REVIEW ARTICLE
Periodontitis and the risk of oral cancer: a meta-analysis of case-control studies
Yan Ma, Nijiati Tuerxun and Gulibaha Maimaitili
Department of Stomatology Xinjiang Medical University, Affiliated Hospital 2, Urumqi, Xinjiang 830063, China
ABSTRACT
Objective: The current studies have yielded inconclusive findings regarding the connection between periodontitis and oral cancer (OC). Therefore, our goal is to elucidate this relationship.
Materials and methods: We conducted a thorough search of electronic databases (EMBASE, PubMed, Web of Science, and Cochrane Library) up to September 2023. The Newcastle-Ottawa Scale (NOS) was applied to assess study quality. To evaluate potential publication bias, both a funnel plot and Egger’s test were employed. Additionally, a sensitivity analysis was conducted to explore the source of heterogeneity when the I2 statistic exceeded 50%.
Results: This systematic review encompassed 16 studies, involving a total of 6,032 OC patients and 7,432 healthy controls. Our meta-analysis, incorporating data from nine studies, revealed a significant correlation between periodontitis and the risk of OC (OR [odds ratio] = 2.94, 95% CI [confidence interval] (2.13, 4.07); five studies, 6,927 participants; low certainty of evidence). Findings also suggested that individuals with more than 15 missing teeth may have a heightened risk of OC (OR = 1.91, 95% CI (1.01, 3.62)). Furthermore, clinical attachment loss (CAL) and decayed, missing, and filled teeth (DMFT) in OC patients were more pronounced compared to the control group (CAL, SMD = 1.94, 95% CI (0.22, 3.66); DMFT, SMD = 0.65, 95% CI (0.12, 1.18)).
Conclusion: Periodontitis may serve as a potential risk factor for OC. However, caution is warranted in interpreting these findings due to the substantial level of heterogeneity.
KEYWORDS: Periodontal diseases; periodontitis; oral cancer; missing teeth; clinical attachment loss; meta-analysis
Citation: ACTA ODONTOLOGICA SCANDINAVICA 2024; VOL. 83: 281–289. DOI: https://doi.org/10.2340/aos.v83.40478.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Odontologica Scandinavica Society. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 24 November 2023; Accepted: 29 February 2024; Published: 14 May 2024.
CONTACT: Gulibaha Maimaitili gulibaha_1972@163.com No. 38, North 2nd Lane, Nanhu East Road, Shuimogou District, Urumqi, Xinjiang, China
Competing interests and funding: The authors have no relevant financial or non-financial interests to disclose.
This work was supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region (2022D01C265).
Supplemental data for this article can be accessed online at https://doi.org/10.2340/aos.v83.40478
Introduction
Periodontitis is a progressive disease that develops over time due to the accumulation of biofilm at the gumline, coupled with the host’s compromised ability to effectively eliminate potentially harmful microflora [1]. Consequently, this process can lead to the sub-gingival colonisation of microbes, persistent inflammation, and damage to adjacent tissues, resulting in the formation of periodontal pockets attributed to the loss of connective tissue attachment and concurrent bone loss [2, 3]. Furthermore, substantial evidence links periodontitis to various systemic conditions, such as diabetes mellitus [4], cardiovascular diseases [5], poor pregnancy outcomes [6], and pulmonary, renal, and immunologic disorders [3]. Periodontal pathogens, such as Fusobacterium nucleatum and Porphyromonas gingivalis, are frequently identified in malignant growths within the oral cavity [7, 8], as well as other types of cancer [9]. These connections have been a subject of investigation for many years, with a recent surge in publications highlighting the connection between periodontitis and cancer risk.
Cancer remains a significant contributor to global mortality, with oral cancer (OC) ranking as the sixth most prevalent form worldwide [10]. The estimated global annual incidence of OC is approximately 500,000 cases, with over 60% of these reported in Asia and Pacific regions, including nations like India, Pakistan, Sri Lanka, Taiwan, and Papua New Guinea [11]. Remarkably, OC constitutes approximately 25% of cancer treatment cases in these high-risk countries [10]. Recognised risk factors for OC include well-established factors such as tobacco and alcohol use [12], as well as systemic conditions like diabetes mellitus [13], metabolic syndrome [14], and chronic inflammation and infection [15]. Moreover, numerous narrative reviews have discussed the links between tooth loss, periodontal disease (PD), and cancer [16–18]. Research has indicated that individuals with PD face an increased risk of developing various cancer types, including pancreatic [19], skin [20], colon [21], and breast [22], compared to those without PD. Dental biofilms and the accompanying dysbiosis are implicated in several oral conditions, including OC [23, 24]. Two meta-analyses evaluating the relationship between PD and OC, with limited numbers of the included studies (ranging from 5 to 11 studies) [25, 26] demonstrated this positive association. A recently published meta-analysis has additionally shown that tooth loss may nearly double the risk of OC [27].
Therefore, with the emergence of recent publications, the objective of this study is to conduct a comprehensive meta-analysis of all pertinent published literature, seeking to explore the potential correlation between PD (specifically, periodontitis) and OC.
