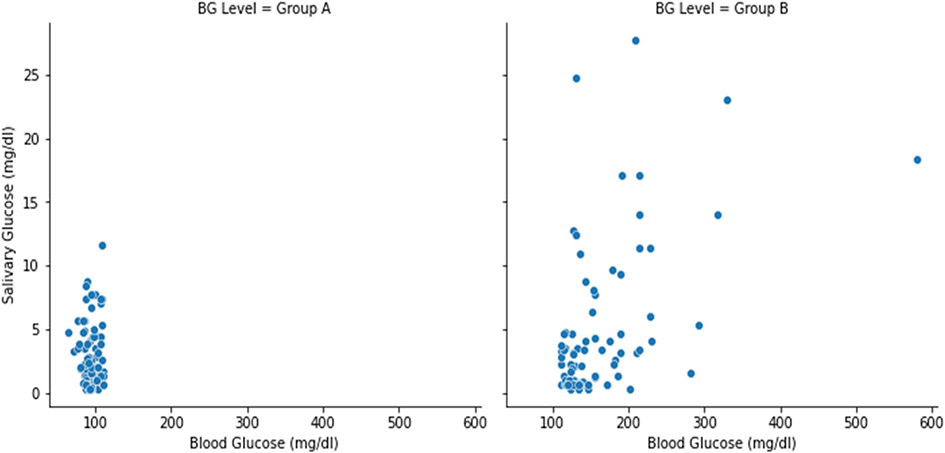
Figure 2. Schematic representation of the relationship between salivary and blood glucose level.
Analysis between blood and salivary glucose with the demographic, socioeconomic, and diet data
The correlation analysis between blood and salivary glucose for age, alcohol consumption, gender, monthly wages, socio-economic status, BMI, waist-to-hip ratio, diet, stress score, exercise scale, and family history was noted. The results based on the Chi-square test showed an association of the following sociodemographic variables with blood glucose levels. Age (p-value = .00), alcohol consumption (p-value= .016), monthly wages (p-value = .009); socioeconomic status (p-value = .019) were positively correlated with the diabetic group with a statistically significant p-value < .05. The unadjusted odds ratio was computed and their statistical significance was tested against the confidence intervals. The statistically significant variables for blood glucose are as follows: The unadjusted odd ratio showed the well-known pattern that persons above the age of 40 are six times more likely to be diabetic. Participants with low monthly wages (<10,000 INR) are four times more likely to be diabetic. Participants from low socioeconomic status are three times more likely to be diabetic (Table 3).
