RESEARCH ARTICLE
The effect of citric acid on mineralisation and vascular endothelial growth factor secretion from apical papilla stem cells
Krasimir Hristova, Nikolay Ishkitievb, Marina Mitevab, Violeta Dimitrovab, Ralitsa Gigovaa, Nataliya Gatevaa and Liliya Angelovac
aDepartment of Pediatric Dentistry, Faculty of Dental Medicine, Medical University of Sofia, Sofia, Bulgaria; bDepartment of Chemistry and Biochemistry, Medical Faculty, Medical University of Sofia, Sofia, Bulgaria; cDepartment of Dental Public Health, Faculty of Dental Medicine, Medical University of Sofia, Sofia, Bulgaria
Abstract
Objective: To investigate the influence of citric acid on the osteogenic and angiogenic potential of stem cells from apical papillae (SCAPs).
Materials and methods: Stem cells from apical papillae were isolated from freshly extracted third permanent molars. These cells were treated with 20 and 100 μM citric acid. Alizarin red staining was used to evaluate mineral deposition. The secreted levels of vascular endothelial growth factor (VEGF) were assessed by ELISA on days 18, 24 and 28. Immunofluorescence analysis was performed to assess the expression of surface markers after exposure to 20 and 100 μM citric acid.
Results: Different mineralisation patterns were observed. Supplemented with citric acid, media showed more diffuse and less dense crystals. On day 18, most VEGF was secreted from the cells with no added citric acid. On day 24, there was a significant increase (p < 0.05) in the levels of VEGF secreted from cells treated with 20 μM citric acid. On day 28, cells from the control group did not secrete VEGF. There was a reduction in the levels of VEGF secreted by cells treated with 20 μM citric acid and a significant increase in the cells exposed to 100 μM citric acid (p < 0.05).
Conclusion: Citric acid can promote the differentiation of SCAPs and angiogenesis.
KEYWORDS: Citric acid; VEGF; regenerative endodontics; stem cells from apical papilla; immature teeth
Citation: ACTA ODONTOLOGICA SCANDINAVICA 2024; VOL. 83: 546–552. DOI: https://doi.org/10.2340/aos.v83.42026.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Odontologica Scandinavica Society. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 13 February 2024; Accepted: 15 September 2024; Published: 1 October 2024.
CONTACT Krasimir Hristov k.christov@fdm.mu-sofia.bg Department of Pediatric Dentistry, Faculty of Dental Medicine, 3 Georgy Sofiyski Blvd, 1431 Sofia, Bulgaria
Competing interests and funding: No potential conflict of interest was reported by the authors.
This study is financed by the European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project № BG-RRP-2.004-0004-C01
Introduction
Regenerative endodontic treatment (RET) is becoming increasingly popular in managing apical periodontitis in immature permanent teeth [1]. This procedure permits the lengthening and thickening of the walls of the root canal, construction of the apex, and in some cases, may even promote the recovery of tooth sensitivity [2]. The most complex aspect of this treatment is the formation of a new pulp-dentin complex, which requires the predictable induction of angiogenesis and neurogenesis [3]. As with conventional endodontic treatments, thorough disinfection of the root canals and removal of the smear layer are of utmost importance for a successful outcome [4]. Removal of the smear layer increases penetration of the irrigant and subsequently enhances root canal disinfection and the release of growth factors [5]. In regenerative endodontics, there are several key considerations in addition to the antibacterial effect of irrigants: low cytotoxicity and the ability to release growth factors included in the dentin [6, 7]. Growth factors from the dentin, which play a key role as biological inducers, are reactivated and released during its demineralisation under the influence of acidic products [8]. In endodontic treatment, this is achieved through using organic acids or chelators as irrigants [9, 10]. Ethylenediaminetetraacetic acid (EDTA) and citric acid are the most commonly used solutions to achieve this goal in clinical practice [11]; these products favour cell adhesion and have no adverse effects on cell proliferation [12, 13]. Stimulation of the expression of growth factors by undifferentiated stem cells is also essential [14]. Therefore, the aim of the present study was to investigate the influence of citric acid on the osteogenic and angiogenic potential of stem cells from apical papillae (SCAPs).
Materials and methods
Isolation of stem cells from apical papillae
Firstly, we isolated SCAPs from freshly extracted tooth germs of the permanent third molars. The growth of the SCAPs was maintained in cell culture. The apical papillae were carefully removed from the tooth’s root and then stored and incubated in an enzymatic digestion solution containing 3 mg/mL of collagenase (Sigma-Aldrich, St. Louis, MO, USA) and 4 mg/mL of dispase at 37°C for 1 hour. A single-cell suspension was obtained by passing the cells through a sterile 70 µm strainer (Falcon, Corning, NY, USA) and seeding the cells into culture dishes containing Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen, Eugene, OR, USA). The culture medium was supplemented with 10% foetal bovine serum, 100 U/mL of penicillin, and 100 µg/mL of streptomycin (Invitrogen). Cells between the third and fifth passages were used for subsequent experiments.