Materials and methods
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, involving a systematic process that encompassed a thorough literature search, meticulous organisation of review documents, rigorous assessment of the quality of each empirical study, comprehensive data synthesis, and meticulous report writing. Additionally, our meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number CRD42023407582.
Literature search
We systematically conducted a literature search across multiple databases, including PubMed (National Library of Medicine, Washington, DC), Embase, Cochrane, and Web of Science, to gather studies specifically investigating the association between periodontitis and OC. The search spanned from the inception of the databases up to September 2023, utilising a strategy that combined Medical Subject Headings (MeSH) terms with their corresponding free-text terms. The MeSH terms employed in the search included ‘oral cancer’, ‘periodontal disease’, ‘tooth loss’, ‘squamous cell carcinoma’, ‘missing teeth’, ‘alveolar bone loss’, ‘clinical attachment loss’, and ‘periodontitis’. The complete search strategy is detailed in Table S1.
Inclusion criteria and exclusion criteria
The selection criteria for this study were established in accordance with the PECO framework, which delineates the key components as follows: P (any population), E (exposed to a history of periodontitis or OC), C (comparator, not exposed to periodontitis or OC), and O (outcome indicators related to OC). In essence, our investigation focused on observational research, comprising both case-control and cohort studies, exploring the association between various indicators of periodontal health and OC. Eligible studies had to meet the following conditions: (1) Clearly defined control and case groups, with patients in the case group having a well-established diagnosis of periodontitis or OC, using specified tools such as the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) [28] for periodontitis and the ICD for Oncology [29] for OC; and (2) inclusion of hazard ratios (HRs), odds ratios (ORs), or risk ratios (both unadjusted and adjusted) accompanied by 95% confidence intervals (CIs), or the provision of sufficient data within the article to facilitate the computation of these ratios.
The exclusion criteria were defined as follows: (1) Studies lacking explicit diagnostic and effectiveness criteria; (2) Studies categorised as meta-analyses, review articles, case reports, conference abstracts, guidelines, letters/response to the editors, or opinion articles; and (3) Studies containing incomplete or inaccurate data that precluded meaningful integration.
Study selection and data extraction
Following the criteria for inclusion and exclusion outlined previously, the study selection process was independently conducted by two researchers, Yan Ma and Nijiati Tuerxun. Initially, all potentially relevant studies were imported into EndNote X9 to identify and eliminate duplicate entries. Subsequently, a screening process ensued, involving the review of titles and abstracts to exclude studies that did not meet the predefined eligibility criteria. Finally, full-text articles underwent additional screening. In cases of disagreements, resolution was attained through discussion or consultation with a third researcher, Gulibaha Maimaitili.
We utilised the Cochrane data extraction form to retrieve the following data and details: (1) Fundamental information including the title, primary author’s name, and publication year; (2) Essential characteristics of the study subjects, encompassing age, gender, case numbers in each group, and diagnostic criteria; and (3) Outcome measures, which encompassed the relationship between tooth loss, squamous cell carcinoma, alveolar bone loss, or clinical attachment loss (CAL), and OC.
Quality assessment of the included studies
To evaluate potential bias and the overall quality of the included studies, we employed the Newcastle–Ottawa Scale (NOS), a widely utilised tool for assessing the methodological quality or risk of bias in case-control studies, in accordance with Cochrane recommendations [30, 31]. The evaluation of publication bias in the included studies was independently conducted by two researchers, Yan Ma and Nijiati Tuerxun, with any discrepancies resolved through consultation or discussion with a third researcher, Gulibaha Maimaitili.
Each included study underwent an evaluation based on specific criteria. For case-control studies, these criteria included selection (scored from 0 to 4), comparability (scored from 0 to 2), and exposure (scored from 0 to 3). For cohort studies, the criteria consisted of selection (scored from 0 to 4), comparability (scored from 0 to 2), and outcome (scored from 0 to 3). The results were then interpreted according to commonly accepted standards and categorised into the following groups: very high risk of bias (0–3 NOS points), high risk of bias (4–6 NOS points), and low risk of bias (7–9 NOS points) [32].
Data analysis
We calculated the combined OR for OC in individuals with PD compared to those without PD, accompanied by the associated 95% CI, using Stata version 16.0. Simultaneously, we assessed the standardised mean difference, along with a 95% CI, to examine various forms of PD between OC patients and healthy controls. Heterogeneity was evaluated by quantifying the proportion of variation attributed to confounding variables, employing I2 statistics. An I2 value exceeding 50% indicated a significant degree of heterogeneity among the included studies. In such instances, a random-effects model was employed, and a sensitivity analysis was conducted to identify the source of this heterogeneity. Conversely, when I2 was below 50%, a fixed-effects model was applied. Additionally, suspicion of publication bias was raised if the p-value was below 0.05.
