SHORT REPORT
Cardiovascular events among patients with prostate cancer treated with abiraterone and enzalutamide
Onur Basera,b,c , Gabriela Samayoad, Archana Dwivedid, Sara AlSalehe, Burhan Cigdemf and Erdi Kizilkayaf
, Gabriela Samayoad, Archana Dwivedid, Sara AlSalehe, Burhan Cigdemf and Erdi Kizilkayaf
aDepartment of Economics, Bogazici University, Bebek, Istanbul, Turkiye; bDepartment of Internal Medicine, University of Michigan, Ann Arbor, MI, USA; cGraduate School of Public Health, City University of New York, New York, USA; dColumbia Data Analytics, New York, New York, USA; eColumbia Data Analytics, Ann Arbor, Michigan, USA; fMergen Analytics, Ankara, Turkey
ABSTRACT
Background and purpose: There is growing concern about the adverse metabolic and cardiovascular effects of abiraterone acetate (AA) and enzalutamide (ENZ), two standard hormonal therapies for prostate cancer. We analysed the risk of cardiovascular adverse events among patients treated with AA and ENZ.
Patients and methods: We used Kythera Medicare data from January 2019 to June 2023 to identify patients with at least one pharmacy claim for AA or ENZ. The index date was the first prescription claim date. Patients were required to have 1 year of data pre- and post-index date. New users excluded those with prior AA or ENZ claims and pre-existing cardiovascular comorbidities. Demographic and clinical variables, including age, socioeconomic status (SES), comorbidity score, prostate-specific comorbidities, and healthcare costs, were analysed. Propensity score matching was employed for risk adjustment.
Results: Of the 8,929 and 8,624 patients in the AA and ENZ cohorts, respectively, 7,647 were matched after adjusting for age, sociodemographic, and clinical factors. Between the matched cohorts (15.54% vs. 14.83%, p < 0.05), there were no statistically significant differences in any cardiovascular event after adjusting for these factors. The most common cardiovascular event in both cohorts was heart failure (5.20% vs. 4.49%), followed by atrial fibrillation (4.42% vs. 3.60%) and hypotension (2.93% vs. 2.48%).
Interpretation: This study provides real-world evidence of the cardiovascular risk of AA and ENZ that may not appear in clinical trial settings. Adjusting for age, baseline comorbidities, and SES, the likelihood of a cardiovascular event did not differ between treatment groups.
KEYWORDS: Prostate cancer; abiraterone; enzalutamide; cardiovascular adverse events; androgen deprivation therapy; hormone therapy
Citation: ACTA ONCOLOGICA 2024, VOL. 63, 137–146. https://doi.org/10.2340/1651-226X.2024.20337.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Oncologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 5 October 2023; Accepted: 29 February 2024; Published: 9 April 2024
CONTACT Onur Baser onur.baser@bogazici.edu.tr Professor of Economics, Bogazici University, Natuk Birkan Building, 224, Bebek, Istanbul, Turkiye.
Competing interests and funding: The authors declare no conflict of interest.
This research received no external funding.
Introduction
Prostate cancer is the second most common cancer in the United States of America (US), comprising 9.5% of all new cancer cases recorded in 2018 [1], and approximately 34,500 deaths each year [2]. It is the sixth-leading cause of cancer mortality in men worldwide [3]. Metastatic castration-resistant prostate cancer (mCRPC) is the most frequent cause of prostate cancer–related death [3]. Men who progress to mCRPC have a poor prognosis, with a median overall survival of 25.6 months [2, 4–6].
Androgen deprivation therapy (ADT) is central to treating locally advanced and metastatic disease [7] by blocking the production of testosterone or curtailing its function to stop prostate cancer growth. Abiraterone acetate (AA), an androgen biosynthesis inhibitor, and enzalutamide (ENZ), an androgen receptor signalling inhibitor, are standard hormonal therapies that are mainstay additions to ADT. Both AA and ENZ have been approved for use in pre-chemotherapy and post-chemotherapy settings, demonstrating satisfactory efficacy and tolerability [3]. Both AA and ENZ have been proven to increase the survival of patients with CRPC and, more recently, of patients with metastatic hormone-sensitive disease naive to hormonal agents [8, 9]. However, adverse drug effects are common with these hormonal therapies and may vary with patient and drug characteristics [10, 11]. Furthermore, cardiovascular disease (CVD) is a primary cause of noncancer mortality in men with prostate cancer [12]. A recent study identified that CVD accounted for almost 30.2% of all deaths among prostate cancer patients [13]. Thus, there is a growing concern about the adverse metabolic and cardiovascular effects [14] of ADT due to the higher risk of CVD associated with the therapy in a population susceptible to CVD.