Immunofluorescence assays
The stem cell properties of SCAPs were demonstrated by assessing the expression levels of several cell surface markers by immunocytochemistry, and the use of several primary antibodies: STRO-1 (Thermo Fisher Scientific, Waltham, MA, USA), CD24, CD31, CD13, and CD146. Except for STRO-1, all antibodies were produced in mice by Sigma-Aldrich (Sigma-Aldrich, St. Louis, Missouri, United States). Cells were washed with phosphate-buffered saline (PBS) (Biolegend, San Diego, CA, USA). Thereafter, non-specific binding sites were blocked by incubation in 2% bovine serum albumin (BSA) in PBS, re-washing with PBS, incubating with the primary antibody for 1 hour at room temperature, washing with PBS, and labelling with an anti-mouse secondary antibody for 1 hour in the dark (Sigma-Aldrich).
Mineralisation assessment
Cells were cultured for 14 days in four different types of media. For the control group, cells were cultured in DMEM, supplemented with 10% foetal bovine serum (Sigma -Aldrich), 100 U/mL of penicillin, 100 µg/mL of streptomycin, and 10 ng/mL of TGFβ (RnD Systems, Minneapolis, MN, USA). Cells in mineralisation group 1 (MM1) were cultured in DMEM supplemented with 50 μg/mL of ascorbic acid (Serva Electrophoresis GmbH, Heidelberg, Germany), 10 nM dexamethasone (Wako Pure Chemicals, Osaka, Japan), and 10 mM β-glycerophosphate (Sigma-Aldrich). Cells in mineralisation group 2 (MM2) were cultured in DMEM supplemented with 50 μg/mL of ascorbic acid, 10 nM dexamethasone, 10 mM β-glycerophosphate, and 20 μM of citric acid. Cells in mineralisation group 3 (MM3) were cultured in DMEM supplemented with 50 μg/mL of ascorbic acid, 10 nM dexamethasone, 10 mM β-glycerophosphate, and 100 μM of citric acid.
Alizarin red staining was used to evaluate mineral deposition. For this purpose, the culture medium was aspirated from the wells of culture plates and the cells were washed three times with PBS. This was followed by their fixation with 10% formalin for 1 hour at room temperature. The solution was then aspirated, and the cells were washed thrice with deionised water. At room temperature and dark conditions, 1 mL of fresh Alizarin red solution (Applichem GmbH, Darmstadt, Germany) was added to each well and incubated for 45 minutes. Subsequently, the solution was removed and the cells were washed thrice with deionised water. Finally, 1 mL of PBS was added to each well and the cells were observed using a phase-contrast light microscope (Leica Camera, Wetzlar, Germany).
Vascular endothelial growth factor expression during cell culture
The levels of vascular endothelial growth factor (VEGF) secreted by cultured cells were determined in three types of culture media: (1) DMEM supplemented with 10% foetal bovine serum, 100 U/mL of penicillin, and 100 µg/mL of streptomycin; (2) DMEM supplemented with 10% foetal bovine serum, 100 U/mL of penicillin, 100 µg/mL of streptomycin, and 20 µM citric acid; and (3) DMEM supplemented with 10% foetal bovine serum, 100 U/mL of penicillin, 100 µg/mL of streptomycin, and 100 µM citric acid. The supernatants from each well were collected on days 18, 24, and 28, and the levels of secreted VEGF were assayed by ELISA (Human VEGF-B ELISA Kit, Sigma Aldrich). Immunofluorescence analysis was also performed to investigate the expression of stem cells and differentiation markers.
Statistical analysis
Each experiment was carried out three times. Data were collated and analysed by Statistical Package for the Social Sciences (SPSS) version 19.0 software (IBM Corp., Armonk, NY, USA). Shapiro-Wilk test was used to determine whether the data set was normally distributed (p > 0.05). Data were compared by analysis of variance (ANOVA), and differences were considered significant at p < 0.05.
Results
Immunofluorescence was used to qualitatively investigate the ability of cells isolated from apical papillae to express surface markers. The resulting images are presented in Figure 1. These images confirm the presence of stem cells in the apical papillae because of the expression of cell markers that are typical for undifferentiated cells. Cells did not express individual markers in a homogenous manner. These results confirm the heterogeneous nature of the cell cultures.