Results
Literature search results
Following the search strategy, a total of 6,843 records were initially identified from the four databases, with an additional four records recognised through relative reviews. After removing 1,409 duplicates, the remaining 5,434 records were screened, leading to the removal of 1,038 records categorised as meta-analyses, reviews, guidelines, or conference abstracts. Subsequently, a review of the titles and abstracts of the remaining records led to the removal of 4,142 records based on the predefined criteria for inclusion and exclusion mentioned earlier. Full-text articles were evaluated, and 58 records were excluded due to unavailability of full texts and relevant outcomes. Ultimately, the present study incorporated a total of 16 studies. The detailed selection process is displayed in Figure 1.
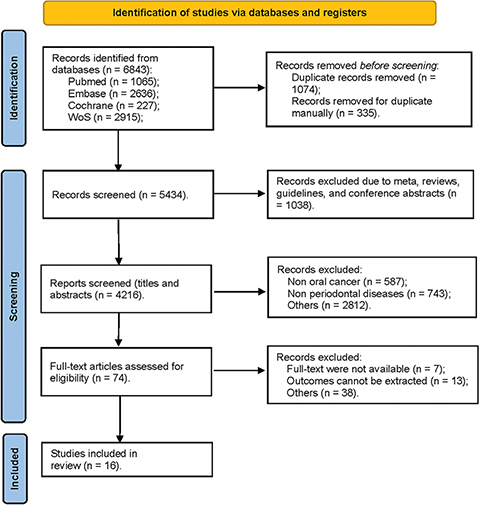
Figure 1. Flow diagram for searching and selecting qualifying studies incorporated in the meta-analysis.
Description of the included studies
Table 1 presents a comprehensive overview of the eligible studies, delineating key characteristics such as the country of origin, study design, sample size, case and control group sizes, types of cases, and outcome indicators. Specifically, the analysis encompasses 12,584 case-control studies on OC, including OC [33–37], oral squamous cell carcinoma (OSCC) [38–43], oral cavity and/or oropharyngeal cancer (OOSCC) [44–46], and pharyngeal cancer [47], and 19,273 healthy controls. Among the included studies, six were conducted in Asia region [35, 36, 39, 40, 47, 48], five in European regions [38, 41–43, 46], two in the United States of America (USA) [37, 45], and three in other countries [33, 44, 49]. Smoking as a known risk factor for both OC and PD has been adjusted in the included studies when evaluating the association between periodontitis and OC. Besides, other adjusted factors such as age, gender, and alcohol consumption have also been adjusted and are presented in Table 2.
The quality assessment of the included studies
The evaluation of bias risk revealed that, among the papers included in the analysis, the mean quality score based on NOS was 8.2 for the 16 case-control studies, with scores ranging from 5 to 9. Fourteen of these studies were classified as having a low risk of bias, while two were deemed to have a high risk of bias. Detailed information is available in Table 3, which outlines the Methodological Quality of Prospective Case-Control Studies according to the NOS criteria.
Meta-analysis
The relationship between PD and OC
We incorporated a total of 16 studies into our qualitative analysis, all examining the relationship between PD and OC. However, for the meta-analysis, we pooled the OR from eight of these articles, specifically those that employed valid instruments to measure periodontitis [33, 34, 37, 39, 41, 45–47]. The pooled OR, generated via the random-effects model, is illustrated in Figure 2. The overall findings indicate a threefold rise in the likelihood of OC in populations afflicted with PD (OR = 2.94, 95% CI (2.13, 4.07), p < 0.001). Nevertheless, we detected variability among the studies included (I2 = 52.4%). No evidence of publication bias was observed (Egger’s test, p = 0.703), and the funnel plot displayed a symmetric distribution (Supplementary Figure S1).
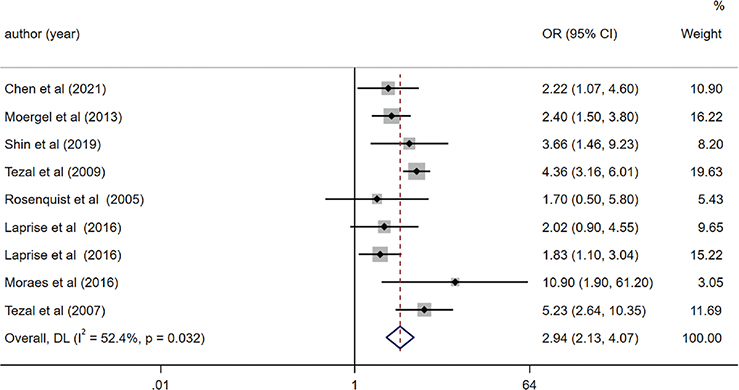
Figure 2. Forest plot of periodontal disease and risk of oral cancer (OC).
We carried out sensitivity analyses by systematically excluding one study at a time.
These analyses consistently verified the reliability of the findings (OR = 2.63, 95% CI (1.92, 3.62), p < 0.001) even after excluding the study [34]. Heterogeneity across the remaining studies was negligible (I2 = 30.7%).