Real-world studies showing the association between cardiovascular events related to hormonal treatments and pre-existing metabolic, cardiovascular, or neurological conditions are limited [7, 10, 15–17]. Furthermore, because differential adverse effects of AA and ENZ and their interactions with patient comorbidities have not been fully elucidated, there is little guidance on how to choose these drugs based on pre-existing conditions [10].
This retrospective cohort study aims to investigate the likelihood of experiencing negative cardiovascular events during treatment with AA and ENZ. The study also intends to provide useful insights that can assist physicians in making informed decisions about patient care.
Patients and methods
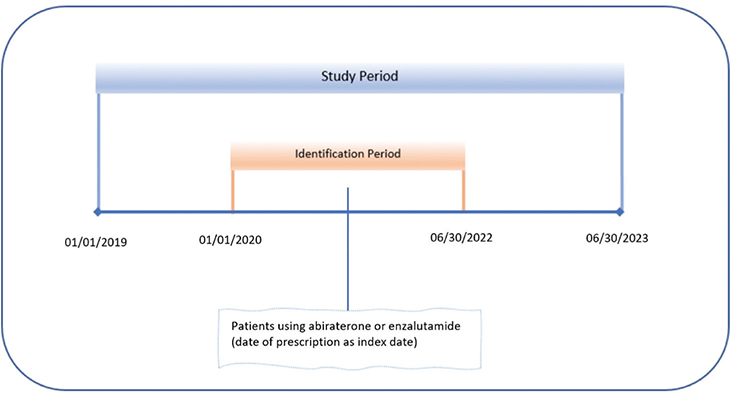
We conducted a retrospective cohort study using Kythera Medicare data from January 2019 to June 2023. Kythera Labs’ data contains medical and pharmacy claims with 79% coverage of all US patients. In addition to commercial and Medicaid plans, the data include both Medicare Fee-for-Service and managed-care patients. Out of 65 million Medicare enrolees, the data contains 58 million random patients. Overall, the data covers approximately 275 million patients, 3 million practitioners, 400,000 organisations, and 1.2 million facilities, generating 9.7 billion healthcare claims. Data include the unique de-identified numbers of patients, age, gender, types of insurance (fee-for-service vs. managed care), zip codes, diagnosis according to the International Classification of Diseases (ICD-10), Current Procedural Terminology (CPT) codes, and National Drug Codes (NDC) for medications. Since each patient is allocated, a unique identifier links all encounters, allowing for longitudinal analysis. The details of the data have been published elsewhere, and the healthcare outcomes derived from these data were compared with other data sets for their validity and consistency [18].
We identified patients with prostate cancer who had at least 1 pharmacy claim for AA or ENZ during the identification period. The first prescription claim date was considered the index date. Patients were required to be in the data at least 1 year pre- and post-index date. Prostate cancer was identified using the appropriate ICD-10 code (ICD 10 C61, Z85.46). Patients with at least 1 claim with a diagnosis of prostate cancer during the baseline are included in the study (Figure 1).
To identify new users, we excluded patients if they had a claim of AA or ENZ prior to the identification period. To distinguish new cases of CVD from ongoing episodes, we excluded patients with cardiovascular comorbidities prior to the index date. Patients with a claim for AA and ENZ at the same time on the index date were also excluded. We analysed a set of demographic and clinical variables at the baseline and during the follow-up period. Age was determined from the relevant file in the data at the index date.
We constructed a summary measure of socioeconomic status (SES) for each US zip code using data on income, education, and occupation from the 5-year estimates for 2021 US Census data [19]. Five-year estimates refer to statistical projections released by the Census Bureau every 5 years, covering various geographic, demographic, and socioeconomic factors. We then linked this information to the enrolees’ zip code of residence in the Kythera files. Previous research identified six variables by factor analysis of census block groups that could be meaningfully combined into a summary SES score. These variables include three measures of wealth/income (log of the median household income, log of the median value of housing units [20], and the percentage of households receiving interest, dividend, or net rental income [21], two measures of education (the percentage of adults ≥ 25 years of age who had completed high school or the percentage of adults ≥ 25 years of age who had completed college) [22], and one measure of occupation/employment (the percentage of employed persons ≥ 16 years of age in executive, managerial, or professional specialty occupations) [22]. The z score for each variable was calculated by subtracting the overall mean and dividing it by the standard deviation. The SES score was then constructed by summing the z scores for each of the six variables.
