ORIGINAL ARTICLE
Progression-free survival after front line, second line and third line in patients with follicular lymphoma treated in clinical practice
Aino Rajamäkia+, Marc Sorigueb+, Roosa E.I. Prusilac, Milla E.L. Kuusistod, Hanne Kuitunene, Esa Jantunena,f, Santiago Mercadalg, Taina Turpeenniemi-Hujanenh, Juan-Manuel Sanchoi, Kaisa Sunelaj* and Outi Kuittinena,h,k*
aInstitute of Clinical Medicine, Faculty of Health Sciences, University of Eastern Finland, Kuopio, Finland; bMedical Department, Trialing Health, Barcelona, Spain; cDepartment of Pediatrics, Kuopio University Hospital, Kuopio, Finland; dDepartment of Internal Medicine, Länsi-Pohja Central Hospital, Kemi, Finland; eDepartment of Oncology, Oulu University Hospital, Oulu, Finland; fDepartment of Medicine, Kuopio University Hospital, Kuopio, Finland; gICO-Hospital Duran I Reynals, L’Hospitalet, Spain; hMedical Research Center, Oulu University Hospital and Translational Medicine Research Unit, University of Oulu, Oulu, Finland;iDepartment of Hematology, ICO-Hospital Germans Trias i Pujol, IJC, UAB, Badalona, Barcelona, Spain; jFinnish Medicines Agency FIMEA, Barcelona, Spain; kDepartment of Oncology, Kuopio University Hospital, Kuopio, Finland
ABSTRACT
Background: The modern-day therapeutic landscape for follicular lymphoma (FL) includes a number of highly effective therapies.
Patients and methods: We set out to determine progression-free survival (PFS) after front line, second line, and third line of therapy on the basis of relevant biological characteristics and therapeutic choices. Patients (n = 743, 51% females, median 60 years old) diagnosed with grade 1–2 FL between 1997 and 2016 in nine institutions were included.
Results: The median PFS1, PFS2, and PFS3 were 8.1 years (95% confidence interval [CI]: 7–9.3 years), 4.2 years (95% CI: 2.8–5.6 years) and 2.2 years (95% CI 1.7–2.8 years). We found longer PFS1 for (1) females, (2) younger age, (3) lower-risk follicular lymphoma international prognostic index (FLIPI), (4) standard intensity (over low intensity) regimens and (5) immunochemotherapy strategies and (6) maintenance rituximab. We found a shorter PFS2 for patients who received front-line immunochemotherapy. Older age at diagnosis correlated with a shorter PFS3. Intensity of front-line chemotherapy, maintenance, or POD24 status did not correlate with PFS2 or PFS3 in this dataset.
Interpretation: With current immunochemotherapy strategies, the natural course of FL is characterized by shorter-lasting remissions after each relapse. It will be interesting to see whether new therapies can alter this pattern.
KEYWORDS: Follicular lymphoma; survival; progression-free survival; treatment
Citation: ACTA ONCOLOGICA 2024, VOL. 63, 267–272. https://doi.org/10.2340/1651-226X.2024.24377.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Oncologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 26 October 2023; Accepted: 13 March 2024; Published: 6 May 2024
CONTACT Marc Sorigue mc.sorigue@zoho.comm Medical Department, Trialing Health, Barcelona, Spain
Supplemental data for this article can be accessed online at https://doi.org/10.2340/1651-226X.2024.24377
+Co-first authors.
*Co-senior authors.
Competing interests and funding: JMS declares Honoraria as a speaker in medical education meetings from: Roche, Janssen, Bristol Myers Squibb, Celgene, Gilead-Kite, Novartis, Incyte, Takeda, Servier, Kern-Pharma, Mundipharma. Honoraria as a participant in advisory boards or consultancy from: Roche, Janssen, Bristol Myers Squibb, Celgene, Gilead-Kite, Novartis, Incyte, Kern-Pharma, Lilly, Beigene, Celltrion, Sandoz, Miltenyi Biomedicine; MELK declares Honoraria as speaker in medical education meeting from Janssen.
None.
Introduction
Follicular lymphoma (FL) is the most common indolent lymphoma, characterized by translocation t(14;18)(q21;q32) and mutations in epigenetic regulators [1]. It most often presents as asymptomatic lymphadenopathy and in patients with a median age of around 60. The modern-day therapeutic landscape includes a number of highly effective strategies, leading to a median overall survival exceeding 20 years [2, 3]. Therefore, FL must be strategically managed, considering not only short-term efficacy and toxicity but also optimal treatment sequencing, long-term toxicities, quality of life, as well as patients’ preferences and values. We aimed to determine progression-free survival (PFS) after front-line, second-line, and third-line therapy based on relevant biological characteristics of the lymphoma, as well as the choice of front-line therapy, with the goal of further characterizing the expected response to second- and third-line therapy for these patients.