The relationship between missing teeth and OC
Among the studies included in the meta-analysis, six specifically investigated the association between tooth loss and OC [35, 36, 41, 42, 45, 46]. We observed a significant link between having more than 15 missing teeth and an elevated risk of OC (OR = 1.91, 95% CI (1.01, 3.62), p = 0.047). However, no significant correlation was found between the number of missing teeth per tooth and the risk of OC (OR = 0.96, 95% CI (0.92, 1.01), p = 0.131). Notably, we identified significant heterogeneity among the studies investigating the impact of having more than 15 missing teeth (I2 = 83.3%). Notably, no indication of publication bias was observed in the included studies (Egger’s test, p = 0.054) (Figure 3).
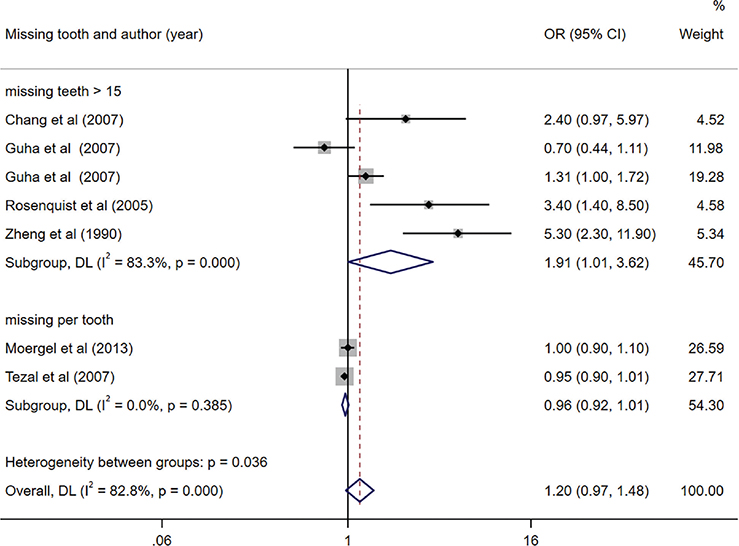
Figure 3. Forest plot of missing teeth and risk of oral cancer (OC).
Assessing the difference of PD between OC and control groups
Among the included studies, only five studies evaluated the difference in PD difference between OC and control groups [33, 34, 37, 38, 45]. Depending on the various kinds of periodontal measurement parameters (i.e., PPD [periodontal pocket depth], ABL [alveolar bone lost], and missing teeth) reported in the included studies, subgroup analysis was conducted explicitly. The combined findings revealed a significant increase in CAL and DMFT (decayed, missing and filled teeth) index within OC group compared to the control group (CAL, SMD = 1.94, 95% CI (0.22, 3.66), p = 0.027; DMFT, SMD = 0.65, 95% CI (0.12, 1.18), p = 0.017). However, the remaining indicators of PD including PPD, ABL, and mean missing teeth, did not significantly differ between the two groups (Figure 4). Additionally, no evidence of publication bias was observed (egger, p = 0.837).
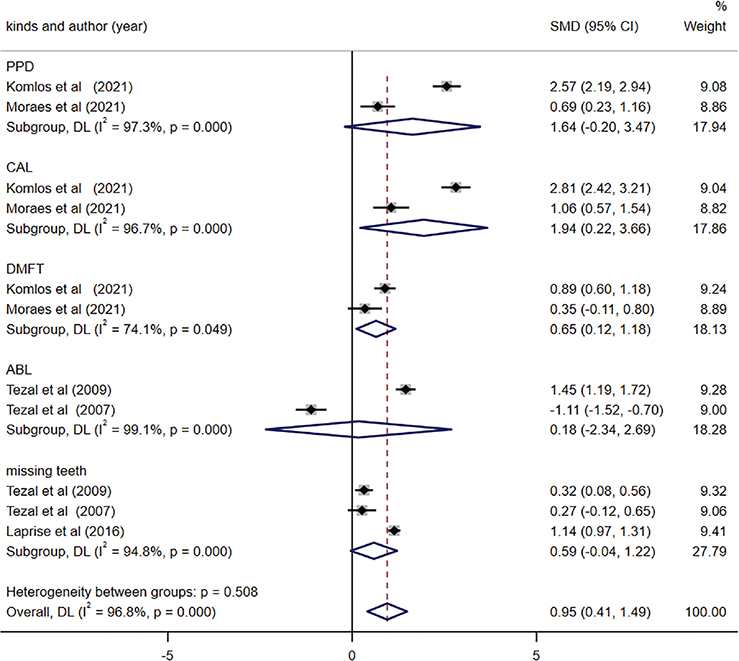
Figure 4. Forest plot of periodontal disease between oral cancer (OC) and control groups.
Discussion
The current study was to assess the association between periodontitis and OC and the findings demonstrated that the risk of OC in patients with periodontitis increased 2.94 times compared to those without PD. Moreover, having more than 15 missing teeth emerged as a potential risk factor for OC, and there were significant differences that were observed in CAL and the DMFT index between OC patients and healthy controls.