Additionally, different clinical measures were derived: updated Charlson Comorbidity Index (CCI), Chronic Disease Score (CDS), and Elixhauser Index. The original CCI encompassed 19 categories of identifiable medical conditions [23]. The original index, which has since adopted several weights, some of which allow outpatient diagnoses to contribute to the score, was translated to the updated ICD-10 codes [23]. The CDS is an aggregate comorbidity measure based on current medication use [24]. The score increases with the number of chronic diseases under treatment and the complexity of the treatment regimen. The Elixhauser Index is based on a comprehensive set of 30 ICD-9-CM comorbidity flags and has been updated to ICD-10 codes [25]. Current coding for the index is available from the Agency for Healthcare Research and Quality. It has been shown that using all these indexes as a proxy of disease severity improves the performance of the outcomes research models [26].
We identified prostate-specific comorbidities (depression, diabetes, gastric acid disorders, hyperlipidemia, osteoporosis, asthma, chronic obstructive pulmonary disease, Alzheimer’s disease, and chronic pain syndrome) [27] and baseline total healthcare cost to proxy for the severity of prostate cancer. The incidence of a cardiovascular event was defined as presence of hypertension, ischemic heart disease, myocardial infarction, heart failure, ventricular arrhythmias, cerebrovascular accidents, peripheral vascular disease, atrial fibrillation, paroxysmal tachycardia, cardiomyopathy, hypotension, pulmonary embolism, atherosclerosis, or aortic aneurysm during the year after initiation of index medication.
Baseline and outcome variables were analysed descriptively. Numbers and percentages were provided for categorical variables; means and standard deviations were provided for continuous variables. Student’s t-tests and Pearson chi-squared tests were used to test statistically significant differences at the 5% level for continuous and categorical variables, respectively. Standardised differences (STDs) were calculated to distinguish practical from statistical significance, and STDs with a value greater than 0.01 were considered significant.
To control the non-random assignment of patients, we constructed logistic regression models that predicted the likelihood of using each medication (the propensity score) and matched patients in each cohort by this score. We used as explanatory variables all demographics (age, SES), clinical characteristics (CCI, CDS, and Elixhauser Index scores), baseline prostate-specific comorbidities, and healthcare costs at baseline. McNemar’s test for categorical variables or a paired Student’s t-test for continuous variables was used to account for the dependence of matched pairs. All analyses were conducted using Pyspark and SparkR on Databricks.
Results
Among the initially identified 26,035 patients in the AA cohort and 24,921 patients in the ENZ cohort, a total of 8,929 patients met the criteria for AA treatment, resulting in a retention rate of approximately 34.28% from the initial cohort. Within the ENZ group, 8,624 patients qualified for treatment, with a retention rate of approximately 34.58% from the initial cohort. The mean patient age was 71.58 years in the AA group and 73.40 years in the ENZ group (p < 0.05). The analysis of comorbidity scores revealed notable differences between the groups. Specifically, both the CCI score (2.19 vs. 2.28), and the Elixhauser score (3.29 vs. 3.43) were significantly higher (p < 0.05) in the ENZ cohort, while the CDS showed no statistically significant difference.
Our results indicated that patients who reside in high-SES score regions were more likely to use AA than ENZ (35.39% vs. 30.61%, p < 0.05). On the contrary, patients who reside in low-SES score regions were more likely to use ENZ than AA (29.15% vs. 35.12%, p < 0.05).
Table 1 compares the baseline characteristics and prevalence of various prostate cancer–specific comorbidities in the treatment groups. Diabetes (17.38% vs. 23.12% was the most prevalent comorbidity among the ENZ group (p < 0.05) and hyperlipidemia (21.02% vs. 21.93%) was the most common comorbidity among the AA group (not significant). Additionally, depression (5.62% vs. 4.87%) and asthma (2.70% vs. 2.18%) were significantly more common in the AA group than in the ENZ cohort (p < 0.05). Although gastric acid disorders were more common in the AA cohort, and chronic obstructive pulmonary disease, osteoporosis, pain syndrome, and Alzheimer’s disease were more prevalent among the ENZ cohort, these differences were not statistically significant. Total baseline health expenditure, however, was slightly higher for the AA group ($25451.16 vs. $21540.64, p < 0.05).
Table 2 describes the incidence of cardiovascular events after initiation of AA or ENZ therapy. Statistically significant differences (p < 0.05) were observed in cerebral infarction (1.27% vs. 1.83%) and peripheral vascular diseases (1.72% vs. 2.60%). Importantly, paroxysmal tachycardia (0.07%) was found only in the AA cohort. The other cardiovascular events showed no significant differences. Heart failure, followed by atrial fibrillation were the most common cardiovascular events for both cohorts. Hypotension in the AA cohort and peripheral vascular diseases in ENZ cohort were the third most common cardiovascular event outcomes for each group.