The assessment of the socio-demographic variables with salivary glucose some differences the in the median for some variables, such as diet, exercise, stress, and family history, the results were not statistically significant (Table 4).
The intergroup comparison by the Mann-Whitney U test between the factors that affect the correlation between salivary and blood glucose levels for both groups A and group B showed that the age of the participants is the most significant factor influencing this correlation. For participants who were above 40 years of age, a statistically significant (with large effect) difference in the median between the salivary glucose levels for non-diabetic (median= 1.337) vs. participants diabetic (median= 3.010) was noted (Table 5 and Figure 3). A positive correlation between blood glucose levels and consumption of fruits and vegetables was noted (Supplementary Table 1). Participants who consumed vegetables (fresh, tinned, or frozen) and pulses like lentils, and kidney beans influenced the correlation between salivary and blood glucose levels. Stress in an individual is also associated with salivary and blood glucose levels (Supplementary Table 2)
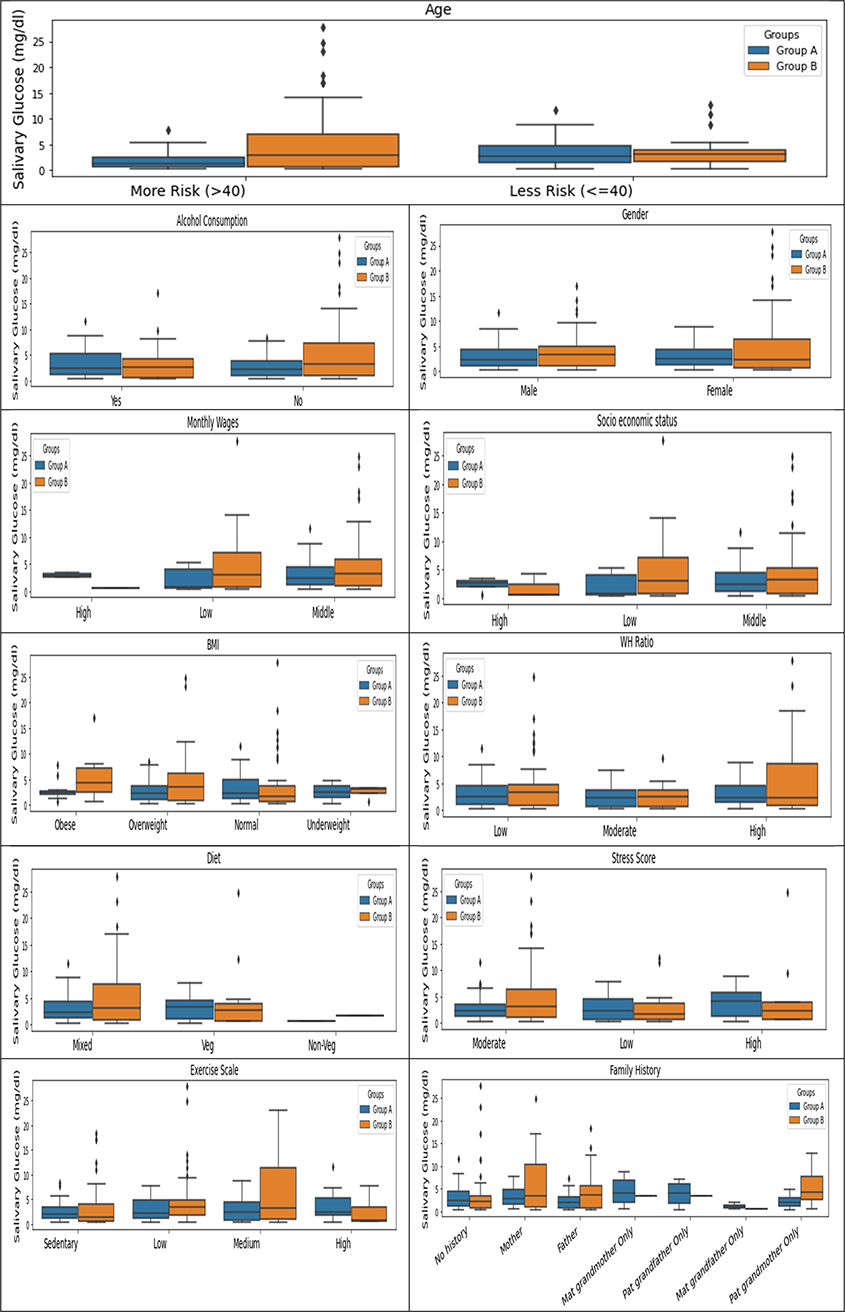
Figure 3. Group-wise analysis of socio-demographic variables with salivary glucose.