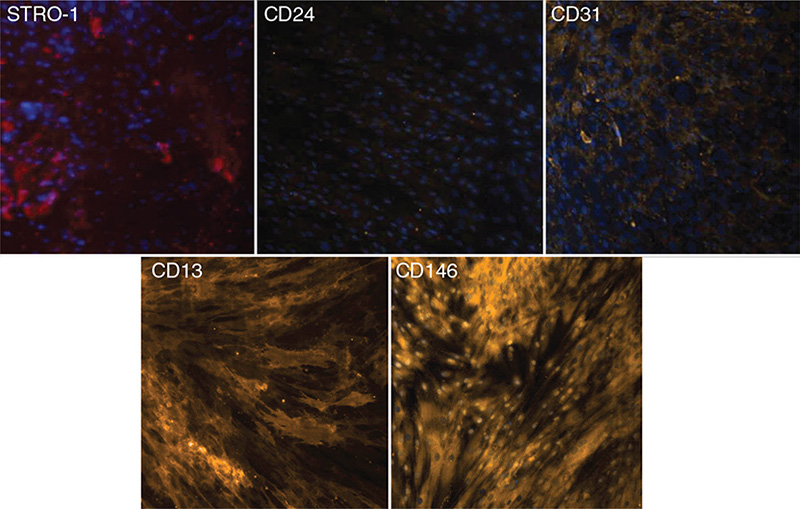
Figure 1. Immunofluorescence study of the expression of cell surface markers (STRO-1, CD24, CD31, CD13, and CD146) in apical papilla stem cells.
Figure 2 shows mineral deposits after the incubation of SCAPs in different osteo-differentiation media, as visualised by Alizarin red staining. Different mineralisation patterns were observed, with cultures with standard osteogenic medium characterised by denser and localised mineral deposits. Cells with different concentrations of citric acid added were more diffuse and less dense. The deposits were non-homogeneous, and their size and density decreased with increasing citric acid concentration.
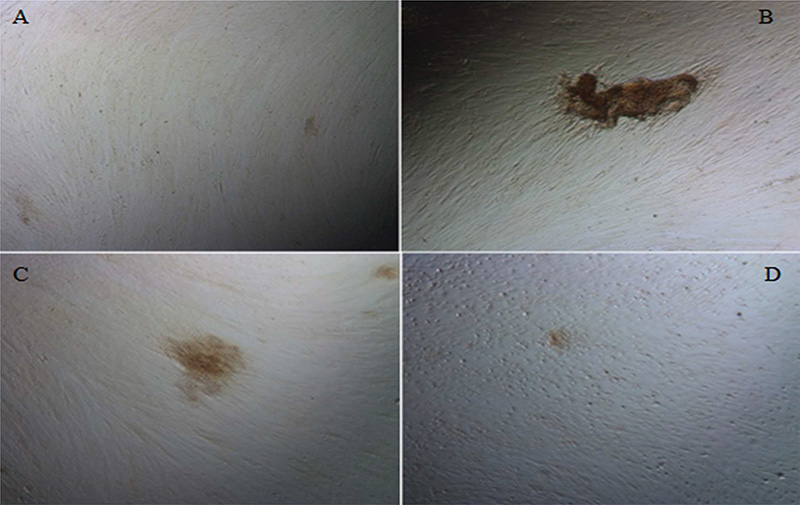
Figure 2. Evaluation of the formation of mineral deposits by SCAPs after exposure to citric acid. (A) Control, (B) Standard mineralisation-inducing medium, (C) Medium supplemented with 20 μM citric acid, and (D) Medium supplemented with 100 μM citric acid. SCAP: stem cells from apical papillae.
Figure 3 shows the levels of VEGF secreted on days 18, 24, and 28. On day 18, the levels of secreted VEGF were the highest from cells cultured in the absence of citric acid; the next highest levels of VEGF were secreted by cells exposed to 100 μM citric acid. There was a significant difference between all individual groups (ANOVA p < 0.05). On day 24, there was a significant increase in the levels of secreted VEGF from cells cultured in a medium supplemented with 20 μM citric acid; however, the levels of VEGF secreted by cells in the control group and those exposed to 100 μM citric acid decreased significantly (p < 0.05). On day 28, cells from the control group did not secrete VEGF. There was a reduction in the levels of VEGF secreted by cells cultured with 20 μM citric acid and a significant increase in the levels of VEGF secreted by cells exposed to 100 μM, although these levels did not reach those produced by cells exposed to 20 μM citric acid (ANOVA, p < 0.05).