There were 7,647 patients matched in each cohort after controlling for age, socio-demographics, and comorbidity factors. When patients were matched, hyperlipidemia (21.50% vs. 20.87%) was the most common comorbidity in both cohorts, followed by diabetes (19.68% vs. 19.72%); the differences were not significant. Propensity score matching created similar samples in terms of demographic and clinical factors. Total healthcare expenditure, however, were slightly higher for the AA group even after matching ($22934.78 vs. $21862.99, p < 0.05) (Table 3).
Table 4 demonstrates that, when demographic and clinical factors are controlled for at the baseline, there was no statistical difference in any cardiovascular event between the cohorts (15.54% vs. 14.83%). The most common cardiovascular event for both cohorts was heart failure (5.20% vs. 4.49%) followed by atrial fibrillation (4.42% vs. 3.60%), and hypotension (2.93% vs. 2.48%); again, the differences were not significant.
Discussion
Prostate cancer is the leading cause of cancer-related mortality, and its treatment often involves ADT such as AA and ENZ [3]. While these therapies have proven effective in managing mCRPC [3, 28] they come with a risk of adverse cardiovascular events [14, 28, 29]. Furthermore, the available literature on the treatment of metastatic prostate cancer with AA and ENZ predominantly comprises clinical trials, with a limited representation of real-world data. Clinical trials often exclude patients with significant comorbidities, posing a challenge in understanding the performance of these treatments in real-world scenarios. Our study showed that after controlling for age, SES, and sociodemographic and clinical factors, there was no significant difference in cardiovascular events between AA and ENZ, in agreement with the existing literature [15, 30, 31] Moreover, investigators such as George et al. have demonstrated that any difference in cardiovascular events for both AA and ENZ is minimal (hazard ratio, 1.23 vs. 1.10; p < 0.05) compared with the control [6].
Additionally, a comprehensive systematic review of real-world studies found few literature reviews on cardiovascular events [10]. Shah et al. found more pronounced cardiovascular events in patients using AA than ENZ therapy [10]; however, some of the articles identified included research populations with pre-existing cardiovascular conditions [10]. By contrast, we excluded patients with pre-existing CVD to distinguish new cases of CVD from ongoing episodes and to make a stronger correlation that the identified cardiovascular events were due to the adverse effects of the medication.
In our study, the median age for prostate cancer was 72 years old, and the most common prostate-specific comorbidity was diabetes for the ENZ group and hyperlipidemia for the AA group. When patients were matched, hyperlipidemia was the most common comorbidity in both cohorts, followed by diabetes. These results are consistent with previous literature [32–34]. Diabetes was found to be 28% higher in the ENZ cohort than in the AA cohort. Additionally, this supports our findings that the CCI and Elixhauser scores were higher in the ENZ group, indicating this cohort was sicker than the AA cohort.
Prostate cancer patients in higher-SES areas tended to use AA, whereas patients located in low-SES areas tended to use ENZ. The baseline health expenditure in the AA cohort was $1071.79 (p < 0.05) more than that in the ENZ cohort. Additional studies should be done to investigate the reason for these differences in SES and cost in depth, as they might be useful in understanding differences in patients’ prescribing patterns and help in better profiling the patients.
After controlling for age, sociodemographic characteristics, and clinical factors, we found that heart failure was the most common cardiovascular event, appearing at a 16% higher rate in the AA cohort than in the ENZ group. This aligns with previous studies that indicate the use of abiraterone was associated with a greater risk of cardiovascular-related hospitalisation compared to ENZ, for heart failure [16]. Furthermore, this aligns with prior research emphasising the need for vigilant monitoring of heart failure and CVD in these patients [35] Notably, only 4 patients in the AA cohort and no patients in the ENZ cohort had paroxysmal tachycardia as an adverse event. Moreover, compared with the ENZ cohort, nearly 23% more patients in the AA group developed atrial fibrillation. These findings have been discussed in previous literature where there is an elevated hazard ratio for arrhythmia-related hospitalisation in AA users than ENZ users [16]. This is further supported by an observational retrospective pharmacovigilance study, which found that AA was linked to a notably higher frequency of atrial tachyarrhythmia and heart failure compared with ENZ and other ADTs, likely due to AA’s tendency to induce hypermineralocorticism [16]. The increased risk of cardiovascular events associated with AA has been linked to its mineralocorticoid excess; to mitigate this adverse effect, it is indicated to prescribe it in combination with prednisone [16]. Therefore, patients predisposed to complications from heart failure, fluid overload, and arrhythmias should use AA with caution.