Discussion
Regular monitoring of DM is vital for early diagnosis, assessing the patient’s compliance, and evaluating if the anti-diabetic medication is working effectively. Blood is the most commonly used biological fluid to check the glycemic load. Many patients do not go for regular monitoring and blood glucose investigations because of the fear of blood withdrawal and needle pricking. Recently, saliva has gained popularity as a potential biological fluid for estimating blood glucose levels [18]. Saliva contains 3 to 25 mmol/l of glucose, which can be correlated with blood glucose levels. Many studies have correlated salivary and glucose levels in patients and shown that salivary fasting glucose levels in patients with DM have a statistically positive correlation to blood glucose levels in diabetic patients as compared to healthy controls [24–33,41–46]. However, a study by Amer et al. did not find any glucose, even in the slightest concentrations in the saliva of healthy individuals. However, the salivary samples obtained from Type 2 DM showed a significant concentration of glucose [19]. These findings are similar to the results obtained from our study, where a statistically significant difference in the distribution of blood glucose levels between diabetic and healthy patients was noted (p-value< .05), but the same could not be represented in the salivary glucose levels. We also noted a weak correlation of 0.4 between the blood glucose and salivary glucose levels for Group B (Diabetic) considering the significance level of p ≤ .05 [19]. The level of salivary glucose does not vary with gender, and the concentration of salivary glucose was not dependent on blood glucose [36]. Although a positive correlation exists between salivary and blood glucose levels in diabetic patients unlike, healthy controls. The varying results between salivary and blood glucose levels are attributed to the fact that salivary and blood glucose are dependent upon many environmental and patient-related factors. Hence if salivary glucose has to be correlated to blood glucose levels, the influence of these variables should be taken into consideration [33–42].
To our knowledge, few studies have assessed the effect of socioeconomic status, stress, physical activity, and diet on the salivary, salivary flow rate, and blood glucose levels [35–42]. We found that factors like age, alcohol consumption, monthly wages (financial status), and socioeconomic status are associated with blood glucose levels. However, the effect of these on salivary glucose is not statistically significant [7]. Patients above 40 years of age showed a statistically significant difference (with a large effect) in the salivary glucose levels for those who are healthy (median= 1.337) vs. patients with DM (median= 3.010). Diet influenced the correlation between salivary and blood glucose levels. For example, if saliva is used to check the glucose level immediately after a meal, higher values may be obtained. This is also noted by previous studies where the correlation between the glucose concentration in saliva and serum was higher after than before the carbohydrate intake. The results were also noted to be independent of factors such as age, gender, and duration of the disease [17,30,34]. The poor correlation between blood and salivary glucose levels in diabetic patients may also linked to the oral retention of carbohydrates, the flow of saliva, glucose utilization by bacteria, the release of carbohydrates from salivary glycoproteins, and contamination of the saliva by gingival crevicular fluid, especially in patients with poor periodontal status [35–38]. Jurysta et al. (2009) evaluated the salivary glucose concentration in unstimulated and mechanically stimulated salivary samples in normal, healthy, and diabetic patients and observed a higher glucose concentration in the saliva of diabetic patients than in healthy controls. Furthermore, no significant difference between unstimulated and stimulated salivary samples when compared with the serum glucose levels in diabetic patients was noted [39].
Based on the existing evidence and efficacy of saliva for blood glucose estimation, many companies have developed kits to test blood glucose using saliva. However, one should note that the sensitivity of these kits may be influenced by factors such as diet, stress, age, and stress levels in individuals. If future salivary kits are to be developed, one should know how patient-related factors such as age, BMI, socioeconomic status, stress, diet, and time of collection of saliva are matched to provide a more objective estimation of blood glucose using saliva. Age, socioeconomic status, and diet (especially consumption of fresh fruits and vegetables, stress, and monthly wages) influence salivary and blood glucose levels. One should also note that calibrating such devices with variables like monthly wages, stress, and diet would be a very difficult task as these variables are very dynamic and sometimes cannot be measured objectively. Hence some margins of error would always remain when saliva is used as a tool for measuring blood glucose. Additionally, one should note that the potential use of saliva in diagnosis as well as in the regular monitoring of diabetic patients cannot be attempted in certain situations including numerous auto-immune and/or inflammatory conditions such as Sjogren’s syndrome, amyloidosis, sarcoidosis, HIV/AIDS, hepatitis C, malignant conditions such as lymphomas and salivary gland agenesis or aplasia. Additionally, the use of saliva as a diagnostic tool is limited in patients with xerostomia or a possible change in salivary composition to the extent of not being reliable for diagnostics as well as in the regular monitoring of the patients. Patients with salivary gland changes after exposure to radiation in the head and neck area cancer also pose such challenges. Similar challenges are faced even in situations wherein the glucose threshold is either exceeded as in hyperglycaemic crises like diabetic ketoacidosis due to xerostomia or in cases of severe hypoglycaemia because blood glucose levels have to cross a minimum threshold to appear in saliva [40–46].