Our findings have established a substantial association between the occurrence of OC and both periodontitis and tooth loss, aligning with the conclusions of the systematic review [50]. Since 2010, Seymour pointed out that inadequate oral health, particularly the degree and severity of PD, with numerous systemic diseases [16]. In 2012, Meisel and colleagues identified a potential connection between PD and premalignant oral lesions [51]. Recent evidence suggests that the degree and severity of PD and the presence of missing teeth may be linked to an increased risk of developing malignant diseases [52, 53]. Periodontitis is characterised as an inflammatory disease influencing the supporting structures of teeth, stands as the leading cause of tooth loss in adults. This chronic inflammatory condition holds the potential to elevate cancer risk by impeding apoptosis and promoting the growth of tumour cells [54]. The chronic inflammation induced by periodontal infections can also disrupt regular cell function and potentially contribute to carcinogenesis [55]. Consequently, periodontitis is considered as an indicator of a specific type of immune response that could impact the growth and advancement of cancer. Recent systematic reviews and meta-analyses exploring the relationship between PD and OC [25, 26, 50] consistently reveal a 2- to 5-fold elevated risk of developing OC among individuals with PD.
Previously, according to the inflammation of the disease, periodontitis was divided into mild, medium and severe forms. Severe periodontitis was often characterized by significant loss of periodontal support tissue, leading to loose or shifted teeth, and in severe cases, tooth dislodgment. In light of the epidemiology, aetiology, and pathogenesis of PD, as well as recent clinical evidence and related knowledge, the American Academy of Periodontology (AAP) and the European Federation of Periodontology (EFP) jointly organised the International Symposium on New Classifications of Periodontal and Periphytal Diseases in November 2017 [1]. As a result, a new classification system was formulated, and a consensus report was officially published in June 2018. Notably, the revised classification system eliminates the previous differentiation between chronic periodontitis and invasive periodontitis. Three distinct forms of periodontitis are now recognized: necrotizing periodontitis, periodontitis as a manifestation of systemic disease, and the forms of the disease previously acknowledged. A new periodontitis classification scheme is further characterised based on a multi-dimensional staging and grading system. Neither the previous classification nor the 2018 new classification changed the clinical definition of periodontitis: an inflammation that is microbial-related, host-mediated, and results in loss of periodontal adhesion. Most articles we retrieved did not use the 2018 new classification of periodontitis, but this did not affect the diagnosis of periodontitis. The aim is to classify the strongest scientific evidence, but in the absence of data, the use of lower-level evidence is inevitable.
We conducted a thorough search for publicly available studies pertaining to tooth loss and the risk of OC. Our investigation demonstrated that tooth loss indeed serves as a risk factor for OC, with an OR of 1.91. It is worth noting that tooth loss can be considered a potential indicator for PD, as the majority of adult tooth loss cases are attributed to periodontitis. However, the count of lost teeth can also be indicative of oral well-being and may arise from factors such as dental cavities, accidents, or orthodontic interventions. Consequently, tooth loss can encompass a range of oral health conditions, making it a somewhat imprecise indicator of periodontitis. Additionally, the prevalence of tooth loss can vary significantly among different population groups [52]. To date, several previous studies [35, 36, 41, 42, 46] have reported associations between tooth loss and the risk of OC; however, no meta-analysis has been conducted to investigate this connection. This might be due to the different categories of missing teeth. Some studies assessed the mean numbers of missing teeth (e.g., [41]), while Rosenquist et al. assessed the missing numbers of teeth over 20 and its risk of OC [46]. Meanwhile, another study used the standard of the number of missing teeth over 16 [42]. Additionally, certain studies examined the correlation between OC risk and the quantity of missing teeth per tooth [41, 45]. Future studies should use more standardised criteria to examine the connection between tooth loss and the risk of OC, and rigorously control for the underlying causes of patients’ missing teeth. Furthermore, the DMFT index is the key indicator of caries experience in dental epidemiology [56]. It represents the sum of decayed, missing (due to caries), and filled teeth in the permanent dentition. Dental caries results from demineralisation of teeth due to lactic acid produced by fermentation of carbohydrates by Gram-positive parthenogenic bacteria [57]. Previous research has highlighted the association between cariogenic bacteria and periodontal health, where reductions in the populations of these commensal bacteria are correlated with an increase in periodontal inflammation and destruction [58, 59], potentially contributing to OC. The meta-analysis results revealed significant differences in the DMFT index between OC patients and healthy controls. This finding aligns with previous studies suggesting an association between dental caries and head and neck cancers, particularly among patients with oral cavity and oropharyngeal cancers [60].
In addition, the findings also revealed a significant increase in CAL among OC patients in comparison to their healthy counterparts. Periodontitis is distinguished by the loss of periodontal ligament attachment, which is a consequence of host-mediated inflammation triggered by microbial activity. CAL, representing this loss of attachment, is determined by assessing the dentition using a standardised periodontal probe that measures from the cementoenamel junction to the base of the gingival sulcus [61]. However, both clinical and radiographic methods for CAL assessment and diagnosis have remained unaltered and are susceptible to inaccuracies, particularly in the initial phases of periodontitis [62]. Periodontal probing, utilised for CAL and probing depth assessment, is subject to various sources of variation, although relatively few articles have been published on this topic. Variability can arise from factors such as operator-related aspects (e.g., insertion force, probe placement, and angulation), probe design (which can affect tactile feedback), and the operator’s level of experience. All these elements contribute to the potential for uncertainty in periodontal measurements [63, 64]. As highlighted by Tezal and colleagues in 2005, the limitations of studies exploring PD as a risk factor for OC include the diversity of study designs, variations in study populations, and differences in measurement methods [65].