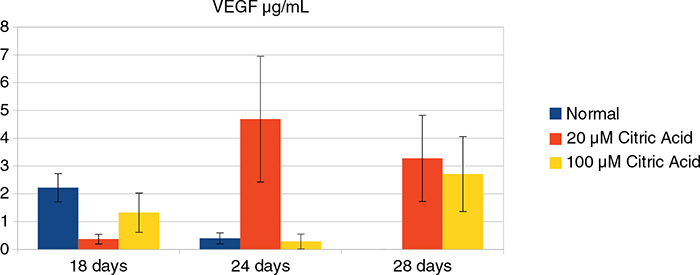
Figure 3. The levels of secreted VEGF in control medium (blue), medium supplemented with 20 μM (red), and 100 μM citric acid (yellow). VEGF: Vascular Endothelial Growth Factor.
Immunofluorescence analysis revealed a weakening of positive signals associated with surface markers when the cells were incubated in a medium containing citric acid; this was most likely because of the initiation of cellular differentiation (Figure 4).
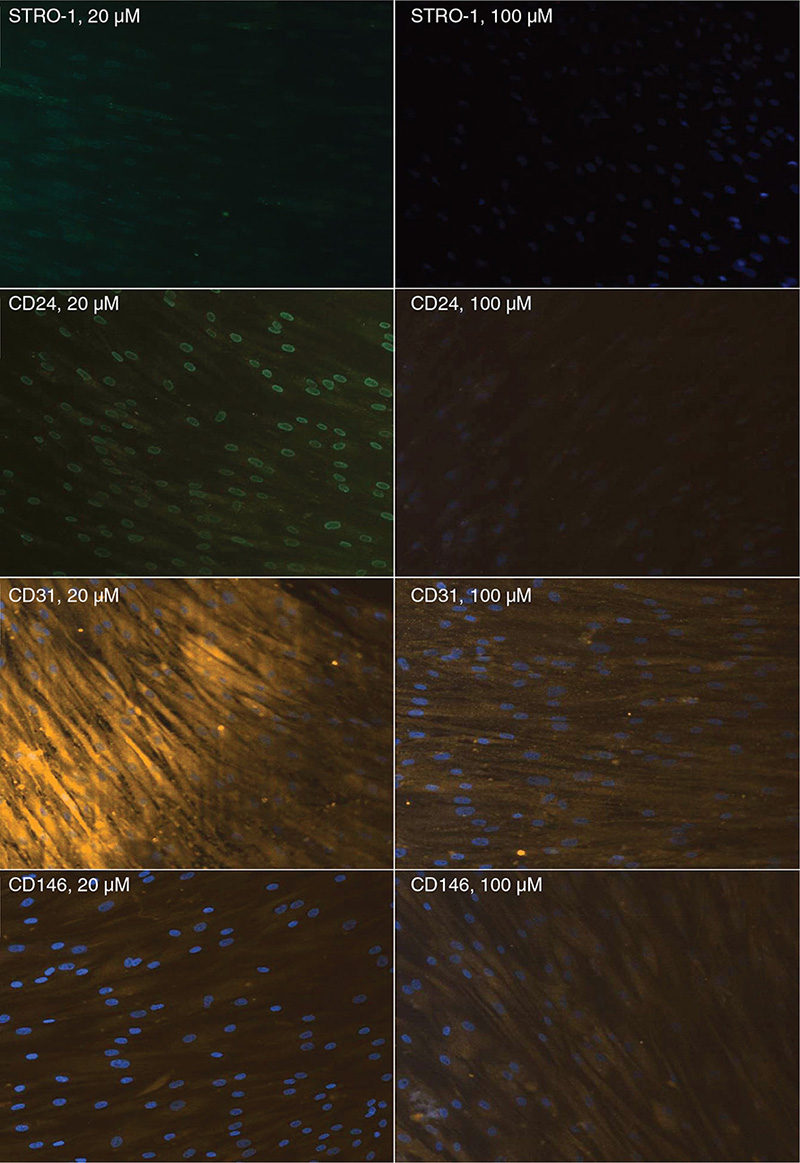
Figure 4. Immunofluorescence analysis of the expression of surface markers by SCAPs after exposure to 20 and 100 μM citric acid. SCAP: stem cells from apical papillae.
Discussion
In the present study, we investigated the osteogenic and angiogenic potential of citric acid on SCAPs. Citric acid and its citrate derivatives represent a metabolic factor that is found in abundance in bone and can be used by mesenchymal stem cells to stimulate osteogenesis by regulating certain metabolic pathways [15]. Citric acid is known to have antioxidant and anti-inflammatory properties [16]. In addition, using 10% citric acid as an irrigant has been shown to promote the release of dentin-incorporated growth factors without affecting cell proliferation and adhesion to the root dentin [12]. In some cases, conditioning dentin with citric acid has been shown to promote the release of greater amount growth factors than EDTA [8, 15]. The concentrated release of growth factors can accelerate tissue regeneration by stimulating cell proliferation and the differentiation of stem cells [17, 18].