However, for healthy and diabetic individuals with good saliva flow rates, saliva can be used as a potential diagnostic tool for blood glucose estimation. Salivary glucose can be correlated to blood glucose levels and this correlation between saliva and blood glucose levels was found to be significant, but based on many factors. Any model or machine analyzing glucose levels using saliva should be calibrated based on these factors. Since the prevalence of DM is rising at a massive rate, the use of a non-invasive method of estimating blood glucose levels is crucial.
Conclusion
Saliva is a potential biological fluid that can be used for glucose estimation. However, a weak correlation between saliva and blood glucose levels in healthy individuals was seen. However, salivary glucose was correlated to serum glucose for individuals with DM. Hence, saliva should only be used as a monitoring tool rather than a diagnostic tool, and that too only in highly diabetic patients.
Acknowledgments
We would like to thank the Department of Haematology and Clinical Pathology laboratory, Kasturba Medical Hospital, Manipal for providing the necessary infrastructure for sample collection.
Ethics approval and consent to participate
The study was conducted in accordance with the tenets of the Helsinki Declaration (as revised in 2013) and has been approved by the Institutional Review Committee of Kasturba Medical College and Kasturba Hospital (IEC no:443/2020). This study was also registered at the clinical trial registry, India with registry no (CTRI/2020/12/029832). The informed consent obtained from study participants was taken in a written and verbal manner explaining the methodology and rationale of the study.
Geolocation information
India
Data availability statement
Data related to the study is available upon request from via email to the corresponding author.
References
[1] Zaccardi F, Webb DR, Yates T, et al. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J. 2016;92(1084):63–69. doi: 10.1136/postgradmedj-2015-133281.
[2] Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183.
[3] Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr. 2019;13(2):1165–1172. doi: 10.1016/j.dsx.2019.01.040.
[4] American Diabetes Association. Introduction: standards of medical care in diabetes-2022. Diabetes Care. 2022; 145(Suppl 1):S1–S2. doi: 10.2337/dc22-Sint.
[5] Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843.
[6] Czupryniak L, Barkai L, Bolgarska S, et al. Self-monitoring of blood glucose in diabetes: from evidence to clinical reality in Central and Eastern Europe–recommendations from the international Central-Eastern European expert group. Diabetes Technol Ther. 2014;16(7):460–475. doi: 10.1089/dia.2013.0302.
[7] Ali SN, Dang-Tan T, Valentine WJ, et al. Evaluation of the clinical and economic burden of poor glycemic control associated with therapeutic inertia in patients with type 2 diabetes in the United States. Adv Ther. 2020;37(2):869–882. doi: 10.1007/s12325-019-01199-8.
[8] Benjamin EM. Self-monitoring of blood glucose: the basics. Clin. Diabetes. 2002;20(1):45–47.
[9] Ginsberg BH. Factors affecting blood glucose monitoring: sources of errors in measurement. J Diabetes Sci Technol. 2009;3(4):903–913. doi: 10.1177/193229680900300438.
[10] Acar N, Ozcelik H, Cevik AA, et al. Low perfusion index affects the difference in glucose level between capillary and venous blood. Ther Clin Risk Manag. 2014;10:985–991. doi: 10.2147/TCRM.S73359.
[11] Wei H, Lan F, He Q, et al. A comparison study between point-of-care testing systems and Central laboratory for determining blood glucose in venous blood. J Clin Lab Anal. 2017;31(3):e22051. doi: 10.1002/jcla.22051.
[12] Al Hayek AA, Robert AA, Babli S, et al. Fear of self-injecting and self-testing and the related risk factors in adolescents with type 1 diabetes: a cross-sectional study. Diabetes Ther. 2017;8(1):75–83. doi: 10.1007/s13300-016-0221-8.
[13] Rosa LS, Mistro S, Oliveira MG, et al. Cost-effectiveness of point-of-care a1c tests in a primary care setting. Front Pharmacol. 2020;11:588309. doi: 10.3389/fphar.2020.588309.