The present investigation, which explores the relationship between PD and the risk of OC, has several inherent limitations. The assessment of PD has proven to be a highly challenging task in previous research, mainly due to the necessity for multiple periodontal measurements and the evolving clinical definitions over time. The adoption of standardised clinical case definitions for population-based studies has been suggested [66, 67], although these definitions have not been widely employed in prior studies. Moreover, periodontitis is a multifaceted disease, and its diagnosis relies on determining CAL, probing depth, and bone loss. The diversity in measurement parameters has resulted in considerable heterogeneity among the studies included in this analysis. Additionally, the varying stages of periodontitis (e.g., mild, moderate, and severe periodontitis) were not assessed due to the limited data available in the incorporated studies. Future research should consider adopting standardized measurements and definitions to categorise and explore periodontitis along with its potential association with OC.
Author contributions
All authors contributed to the study conception and design. Writing – original draft preparation: Yan Ma; Writing – review and editing: Yan Ma; Conceptualisation: Nijiati Tuerxun; Methodology: Yan Ma; Formal analysis and investigation: Yan Ma] Funding acquisition: Yan Ma; Resources: Gulibaha Maimaitili; Supervision: Gulibaha Maimaitili and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
References
[1] Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodontol. 2018;45(Suppl 20):S162–70.
[2] Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet (London, England). 2005;366:1809–20. https://doi.org/10.1016/S0140-6736(05)67728-8
[3] Arigbede AO, Babatope BO, Bamidele MK. Periodontitis and systemic diseases: a literature review. J Indian Soc Periodontol 2012;16:487–91. https://doi.org/10.4103/0972-124X.106878
[4] Sanz M, Ceriello A, Buysschaert M, et al. Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 2018;45:138–49. https://doi.org/10.1111/jcpe.12808
[5] Liccardo D, Cannavo A, Spagnuolo G, et al. Periodontal disease: a risk factor for diabetes and cardiovascular disease. Int J Mol Sci. 2019;20(6):1414. https://doi.org/10.3390/ijms20061414
[6] Figuero E, Han YW, Furuichi Y. Periodontal diseases and adverse pregnancy outcomes: mechanisms. Periodontol 2000. 2020;83:175–88. https://doi.org/10.1111/prd.12295
[7] Katz J, Onate MD, Pauley KM, et al. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int J Oral Sci. 2011;3:209–15. https://doi.org/10.4248/IJOS11075
[8] Al-Hebshi NN, Nasher AT, Maryoud MY, et al. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci Rep. 2017;7:1834. https://doi.org/10.1038/s41598-017-02079-3
[9] Bracci PM. Oral health and the oral microbiome in pancreatic cancer: an overview of epidemiological studies. Cancer J. 2017;23:310–14. https://doi.org/10.1097/PPO.0000000000000287
[10] Bawaskar HS, Bawaskar PH. Oral diseases: a global public health challenge. Lancet (London, England). 2020;395:185–6. https://doi.org/10.1016/S0140-6736(19)33016-8
[11] Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. https://doi.org/10.3322/caac.21166
[12] Sarode G, Maniyar N, Sarode SC, et al. Epidemiologic aspects of oral cancer. Dis Mon. 2020;66:100988. https://doi.org/10.1016/j.disamonth.2020.100988
[13] Ramos-Garcia P, Roca-Rodriguez MDM, Aguilar-Diosdado M, et al. Diabetes mellitus and oral cancer/oral potentially malignant disorders: a systematic review and meta-analysis. Oral Dis. 2021;27:404–21. https://doi.org/10.1111/odi.13289
[14] Kapila YL. Oral health’s inextricable connection to systemic health: special populations bring to bear multimodal relationships and factors connecting periodontal disease to systemic diseases and conditions. Periodontol 2000. 2021;87:11–16. https://doi.org/10.1111/prd.12398
[15] Katsanos KH, Roda G, Brygo A, et al. Oral cancer and oral precancerous lesions in inflammatory bowel diseases: a systematic review. J Crohns Colitis. 2015;9:1043–52. https://doi.org/10.1093/ecco-jcc/jjv122
[16] Seymour RA. Is oral health a risk for malignant disease? Dent Update. 2010;37:279–80, 82–3. https://doi.org/10.12968/denu.2010.37.5.279
[17] Meyer MS, Joshipura K, Giovannucci E, et al. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. 2008;19:895–907. https://doi.org/10.1007/s10552-008-9163-4
[18] Fitzpatrick SG, Katz J. The association between periodontal disease and cancer: a review of the literature. J Dent. 2010;38:83–95. https://doi.org/10.1016/j.jdent.2009.10.007