Of the various surface cell markers, CD24, a pluripotency marker, has been shown to be directly associated with and predominantly expressed in SCAPs, although expression levels are usually low [19]. This marker could not be detected in other types of mesenchymal stem cells, including cells from dental pulp [20, 21]. CD24 is used as an indicator for an undifferentiated cell state; therefore, its presence indicates the stemness of cells. As the level of alkaline phosphatase increases, the expression level of CD24 decreases, thus indicating that cells have begun to leave the undifferentiated state and enter that of the osteoblastic nature/lineage [22]. On the other hand, regardless of differentiation, once cells reach the 10th passage, CD24 expression disappears. Given that CD24 is considered as an identifying marker of SCAPs, this finding suggests that the stem cell properties of SCAPs are reduced with passage [23]. Some of the cells in the present study expressed CD24; we also observed that the signal intensity weakened after incubation in a citric acid medium, probably because of the initiation of differentiation processes (Figures 1 and 4). Our results are consistent with publications in the specialised literature concerning the expression of the CD24 marker by SCAPs [20, 21] but in medium supplemented with citric acid.
The analysis of CD146 expression is vital because of the fact that CD146 is the most commonly used marker to characterise perivascular pluripotent stem cells in connective tissue [24]. As with all markers, the expression of CD146 is not homogeneous and requires fine cell sorting to obtain a pure population [25]. Furthermore, because of variations in their expression, neither CD146 nor STRO-1 can be used alone as specific markers of dental stem cells [26]. In an isolated population of SCAPs, CD146-positive cells predominated over STRO-1-positive cells [27]. STRO-1-positive cells have been shown to exhibit neurogenic characteristics, staining positive for beta-3-tubulin, nestin, and neuron-specific enolase, which are all neuronal stem markers. This supports the claim that these cells are oriented towards specific cell lineages depending on the proportional expression of certain factors [28].
Stem cells from apical papillae have the potential for multi-linear differentiation, extracellular matrix synthesis, and mineralisation. These cells can differentiate into odontoblasts/osteoblasts, adipocytes, chondrocytes, and neurocytes in vitro in the presence of established cell line-specific active substances such as growth factors and cytokines [29–31]. This property may be because of the presence of certain factors in heterogeneous cell culture: (1) multipotent stem cells, (2) precursor cells such as odontoblasts/preosteoblasts/preadipocytes, chondroblasts or neural cell precursors, or (3) a combination of all of these factors [32].
The potential of SCAPs to undergo odontoblastic/osteoblastic differentiation was investigated 30 days after induction with an osteogenic medium, where they could form small round Alizarin red–positive nodules which were indicative of calcium deposition in vitro [33]. Similar to dental pulp stem cells, SCAPs possess odontogenic potential in vitro. These cells express lower levels of dentin sialoprotein (DSP), matrix extracellular phosphoglycoprotein (MEPE), transforming growth factor (TGF)β-II receptor, fibroblast growth factor receptor (FGFR1), FGFR3, and melanoma-associated glycoprotein (CD146/MUC18) when compared to dental pulp stem cells [34]. Upon the transplantation of SCAPs with hydroxylapatite/tricalcium phosphate (HA/TCP) granules into immunocompromised mice, these cells underwent differentiation into odontoblasts, thus regenerating a dentin/pulp-like structure and connective tissue. Furthermore, the co-transplantation of SCAPs and periodontal ligament stem cells with HA/TCP granules resulted in the formation of dentin and periodontal ligament [35]. It was confirmed in the current study that SCAPs possessed the potential for osteogenic/odontogenic differentiation. This was demonstrated by visualising mineral deposits after alizarin red staining (Figure 2). In the medium supplemented with citric acid, these deposits were smaller in size and density, probably because of the lower pH of the environment. On the other hand, citrate supplementation increased the production of collagen and mineralisation, thus influencing the organisation of the mineral matrix [36]. This in vitro study proves that irrigation of the root canal with citric acid may promote the process of cell differentiation (Figure 3).
Vascular endothelial growth factor is a vascular endothelial cell mitogenic factor that modulates physiological angiogenesis, vascular permeability, cell migration, proliferation, and vasodilation. In addition, VEGF is a positive regulator of bone development, skeletal growth and fracture healing, and can also stimulate the proliferation and differentiation of osteoblasts [37, 38]. Under appropriate conditions, VEGF can induce the differentiation of stem cells into endothelial cells while modulating tooth development and dentinogenesis [39]. Research has shown that VEGF is synthesised by human pulp cells under physiological conditions and at greater levels in pathological conditions, thus leading to the increased proliferation and expression of alkaline phosphatase [40, 41]. FGF-2, platelet-derived growth factor (PDGF), and TGFβ are released after the application of orthodontic force [42] and act synergistically on pulp angiogenesis both in vitro and in vivo [43]. Hydrogels loaded with VEGF are known to increase proliferation, alkaline phosphatase activity, mineralisation and osteogenesis in undifferentiated stem cells [44].