[14] Bain SC, Bekker Hansen B, Hunt B, et al. Evaluating the burden of poor glycemic control associated with therapeutic inertia in patients with type 2 diabetes in the UK. J Med Econ. 2020;23(1):98–105. doi: 10.1080/13696998.2019.1645018.
[15] Snoek FJ, Mollema ED, Heine RJ, et al. Development and validation of the diabetes fear of injecting and self-testing questionnaire (D-FISQ): first findings. Diabet Med. 1997;14(10):871–876. doi: 10.1002/(SICI)1096-9136(199710)14:10<871::AID-DIA457>3.0.CO;2-Y.
[16] Farhan SA, Shaikh AT, Zia M, et al. Prevalence and predictors of home use of glucometers in diabetic patients. Cureus. 2017;9(6):e1330. doi: 10.7759/cureus.1330.
[17] Tonyushkina K, Nichols JH. Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol. 2009;3(4):971–980. doi: 10.1177/193229680900300446.
[18] Brown DL. Congenital bleeding disorders. Curr Probl Pediatr Adolesc Health Care. 2005;35(2):38–62. doi: 10.1016/j.cppeds.2004.12.001.
[19] Panchbhai AS. Correlation of salivary glucose level with blood glucose level in diabetes mellitus. J Oral Maxillofac Res. 2012;3(3):e3. doi: 10.5037/jomr.2012.3303.
[20] Amer S, Yousuf M, Siddqiui PQ, et al. Salivary glucose concentrations in patients with diabetes mellitus–a minimally invasive technique for monitoring blood glucose levels. Pak J Pharm Sci. 2001;14(1):33–37.
[21] Puttaswamy KA, Puttabudhi JH, Raju S. Correlation between salivary glucose and blood glucose and the implications of salivary factors on the oral health status in type 2 diabetes mellitus patients. J Int Soc Prev Community Dent. 2017;7(1):28–33. doi: 10.4103/2231-0762.200703.
[22] Mascarenhas P, Fatela B, Barahona I. Effect of diabetes mellitus type 2 on salivary glucose–a systematic review and meta-analysis of observational studies. PLOS One. 2014;9(7):e101706. doi: 10.1371/journal.pone.0101706.
[23] Kaufman E, Lamster IB. The diagnostic applications of saliva–a review. Crit Rev Oral Biol Med. 2002;13(2):197–212. doi: 10.1177/154411130201300209.
[24] Yoshizawa JM, Schafer CA, Schafer JJ, et al. Salivary biomarkers: toward future clinical and diagnostic utilities. Clin Microbiol Rev. 2013;26(4):781–791. doi: 10.1128/CMR.00021-13.
[25] Nagalaxmi V, Priyanka V. Can saliva be a marker for predicting type 1 diabetes mellitus? - a pilot study. JIAOMR. 2011;23:579–582. doi: 10.5005/jp-journals-10011-1226.
[26] Lee YH, Wong DT. Saliva: an emerging biofluid for early detection of diseases. Am J Dent. 2009;22(4):241–248.
[27] Mrag M, Kassab A, Omezzine A, et al. Saliva diagnostic utility in patients with type 2 diabetes: future standard method. J Med Biochem. 2020;39(2):140–148. doi: 10.2478/jomb-2019-0019.
[28] Lima-Aragão MV, de Oliveira-Junior Jde J, Maciel MC, et al. Salivary profile in diabetic patients: biochemical and immunological evaluation. BMC Res Notes. 2016;9(1):103. doi: 10.1186/s13104-016-1881-1.
[29] Gupta S, Nayak MT, Sunitha JD, et al. Correlation of salivary glucose level with blood glucose level in diabetes mellitus. J Oral Maxillofac Pathol. 2017;21(3):334–339. doi: 10.4103/jomfp.JOMFP_222_15.
[30] Satish BN, Srikala P, Maharudrappa B, et al. Saliva: a tool in assessing glucose levels in diabetes mellitus. J Int Oral Health. 2014;6(2):114–117.