Although our study aligns with the individual cardiovascular findings for AA users, it is important to note that these findings were not statistically significant when controlled for sociodemographic and clinical factors. Furthermore, studies mentioned before like Hu, J. et al. comparative study between AA and ENZ included patients with pre-existing CVD which is different from our study population that excluded patients with pre-existing CVD and might account for the difference in their results with ours.
Notably, our study found hypotension as the third most common cardiovascular event in the AA group. This finding differentiates with preexisting literature that associates hypertension with AA and ENZ [7, 10, 36, 37]. A possible reason for this might be that hypertension was diagnosed using the ICD-10 code I15.9, which is used for secondary hypertension and is the most appropriate code for this scenario. Secondary hypertension is a type of hypertension that, by definition, is caused by an identifiable underlying primary cause (in this case, the use of medication AA and ENZ). However, primary hypertension accounts for 90%–95% of hypertension cases among adults, therefore it is prone to be coded more frequently in claims studies with its corresponding ICD-10 code, I10 [38]; this may account for the underdiagnosis of hypertension in our study [39]. Most frequent code used in claims studies is I10; therefore, we believe this might account for the underdiagnosis of hypertension in our study.
On the other hand, based on the pharmacovigilance study, patients who were administered ENZ had a lower incidence of cardiac events than those who were given AA [35]. While ENZ has been linked to hypertension [15], ischemic heart disease, and atrial fibrillation [40], our study specifically found a higher prevalence of peripheral vascular diseases and cerebrovascular disease among ENZ users. This aligns with previous research that indicates a greater association among ENZ users with peripheral vascular disease [10, 16]. Gupta et al. discuss how a few ADT studies have demonstrated to be associated with nonfatal cardiovascular events such as peripheral artery disease [14, 41, 42]. Although they do not establish whether peripheral artery disease is more common with ENZ, they state that there is an increased risk associated with ADT [14]. Additionally, although several meta-analyses have reported an increased risk of stroke in men with prostate cancer treated with ADT, they do not differentiate between AA and ENZ [14]. However, Jason Hu et al.’s study did suggest that ENZ users may be at greater risk of cerebrovascular stroke compared to ABI users, although this was also not statistically significant [16] This finding was very similar to our results. Furthermore, ENZ is known for its capacity to cross the blood-brain barrier and has also been associated with a multitude of adverse events relating to the CNS [16, 43]. It is important to mention, that several studies have mentioned the effect of ENZ in the CNS like seizures, fatigue, reduced cognitive function [44]; but few have looked at the effect on cerebrovascular disease. A call for further research on this is warranted.
Limitations
This study has several limitations related to the use of administrative data sets, which may be subject to inaccurate coding of patient clinical diagnoses and procedures, as well as clinical information limited to conditions and treatments defined by ICD-10-CM codes and NDC codes. Since the analysis was done on the review of claims data that were not originally designed for research, some information is bound to be missing. Firstly, as with most claims-based data sources, there is a time lag between an individual’s receipt of services and when the files become available for research. The data may not be generalisable to the entire population, as some information may be missed in processing or reimbursement. Additionally, not all health data are captured in the claims. Both AA and ENZ have been proven to increase the survival of patients with metastatic hormone-sensitive disease naive to hormonal agents (i.e. a patient who has never used ADT therapy before); therefore, we focussed on patients who initiated treatment with these drugs to capture these populations [15]. We do recognise this can be a selection bias as we did not control for patients who might have used previous ADT therapy, which can be a limitation of our study.
It is important to note that although these medications might have had an effect on the incidence of CVD, we did control for this by excluding any patient who had previous CVD in the baseline period.
Findings of higher diabetes incidence in the ENZ cohort vs. the AA cohort could have been influenced by selection bias and should be interpreted with caution. Further, the absence of substantial differences between these drugs with regard to cardiovascular adverse events could be accounted for by improved treatment selection by physicians based on patient comorbidities which should be considered alongside these results.
Moreover, our study has potential limitations related to the use of area-based socioeconomic measures. Ideally, we would have been able to evaluate both individual and area-based socioeconomic factors, as relying solely on area-based measures can lead to misclassification of individuals across different socioeconomic strata within the same area. This misclassification occurs randomly, and its bias direction is known. If socioeconomic disparities exist within specific geographic regions, incorporating individual-level socioeconomic data would likely provide a more comprehensive understanding. Nonetheless, area-based measures offer valuable contextual information, taking into account various factors affecting all residents within an area, such as geographical location and the quality of public amenities such as hospitals. Our estimates would have been more precise if we had had access to more detailed individual-level socioeconomic data, allowing for a more nuanced examination of socioeconomic disparities.