Vascular endothelial growth factor induces the differentiation of dental pulp stem cells into endothelial cells. Under osteogenic conditions, treating dental pulp stem cells with VEGF leads to their differentiation into osteoblasts, as demonstrated by Alizarin red staining and an increase in alkaline phosphatase activity [45]. VEGF-modified dental pulp stem cells exhibit increased myelination, and an increased thickness and diameter of axons in nerve tissue [46]. Exposing SCAPs to VEGF is known to induce the expression of factors that are typical of odontoblasts and neuroblasts, thus providing evidence for the critical role of this VEGF in regenerative endodontic procedures [3]. In the present study, adding citric acid to the culture medium over the first 18 days of SCAPs culture did not increase the levels of secreted VEGF when compared to cells cultured in a standard medium (the control group). However, a significant increase in the levels of VEGF were observed after the 24th day in cells exposed to citric acid; 20 µM citric acid had a better effect than 100 µM (Figure 3). Collectively, the results of the study show that the use of citric acid as an irrigant solution in the protocols of regenerative endodontic procedures may have a positive effect on cell proliferation, differentiation, and angiogenesis by increasing the amount of VEGF released.
Conclusions
Using citric acid as an irrigant in the protocols of regenerative endodontics, in addition to a disinfecting and conditioning effect, stimulates the differentiation of stem cells from the apical papillae. Furthermore, citric acid significantly increases the levels of VEGF after the 28th day of SCAPs culture; this may stimulate angiogenesis in regenerative endodontic procedures and contribute to the successful outcome of the treatment over the long term.
Ethical statement
All parents gave written informed consent for using their children’s teeth for research purposes, and all molars were irreversibly anonymised immediately after extraction. Approval from the Ethical Committee of Medical University, Sofia’s Council of Medical Science was also obtained.
References
[1] Murray PE. Review of guidance for the selection of regenerative endodontics, apexogenesis, apexification, pulpotomy, and other endodontic treatments for immature permanent teeth. Int Endod J. 2023;56(Suppl 2):188–199. https://doi.org/10.1111/iej.13809.
[2] Li J, Zheng L, Daraqel B, et al. The efficacy of concentrated growth factor and platelet-rich fibrin as scaffolds in regenerative endodontic treatment applied to immature permanent teeth: a retrospective study. BMC Oral Health. 2023;23(1):482. https://doi.org/10.1186/s12903-023-03164-y.
[3] Shen Z, Tsao H, LaRue S, et al. Vascular endothelial growth factor and/or nerve growth factor treatment induces expression of dentinogenic, neuronal, and healing markers in stem cells of the apical papilla. J Endod. 2021;47(6):924–931. https://doi.org/10.1016/j.joen.2021.02.011.
[4] Fouad AF, Diogenes AR, Torabinejad M, et al. Microbiome changes during regenerative endodontic treatment using different methods of disinfection. J Endod. 2022;48(10):1273–1284. https://doi.org/10.1016/j.joen.2022.07.004.
[5] Rahmati A, Karkehabadi H, Rostami G, et al. Comparative effects of Er:YAG laser, and EDTA, MTAD, and QMix irrigants on adhesion of stem cells from the apical papilla to dentin: a scanning electron microscopic study. J Clin Exp Dent. 2022;14(4):e310–e315. https://doi.org/10.4317/jced.59129.
[6] Wei X, Yang M, Yue L, et al. Expert consensus on regenerative endodontic procedures. Int J Oral Sci. 2022;14(1):55. https://doi.org/10.1038/s41368-022-00206-z.
[7] da Silva Magalhães K, Kuerten Gil AC, Goulart TS, et al. Efficacy of disinfection procedures performed prior to regenerative endodontic therapy: an integrative review. Aust Endod J. 2023;49(2):418–427. https://doi.org/10.1111/aej.12670.
[8] Sadaghiani L, Alshumrani AM, Gleeson HB, et al. Growth factor release and dental pulp stem cell attachment following dentine conditioning: an in vitro study. Int Endod J. 2022;55(8):858–869. https://doi.org/10.1111/iej.13781.
[9] Arslan H, Ahmed HMA, Şahin Y, et al. Regenerative endodontic procedures in necrotic mature teeth with periapical radiolucencies: a preliminary randomized clinical study. J Endod. 2019;45(7):863–872. https://doi.org/10.1016/j.joen.2019.04.005.
[10] Ballal NV, Narkedamalli R, Ruparel NB, et al. Effect of maleic acid root conditioning on release of transforming growth factor beta 1 from infected root canal dentin. J Endod. 2022;48(5):620–624. https://doi.org/10.1016/j.joen.2022.02.007.