[19] Maisonneuve P, Amar S, Lowenfels AB. Periodontal disease, edentulism, and pancreatic cancer: a meta-analysis. Ann Oncol. 2017;28:985–95. https://doi.org/10.1093/annonc/mdx019
[20] Mei T, Noguchi H, Kuraji R, et al. Effects of periodontal pathogen-induced intestinal dysbiosis on transplant immunity in an allogenic skin graft model. Sci Rep. 2023;13:544. https://doi.org/10.1038/s41598-023-27861-4
[21] Momen-Heravi F, Babic A, Tworoger SS, et al. Periodontal disease, tooth loss and colorectal cancer risk: results from the nurses’ health study. Int J Cancer. 2017;140:646–52. https://doi.org/10.1002/ijc.30486
[22] Shi T, Min M, Sun C, et al. Periodontal disease and susceptibility to breast cancer: a meta-analysis of observational studies. J Clin Periodontol. 2018;45:1025–33. https://doi.org/10.1111/jcpe.12982
[23] Michaud DS, Liu Y, Meyer M, et al. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9:550–8. https://doi.org/10.1016/S1470-2045(08)70106-2
[24] Ahn J, Chen CY, Hayes RB. Oral microbiome and oral and gastrointestinal cancer risk. Cancer Causes Control. 2012;23:399–404. https://doi.org/10.1007/s10552-011-9892-7
[25] Yao QW, Zhou DS, Peng HJ, et al. Association of periodontal disease with oral cancer: a meta-analysis. Tumour Biol. 2014;35:7073–7. https://doi.org/10.1007/s13277-014-1951-8
[26] Ye L, Jiang Y, Liu W, et al. Correlation between periodontal disease and oral cancer risk: a meta-analysis. J Cancer Res Ther. 2016;12:C237–40. https://doi.org/10.4103/0973-1482.200746
[27] Gonde N, Rathod S, Kolte A, et al. Association between tooth loss and risk of occurrence of oral cancer – a systematic review and meta-analysis. Dent Res J. 2023;20:4. https://doi.org/10.4103/1735-3327.367903
[28] Slee VN. The international classification of diseases: ninth revision (ICD-9). Ann Intern Med. 1978;88:424–6. https://doi.org/10.7326/0003-4819-88-3-424
[29] Trott PA. International classification of diseases for oncology. J Clin Pathol. 1977 Aug;30(8):782. https://doi.org/10.1136/jcp.30.8.782-c
[30] Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. https://doi.org/10.1007/s10654-010-9491-z
[31] Luchini C, Veronese N, Nottegar A, et al. Assessing the quality of studies in meta-research: review/guidelines on the most important quality assessment tools. Pharm Stat. 2021;20:185–95. https://doi.org/10.1002/pst.2068
[32] Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. https://doi.org/10.1186/1471-2288-14-45
[33] Laprise C, Shahul HP, Madathil SA, et al. Periodontal diseases and risk of oral cancer in Southern India: results from the HeNCe Life study. Int J Cancer. 2016;139:1512–19. https://doi.org/10.1002/ijc.30201
[34] Moraes RC, Dias FL, Figueredo CM, et al. Association between chronic periodontitis and oral/oropharyngeal cancer. Braz Dent J. 2016;27:261–6. https://doi.org/10.1590/0103-6440201600754
[35] Chang JS, Lo HI, Wong TY, et al. Investigating the association between oral hygiene and head and neck cancer. Oral Oncol. 2013;49:1010–17. https://doi.org/10.1016/j.oraloncology.2013.07.004
[36] Zheng TZ, Boyle P, Hu HF, et al. Dentition, oral hygiene, and risk of oral cancer: a case-control study in Beijing, People’s Republic of China. Cancer Causes Control. 1990;1:235–41. https://doi.org/10.1007/BF00117475
[37] Tezal M, Sullivan MA, Hyland A, et al. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prevent. 2009;18:2406–12. https://doi.org/10.1158/1055-9965.EPI-09-0334
[38] Komlos G, Csurgay K, Horvath F, et al. Periodontitis as a risk for oral cancer: a case-control study. BMC Oral Health. 2021;21:640. https://doi.org/10.1186/s12903-021-01998-y
[39] Shin YJ, Choung HW, Lee JH, et al. Association of periodontitis with oral cancer: a case-control study. J Dent Res. 2019;98:526–33. https://doi.org/10.1177/0022034519827565
[40] Narayan TV, Revanna GM, Hallikeri U, et al. Dental caries and periodontal disease status in patients with oral squamous cell carcinoma: A screening study in urban and semiurban population of Karnataka. J Maxillofac Oral Surg. 2014;13:435–43. https://doi.org/10.1007/s12663-013-0540-5
[41] Moergel M, Kämmerer P, Kasaj A, et al. Chronic periodontitis and its possible association with oral squamous cell carcinoma – a retrospective case control study. Head Face Med. 2013;9:39. https://doi.org/10.1186/1746-160X-9-39
[42] Guha N, Boffetta P, Wünsch Filho V, et al. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case-control studies. Am J Epidemiol. 2007;166:1159–73. https://doi.org/10.1093/aje/kwm193