Conclusion
This study provides real-world evidence of the cardiovascular risk of prostate cancer patients treated with AA and ENZ that may not be apparent in clinical trial settings. After adjusting for prostate-specific comorbidities, age, SES, the likelihood of a cardiovascular event did not differ between AA and ENZ users. Our research may be of interest to clinicians treating patients with mCRPC, due to the high incidence of CVD in this population. This analysis adds to the discussion regarding the differences observed between AA and ENZ in terms of treatment-associated cardiovascular events and may aid clinicians when making informed treatment decisions in prescribing AA or ENZ.
Acknowledgement
The authors thank A.E. and M.M. for their assistance in editing the manuscript.
Author contributions
O.B. provided the supervision, conceptualisation, methodology, validation, and visualisation of the research, and participated in the writing process from the original draft preparation to the reviewing and editing of the manuscript. G.S. participated in the project management, supervision, methodology, analysis, investigation of the literature review, and in the writing process from the original draft preparation to the reviewing and editing of the manuscript. A.D. participated in the methodology, analysis, investigation of the literature review, and in the writing process from the original draft preparation to the reviewing and editing of the manuscript. S.A. participated in the investigation of the literature review and in the writing process from the original draft preparation to the reviewing and editing of the manuscript. B.C. participated in the investigation of the data, methodology, software, validation, analysis, and data curation. E.K. participated in the investigation of the data, methodology, software, validation, analysis, and data curation.
Ethics declarations
All data are de-identified and used in full compliance with the Health Insurance Portability and Accountability Act (HIPAA). The study was conducted under the research provisions of Privacy Rule 45 CFR 164.514(e) and was exempt from Institutional Review Board review as per 45 CFR §46.101(b)(4). Informed consent was not required as the data were from an anonymous, de-identified database.
Data availability
Data are not publicly available due to privacy and ethical restrictions.
References
[1] Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10(2):63–89. https://doi.org/10.14740/wjon1191
[2] Hung A, Candelieri D, Li Y, et al. Tumor testing and treatment patterns in veterans with metastatic castration-resistant prostate cancer. Semin Oncol. 2023;50(1–2):11–24. https://doi.org/10.1053/j.seminoncol.2023.03.001
[3] Zhang W, Wu T-Y, Chen Q, et al. Indirect comparison between abiraterone acetate and enzalutamide for the treatment of metastatic castration-resistant prostate cancer: a systematic review. Asian J Androl. 2017;19(2):196. https://doi.org/10.4103/1008-682X.178483
[4] Freedland SJ, Davis M, Epstein AJ, Arondekar B, Ivanova JI. Real-world treatment patterns and overall survival among men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) in the US Medicare population. Prostate Cancer Prostatic Dis. 2023: 1–7 https://doi.org/10.1038/s41391-023-00725-8
[5] Halwani AS, Rasmussen KM, Patil V, et al., editors. Real-world practice patterns in veterans with metastatic castration-resistant prostate cancer. Urol Oncol Semin Original Investig. 2020; 38(1):1.e1–1.e10. https://doi.org/10.1016/j.urolonc.2019.09.027
[6] George DJ, Sartor O, Miller K, et al. Treatment patterns and outcomes in patients with metastatic castration-resistant prostate cancer in a real-world clinical practice setting in the United States. Clin Genitourinary Cancer. 2020;18(4):284–94. https://doi.org/10.1016/j.clgc.2019.12.019
[7] Serrano Domingo JJ, Alonso Gordoa T, Lorca Álvaro J, et al. The effect of medical and urologic disorders on the survival of patients with metastatic castration resistant prostate cancer treated with abiraterone or enzalutamide. Therap Adv Urol. 2021;13:17562872211043341. https://doi.org/10.1177/17562872211043341