[11] Tonini R, Salvadori M, Audino E, et al. Irrigating solutions and activation methods used in clinical endodontics: a systematic review. Front Oral Health. 2022;3:838043. https://doi.org/10.3389/froh.2022.838043.
[12] Atesci AA, Avci CB, Tuglu MI, et al. Effect of different dentin conditioning agents on growth factor release, mesenchymal stem cell attachment and morphology. J Endod. 2020;46(2):200–208. https://doi.org/10.1016/j.joen.2019.10.033.
[13] Ivica A, Zehnder M, Mateos JM, et al. Biomimetic conditioning of human dentin using citric acid. J Endod. 2019;45(1):45–50. https://doi.org/10.1016/j.joen.2018.09.015.
[14] Shamszadeh S, Shirvani A, Asgary S. The role of growth factor delivery systems on cellular activities of dental stem cells: a systematic review (Part II). Curr Stem Cell Res Ther. 2024;19(4):587–610. https://doi.org/10.2174/1574888X17666220609093939.
[15] Gómez-Delgado M, Camps-Font O, Luz L, et al. Update on citric acid use in endodontic treatment: a systematic review. Odontology. 2023;111(1):1–19. https://doi.org/10.1007/s10266-022-00744-2.
[16] Wu X, Dai H, Xu C, et al. Citric acid modification of a polymer exhibits antioxidant and anti-inflammatory properties in stem cells and tissues. J Biomed Mater Res A. 2019;107(11):2414–2424. https://doi.org/10.1002/jbm.a.36748.
[17] Yu S, Zheng Y, Guo Q, et al. Mechanism of pulp regeneration based on concentrated growth factors regulating cell differentiation. Bioengineering (Basel). 2023;10(5):513. https://doi.org/10.3390/bioengineering10050513.
[18] Tabatabaei F, Aghamohammadi Z, Tayebi L. In vitro and in vivo effects of concentrated growth factor on cells and tissues. J Biomed Mater Res A. 2020;108(6):1338–1350. https://doi.org/10.1002/jbm.a.36906.
[19] Liu C, Xiong H, Chen K, et al. Long-term exposure to pro-inflammatory cytokines inhibits the osteogenic/dentinogenic differentiation of stem cells from the apical papilla. Int Endod J. 2016;49(10):950–959. https://doi.org/10.1111/iej.12551.
[20] Abe S, Kaida A, Kanemaru K, et al. Differences in the stemness characteristics and molecular markers of distinct human oral tissue neural crest-derived multilineage cells. Cell Prolif. 2022;55(10):e13286. https://doi.org/10.1111/cpr.13286.
[21] Liang J, Zhao YJ, Li JQ, et al. A pilot study on biological characteristics of human CD24(+) stem cells from the apical papilla. J Dent Sci. 2022;17(1):264–275. https://doi.org/10.1016/j.jds.2021.01.012.
[22] Dong R, Yao R, Du J, et al. Depletion of histone demethylase KDM2A enhanced the adipogenic and chondrogenic differentiation potentials of stem cells from apical papilla. Exp Cell Res. 2013;319(18):2874–2882. https://doi.org/10.1016/j.yexcr.2013.07.008.
[23] Zhang W, Zhang X, Ling J, et al. Proliferation and odontogenic differentiation of BMP2 gene-transfected stem cells from human tooth apical papilla: an in vitro study. Int J Mol Med. 2014;34(4):1004–1012. https://doi.org/10.3892/ijmm.2014.1862.
[24] Matsui M, Kobayashi T, Tsutsu T. CD146 positive human dental pulp stem cells promote regeneration of dentin/pulp-like structures. Hum Cell. 2018;31(2):127–138. https://doi.org/10.1007/s13577-017-0198-2.
[25] Turrioni AP, Oliveira Neto NF, Xu Y, et al. Proliferation rate and expression of stem cells markers during expansion in primary culture of pulp cells. Braz Oral Res. 2021;35:e128. https://doi.org/10.1590/1807-3107bor-2021.vol35.0128.
[26] Aydin S, Şahin F. Stem cells derived from dental tissues. Adv Exp Med Biol. 2019;1144:123–132. https://doi.org/10.1007/5584_2018_333.
[27] Diederich A, Fründ HJ, Trojanowicz B, et al. Influence of ascorbic acid as a growth and differentiation factor on dental stem cells used in regenerative endodontic therapies. J Clin Med. 2023;12(3):1196. https://doi.org/10.3390/jcm12031196.
[28] Retana-Lobo C, Reyes-Carmona J. Immunohistochemical characterization of stem cell, vascular, neural, and differentiation markers in the apical papilla and dental pulp of human teeth at various stages of root development. J Histotechnol. 2023;46(1):17–27. https://doi.org/10.1080/01478885.2022.2122665.