[43] Bundgaard T, Wildt J, Frydenberg M, et al. Case-control study of squamous cell cancer of the oral cavity in Denmark. Cancer Causes Control. 1995;6:57–67. https://doi.org/10.1007/BF00051681
[44] De Rezende CP, Ramos MB, Daguíla CH, et al. Oral health changes in patients with oral and oropharyngeal cancer. Braz J Otorhinolaryngol. 2008;74:596–600. https://doi.org/10.1016/S1808-8694(15)30609-1
[45] Tezal M, Sullivan MA, Reid ME, et al. Chronic periodontitis and the risk of tongue cancer. Arch Otolaryngol Head Neck Surg. 2007;133:450–4. https://doi.org/10.1001/archotol.133.5.450
[46] Rosenquist K, Wennerberg J, Schildt EB, et al. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Oto-laryngol. 2005;125:1327–36. https://doi.org/10.1080/00016480510012273
[47] Chen PJ, Chen YY, Lin CW, et al. Effect of periodontitis and scaling and root planing on risk of pharyngeal cancer: a nested case – control study. Int J Environ Res Public Health. 2021;18:1–12. https://doi.org/10.3390/ijerph18010008
[48] Hiraki A, Matsuo K, Suzuki T, et al. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prevent. 2008;17:1222–7. https://doi.org/10.1158/1055-9965.EPI-07-2761
[49] Garrote LF, Herrero R, Reyes RM, et al. Risk factors for cancer of the oral cavity and oro-pharynx in Cuba. Br J Cancer. 2001;85:46–54. https://doi.org/10.1054/bjoc.2000.1825
[50] Javed F, Warnakulasuriya S. Is there a relationship between periodontal disease and oral cancer? A systematic review of currently available evidence. Crit Rev Oncol Hematol. 2016;97:197–205. https://doi.org/10.1016/j.critrevonc.2015.08.018
[51] Meisel P, Holtfreter B, Biffar R, et al. Association of periodontitis with the risk of oral leukoplakia. Oral Oncol. 2012;48:859–63. https://doi.org/10.1016/j.oraloncology.2012.02.022
[52] Michaud DS, Fu Z, Shi J, et al. Periodontal disease, tooth loss, and cancer risk. Epidemiol Rev. 2017;39:49–58. https://doi.org/10.1093/epirev/mxx006
[53] Dhingra K. Is periodontal disease a risk factor for oral cancer? Evidence-based dentistry. Evid Based Dent. 2022;23:20–1. https://doi.org/10.1038/s41432-022-0245-z
[54] Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–35. https://doi.org/10.1016/j.cell.2006.02.016
[55] Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. https://doi.org/10.1038/nature01322
[56] Dholam KP, Sharma MR, Gurav SV, et al. Oral and dental health status in patients undergoing neoadjuvant chemotherapy for locally advanced head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;132:539–48. https://doi.org/10.1016/j.oooo.2021.07.018
[57] Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90:294–303. https://doi.org/10.1177/0022034510379602
[58] Kõll-Klais P, Mändar R, Leibur E, et al. Oral lactobacilli in chronic periodontitis and periodontal health: species composition and antimicrobial activity. Oral Microbiol Immunol. 2005;20:354–61. https://doi.org/10.1111/j.1399-302X.2005.00239.x
[59] Teanpaisan R, Piwat S, Dahlén G. Inhibitory effect of oral Lactobacillus against oral pathogens. Lett Appl Microbiol. 2011;53:452–9. https://doi.org/10.1111/j.1472-765X.2011.03132.x
[60] Tezal M, Scannapieco FA, Wactawski-Wende J, et al. Dental caries and head and neck cancers. JAMA Otolaryngol Head Neck Surg. 2013;139:1054–60. https://doi.org/10.1001/jamaoto.2013.4569
[61] Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 2018;89(Suppl. 1):S159–72. https://doi.org/10.1002/JPER.18-0006
[62] Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions – introduction and key changes from the 1999 classification. J Clin Periodontol. 2018;45(Suppl. 20):S1–8. https://doi.org/10.1111/jcpe.12935
[63] Erriu M, Genta G, Pili FM, et al. Probing depth in periodontal pockets: in vitro evaluation of contributions to variability due to probe type and operator skill. Proc Inst Mech Eng H. 2015;229:743–9. https://doi.org/10.1177/0954411915606170
[64] Al Shayeb KN, Turner W, Gillam DG. Periodontal probing: a review. Prim Dent J. 2014;3:25–9. https://doi.org/10.1308/205016814812736619
[65] Tezal M, Grossi SG, Genco RJ. Is periodontitis associated with oral neoplasms? J Periodontol. 2005;76:406–10. https://doi.org/10.1902/jop.2005.76.3.406
[66] Holtfreter B, Albandar JM, Dietrich T, et al. Standards for reporting chronic periodontitis prevalence and severity in epidemiologic studies: proposed standards from the Joint EU/USA Periodontal Epidemiology Working Group. J Clin Periodontol. 2015;42:407–12. https://doi.org/10.1111/jcpe.12392
[67] Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(Suppl. 7S):1387–99. https://doi.org/10.1902/jop.2007.060264