[8] Recine F, Sternberg CN. Hormonal therapy and chemotherapy in hormone-naive and castration resistant prostate cancer. Transl Androl Urol. 2015;4(3):355–64. https://doi.org/10.3978/j.issn.2223-4683.2015.04.11
[9] American Urological Association. Advanced prostate cancer: AUA/SUO guideline 2023 [Internet]. [cited 15-12-2023]. Available from: https://www.auanet.org/guidelines-and-quality/guidelines/advanced-prostate-cancer
[10] Shah YB, Shaver AL, Beiriger J, et al. Outcomes following abiraterone versus enzalutamide for prostate cancer: a scoping review. Cancers (Basel). 2022;14(15):3773. https://doi.org/10.3390/cancers14153773
[11] Ingrosso G, Detti B, Scartoni D, et al., editors. Current therapeutic options in metastatic castration-resistant prostate cancer. Semin Oncol. 2018;45(5–6):303–15. https://doi.org/10.1053/j.seminoncol.2018.10.001
[12] Kakarla M, Ausaja Gambo M, Yousri Salama M, et al. Cardiovascular effects of androgen deprivation therapy in prostate cancer patients: a systematic review. Cureus. 2022;14(6):e26209. https://doi.org/10.7759/cureus.26209
[13] Ye Y, Zheng Y, Miao Q, Ruan H, Zhang X. Causes of death among prostate cancer patients gaed 40 years and older in the United States. Front Oncol. 2022;12:914875. https://doi.org/10.3389/fonc.2022.914875
[14] Gupta D, Lee Chuy K, Yang JC, Bates M, Lombardo M, Steingart RM. Cardiovascular and metabolic effects of androgen-deprivation therapy for prostate cancer. J Oncol Pract. 2018;14(10):580–7. https://doi.org/10.1200/JOP.18.00178
[15] Iacovelli R, Ciccarese C, Bria E, et al. The cardiovascular toxicity of abiraterone and enzalutamide in prostate cancer. Clin Genitourinary Cancer. 2018;16(3):e645–53. https://doi.org/10.1016/j.clgc.2017.12.007
[16] Hu J, Aprikian AG, Vanhuyse M, Dragomir A. Comparative cardiovascular safety of novel hormonal agents in metastatic castration-resistant prostate cancer using real-world sata. Clin Genitourin Cancer. 2022;20(1):17–24. https://doi.org/10.1016/j.clgc.2021.08.009
[17] Schoen MW, Carson KR, Eisen SA, et al. Survival of veterans treated with enzalutamide and abiraterone for metastatic castrate resistant prostate cancer based on comorbid diseases. Prostate Cancer Prostatic Dis. 2023;26(4):743–50. https://doi.org/10.1038/s41391-022-00588-5
[18] Baser O, Samayoa G, Yapar N, Baser E, Mete F. Use of open claims vs closed claims in health outcomes research. J Health Econ Outcomes Res. 2023;10(2):44–52. https://doi.org/10.36469/jheor.2023.87538
[19] United States Census Bureau [Internet]. [9-12-2023]. Available from: https://www.census.gov/.
[20] United States Census Bureau. Selected housing characteristics [Internet]. [9-12-2023]. Available from: https://data.census.gov/table?q=housing+value&g=010XX00US,$8600000&tid=ACSDP5Y2021.DP04
[21] United States Census Bureau. Interest, dividends, or net rental income in the past 12 months for households [Internet]. [9-12-2023]. Available from: https://data.census.gov/table?q=interest,+dividend,+or+rental+income+in+household&g=010XX00US,$8600000&tid=ACSDT5Y2021.B19054
[22] United States Census Bureau. Selected population profile in the United States [Internet]. [9-12-2023]. Available from: https://data.census.gov/table?q=occupations+&t=Educational+Attainment&g=010XX00US,$8600000&tid=ACSSPP1Y2021.S0201
[23] Glasheen WP, Cordier T, Gumpina R, Haugh G, Davis J, Renda A. Charlson comorbidity index: ICD-9 update and ICD-10 translation. Am Health Drug Benefits. 2019;12(4):188–97.
[24] Putnam KG, Buist DS, Fishman P, et al. Chronic disease score as a predictor of hospitalization. Epidemiology. 2002;13(3):340346. https://doi.org/10.1097/00001648-200205000-00016
[25] Mehta HB, Sura SD, Adhikari D, et al. Adapting the Elixhauser comorbidity index for cancer patients. Cancer. 2018;124(9):2018–25. https://doi.org/10.1002/cncr.31269
[26] Baser O, Palmer L, Stephenson J. The estimation power of alternative comorbidity indices. Value Health. 2008;11(5):946–55. Epub 20080516. doi: 10.1111/j.1524-4733.2008.00343.x. PubMed PMID: 18489502.