[31] Golamari UMR, Natarajan MSS, Lakshmanan A, et al. Correlation between salivary glucose and blood glucose levels in diabetic and non-diabetic individuals. Int J Adv Med. 2019;6(4):1220–1225. doi: 10.18203/2349-3933.ijam20193274.
[32] Caixeta DC, Pennisi PRC, Moura DV, et al. Association of salivary alpha-2-macroglobulin with glycemia and glycated hemoglobin in type 2 diabetes mellitus: a systematic review and meta-analysis study. Sao Paulo Med J. 2022;140(6):818–828. doi: 10.1590/1516-3180.2021.0816.R2.19052022.
[33] Borg Andersson A, Birkhed D, Berntorp K, et al. Glucose concentration in parotid saliva after glucose/food intake in individuals with glucose intolerance and diabetes mellitus. Eur J Oral Sci. 1998; 106(5):931–937. doi: 10.1046/j.0909-8836.1998.eos106505.x.
[34] Vasconcelos AC, Soares MS, Almeida PC, et al. Comparative study of the concentration of salivary and blood glucose in type 2 diabetic patients. J Oral Sci. 2010;52(2):293–298. doi: 10.2334/josnusd.52.293.
[35] Van Holle V, De Bourdeaudhuij I, Deforche B, et al. Assessment of physical activity in older belgian adults: validity and reliability of an adapted interview version of the long international physical activity questionnaire (IPAQ-L). BMC Public Health. 2015;15(1):433. doi: 10.1186/s12889-015-1785-3.
[36] England CY, Thompson JL, Jago R, et al. Development of a brief, reliable and valid diet assessment tool for impaired glucose tolerance and diabetes: the UK diabetes and diet questionnaire. Public Health Nutr. 2017;20(2):191–199. doi: 10.1017/S1368980016002275.
[37] Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. doi: 10.2307/2136404.
[38] Henson BS, Wong DT. Collection, storage, and processing of saliva samples for downstream molecular applications. Methods Mol Biol. 2010;666:21–30. doi: 10.1007/978-1-60761-820-1_2.
[39] Gomar-Vercher S, Simón-Soro A, Montiel-Company JM, et al. Stimulated and unstimulated saliva samples have significantly different bacterial profiles. PLOS One. 2018;13(6):e0198021. doi: 10.1371/journal.pone.0198021.
[40] Jurysta C, Bulur N, Oguzhan B, et al. Salivary glucose concentration and excretion in normal and diabetic subjects. J Biomed Biotechnol. 2009;2009:430426–430426. doi: 10.1155/2009/430426.
[41] WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy. Geneva: World Health Organization; 2010. 5 (Accessed on 10th February, 2023). https://www.ncbi.nlm.nih.gov/books/NBK138661/?report=reader.
[42] Abikshyeet P, Ramesh V, Oza N. Glucose estimation in the salivary secretion of diabetes mellitus patients. Diabetes Metab Syndr Obes. 2012;5:149–154. doi: 10.2147/DMSO.S32112.
[43] Kumar A, Kumar T, Bhargava M, et al. Salivary and serum glucose levels in diabetes mellitus patients versus control – a randomised control trial. J Med Life. 2020;13(2):235–240. doi: 10.25122/jml-2020-0062.
[44] Ragunathan H, Aswath N, Sarumathi T. Salivary glucose estimation: a noninvasive method. Indian J Dent Sci. 2019;11(1):25–27. doi: 10.4103/IJDS.IJDS_78_18.
[45] K M P, Johnson P, Ganesh M, et al. Evaluation of salivary profile among adult type 2 diabetes mellitus patients in South India. J Clin Diagn Res. 2013;7(8):1592–1595. doi: 10.7860/JCDR/2013/5749.3232.
[46] Tiongco RE, Bituin A, Arceo E, et al. Salivary glucose as a non-invasive biomarker of type 2 diabetes mellitus. J Clin Exp Dent. 2018;10(9):e902–e907. doi: 10.4317/jced.55009.