[29] Li FC, Shahin-Shamsabadi A, Selvaganapathy PR, et al. Engineering a novel stem cells from apical papilla-macrophages organoid for regenerative endodontics. J Endod. 2022;48(6):741–748. https://doi.org/10.1016/j.joen.2022.02.011.
[30] Savoj S, Esfahani MHN, Karimi A, et al. Integrated stem cells from apical papilla in a 3D culture system improve human embryonic stem cell derived retinal organoid formation. Life Sci. 2022;291:120273. https://doi.org/10.1016/j.lfs.2021.120273.
[31] Liu Z, Yan N, Chen Y, et al. Hepatocyte growth factor promotes differentiation potential and stress response of human stem cells from apical papilla. Cells Tissues Organs. 2024;213(1):40–54. https://doi.org/10.1159/000527212.
[32] Zou J, Mao J, Shi X. Influencing factors of pulp-dentin complex regeneration and related biological strategies. Zhejiang Da Xue Bao Yi Xue Ban. 2022;51(3):350–361. https://doi.org/10.3724/zdxbyxb-2022-0046.
[33] Camassari JR, de Sousa ITC, Cogo-Müller K, et al. The self-assembling peptide P(11)-4 influences viability and osteogenic differentiation of stem cells of the apical papilla (SCAP). J Dent. 2023;134:104551. https://doi.org/10.1016/j.jdent.2023.104551.
[34] Liu Q, Gao Y, He J. Stem cells from the apical papilla (SCAPs): past, present, prospects, and challenges. Biomedicines. 2023;11(7):2047. https://doi.org/10.3390/biomedicines11072047.
[35] Sonoyama W, Liu Y, Fang D, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;20(1):e79. https://doi.org/10.1371/journal.pone.0000079.
[36] Perut F, Graziani G, Columbaro M, et al. Citrate supplementation restores the impaired mineralisation resulting from the acidic microenvironment: an in vitro study. Nutrients. 2020;12(12):3779. https://doi.org/10.3390/nu12123779.
[37] McKenzie JA, Galbreath IM, Coello AF, et al. VEGFA from osteoblasts is not required for lamellar bone formation following tibial loading. Bone. 2022;163:116502. https://doi.org/10.1016/j.bone.2022.116502.
[38] Zhang R, Liu Y, Qi Y, et al. Self-assembled peptide hydrogel scaffolds with VEGF and BMP-2 enhanced in vitro angiogenesis and osteogenesis. Oral Dis. 2022;28(3):723–733. https://doi.org/10.1111/odi.13785.
[39] Brunello G, Zanotti F, Scortecci G, et al. Dentin particulate for bone regeneration: an in vitro study. Int J Mol Sci. 2022;23(16):9283. https://doi.org/10.3390/ijms23169283.
[40] Xu F, Qiao L, Zhao Y, et al. The potential application of concentrated growth factor in pulp regeneration: an in vitro and in vivo study. Stem Cell Res Ther. 2019;10(1):134. https://doi.org/10.1186/s13287-019-1247-4.
[41] Janebodin K, Chavanachat R, Hays A, et al. Silencing VEGFR-2 hampers odontoblastic differentiation of dental pulp stem cells. Front Cell Dev Biol. 2021;9:665886. https://doi.org/10.3389/fcell.2021.665886.
[42] Isola G, Matarese G, Cordasco G, et al. Mechanobiology of the tooth movement during the orthodontic treatment: a literature review. Minerva Stomatol. 2016;65(5):299–327.
[43] Kim SK, Lee J, Song M, et al. Combination of three angiogenic growth factors has synergistic effects on sprouting of endothelial cell/mesenchymal stem cell-based spheroids in a 3D matrix. J Biomed Mater Res B Appl Biomater. 2016;104(8):1535–1543. https://doi.org/10.1002/jbm.b.33498.
[44] Elango J. Proliferative and osteogenic supportive effect of VEGF-loaded collagen-chitosan hydrogel system in bone marrow derived mesenchymal stem cells. Pharmaceutics. 2023;15(4):1297. https://doi.org/10.3390/pharmaceutics15041297.
[45] D’Alimonte I, Nargi E, Mastrangelo F. Vascular endothelial growth factor enhances in vitro proliferation and osteogenic differentiation of human dental pulp stem cells. J Biol Regul Homeost Agents. 2011;25(1):57–69.
[46] Xu W, Xu X, Yao L, et al. VEGFA-modified DPSCs combined with LC-YE-PLGA NGCs promote facial nerve injury repair in rats. Heliyon. 2023;9(4):e14626. https://doi.org/10.1016/j.heliyon.2023.e14626.