[27] Ng HS, Koczwara B, Roder D, Vitry A. Development of comorbidities in men with prostate cancer treated with androgen deprivation therapy: an Australian population-based cohort study. Prostate Cancer Prostatic Dis. 2018;21(3):403–10. https://doi.org/10.1038/s41391-018-0036-y
[28] Henríquez I, Roach M 3rd, Morgan TM, et al. Current and emerging therapies for metastatic castration-resistant prostate cancer (mCRPC). Biomedicines. 2021;9(9):1247. https://doi.org/10.3390/biomedicines9091247
[29] Fradin J, Kim FJ, Lu-Yao GL, Storozynsky E, Kelly WK. Review of cardiovascular risk of androgen deprivation therapy and the influence of race in men with prostate cancer. Cancers (Basel). 2023;15(8). https://doi.org/10.3390/cancers15082316
[30] Cao B, Kim M, Reizine NM, Moreira DM. Adverse events and androgen receptor signaling inhibitors in the treatment of prostate cancer: a systematic review and multivariate network meta-analysis. Eur Urol Oncol. 2023;6(3):237–50. https://doi.org/10.1016/j.euo.2023.01.001
[31] Conover MM, Weaver J, Fan B, et al. Cardiovascular outcomes among patients with castration-resistant prostate cancer: a comparative safety study using US administrative claims data. Prostate. 2023;83(7):729–39. https://doi.org/10.1002/pros.24510
[32] Jefferson M, Drake RR, Lilly M, Savage SJ, Tucker Price S, Hughes Halbert C. Co-morbidities in a retrospective cohort of prostate cancer patients. Ethn Dis. 2020;30(Suppl 1):185–92. https://doi.org/10.18865/ed.30.S1.185
[33] Su YL, Chou CL, Rau KM, Lee CT. Asthma and risk of prostate cancer: a population-based case-cohort Study in Taiwan. Medicine (Baltimore). 2015;94(36):e1371. https://doi.org/10.1097/MD.0000000000001371
[34] Chhatre S, Gallo JJ, Guzzo T, et al. Trajectory of depression among prostate cancer patients: a secondary analysis of a randomized controlled trial. Cancers (Basel). 2023;15(7). https://doi.org/10.3390/cancers15072124
[35] Cone EB, Reese S, Marchese M, et al. Cardiovascular toxicities associated with abiraterone compared to enzalutamide – a pharmacovigilance study. EClinicalMedicine. 2021;36:100887. https://doi.org/10.1016/j.eclinm.2021.100887
[36] Scailteux LM, Despas F, Balusson F, et al. Hospitalization for adverse events under abiraterone or enzalutamide exposure in real-world setting: A French population-based study on prostate cancer patients. Br J Clin Pharmacol. 2022;88(1):336–46. https://doi.org/10.1111/bcp.14972
[37] Lee HY, Chen HL, Teoh JY, et al. Abiraterone and enzalutamide had different adverse effects on the cardiovascular system: a systematic review with pairwise and network meta-analyses. Prostate Cancer Prostatic Dis. 2021;24(1):244–52. https://doi.org/10.1038/s41391-020-00275-3
[38] Sidelnikov E, Dornstauder E, Jacob C, et al. Healthcare resource utilization and costs of cardiovascular events in patients with atherosclerotic cardiovascular disease in Germany – results of a claims database study. J Med Econ. 2022;25(1):1199–206. https://doi.org/10.1080/13696998.2022.2141964
[39] Alexander MR. 2022 [Internet] [21-12-2023]. Available from: https://emedicine.medscape.com/article/241381-overview#:~:text=and%20endocrine%20causes.-,Primary%
20or%20essential%20hypertension%20accounts%20for%2090%2D95%25%20of%20adult,inadequate%20
medication%20or%20poor%20compliance
[40] Bishesh Shrestha SGaASN. Cardiovascular adverse events associated with androgen receptor-targeted therapy used in the treatment of prostate cancer. Circulation. 2020;142:A16180. https://doi.org/10.1161/circ.142.suppl_3.16180
[41] Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121(6):833–40. https://doi.org/10.1161/CIRCULATIONAHA.109.192695
[42] O’Farrell S, Garmo H, Holmberg L, Adolfsson J, Stattin P, Van Hemelrijck M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33(11):1243–51. https://doi.org/10.1200/JCO.2014.59.1792
[43] Ryan C, Wefel JS, Morgans AK. A review of prostate cancer treatment impact on the CNS and cognitive function. Prostate Cancer Prostatic Dis. 2020;23(2):207–19. https://doi.org/10.1038/s41391-019-0195-5
[44] Pilon D, Behl AS, Ellis LA, Robitaille MN, Lefebvre P, Dawson NA. Assessment of real-world central nervous system events in patients with advanced prostate cancer using abiraterone acetate, bicalutamide, enzalutamide, or chemotherapy. Am Health Drug Benefits. 2017;10(3):143–53.