Patients and methods
Patients diagnosed with grade 1–2 FL between 1997 and 2016 in nine institutions (seven Finnish [including four university hospitals and three central hospitals] and two Spanish university hospitals) were included [4]. Supplementary Figure 1 shows their inclusion by calendar year. Patients were treated based on the clinical guidelines in place at the time [5–7] with the ultimate decision made by agreement between patient and physician, according to standard practice. In-depth characterization of this cohort has been published earlier [4, 8]. All patients with FL were considered for inclusion. The following patients were excluded: (1) those with grade 3 FL. This was done for two reasons. Firstly, because the subtype (3a vs. 3b) was not reported for all patients in our dataset. Secondly, because despite the general idea that grade 1–2 FL and grade 3a FL should be treated similarly, some physicians may approach them differently (i.e. generally favor anthracycline-based front-line and avoid bendamustine- or lenalidomide-based regimens for grade 3a FL [9, 10]). (2) Patients with composite lymphoma at diagnosis and histological transformation before front-line therapy. Those with documented histological transformations during follow-up (n = 10, out of 105 patients with a biopsy at the time of relapse; 62/282 (22%) in first relapse, 39/111 (35%) in second relapse, and 4/41 (12%) in third relapse) were not excluded. 3) Patients who underwent watchful waiting and were never actively treated. For those who underwent watchful waiting and were subsequently treated, the first active therapy was considered the first line (and thus included in the PFS1 analysis). This study was conducted according to the declaration of Helsinki, was approved by the review board of the Northern Ostrobothnia Hospital District, and is reported according to the STROBE statement for observational studies [11].
Median and interquartile range and proportions and percentages are given for quantitative and qualitative variables, respectively. For this analysis centering of treatment effectiveness, PFS1 was calculated from the date of front-line treatment start until the date of relapse/progression (detected either due to patient symptoms or on routine scans) or death. PFS2 and PFS3 were calculated from the start of second-line and third-line treatment, respectively, until the time of progression or relapse after those treatment lines or death. For all endpoints, the patients were censored at the time of the last follow-up if relapse, progression, or death had not occurred.
The Kaplan Meyer method was used to draw survival curves, and the log-rank test was used to compare them. Both SPSS and R software (ggplot2, survival, survminer packages) were used for the current study. A formal sample size calculation was not undertaken; rather, all patients diagnosed within the predefined time period and meeting inclusion criteria were included in the study.
Results
Seven-hundred and forty-three patients were included. The median age was 60, with 51% being female, and 40% had high-risk FLIPI scores (Supplementary Tables 1 and 2). Table 1 details the treatments given, and Figure 1 shows the PFS after front-line, second-line, and third-line treatment, which were a median of 8.1 years (95% confidence interval [CI]: 7–9.3), 4.2 years (2.8–5.6) and 2.2 years (1.7–2.8), respectively.
| Characteristic | Front-line | Second-line | Third-line |
| Patients treated, n (%) | 697 (94) | 231 (31) | 72 (10) |
| Immunochemotherapy | |||
| Any, n (%) | 493 (71) | 130 (56) | 44 (61) |
| Anthracycline-based, n | 350 | 47 | 3 |
| Bendamustine, n | 60 | 40 | 15 |
| Fludarabine-based, n | 10 | 10 | 2 |
| Platinum-based, n | - | 4 | 9 |
| Low intensity regimensa, n | 58 | 22 | 10 |
| Otherb, n | 15 | 7 | 5 |
| Chemotherapy without rituximab, n (%) | |||
| Any, n (%) | 69 (10) | 52 (23) | 16 (22) |
| Anthracycline-based, n | 25 | 10 | - |
| Bendamustine, n | 1 | 4 | 1 |
| Fludarabine-based, n | 11 | 17 | 1 |
| Platinum-based regimen, n | - | 6 | - |
| Low intensity regimensa, n | 30 | 12 | 4 |
| Otherb, n | 2 | 3 | 10 |
| Rituximab-monotherapy, n (%) | 28 (4) | 14 (6) | 4 (6) |
| Radiation therapy only, n (%) | 91 (13) | 34 (15) | 7 (10) |
| Surgical removal only, n (%) | 14 (2) | 1 (0.4) | - |
| Stem-cell transplantation consolidation, n (%) | 44 (6) | 31 (13) | 7 (10) |
| Rituximab maintenance (after immunochemotherapy), n (%) | 207 (42)c | 47 (36)c | 11 (25)c |
| Median follow-up, years (95% CI) | 6.4 (6.1–6.9) | 5.6 (4.9–5.6) | 3.4 (2.4–6.3) |
| aAlkylator-based treatments (cyclophosphamide alone, in combination with prednisone, or with vincristine and prednisone [CVP], chlorambucil) or gemcitabine. | |||
| bOther therapies include in front-line: radioimmunotherapy (90Y-ibritumomab tiuxetan), and bortezomib; in second line: MINE/MIME (mesna, ifosfamide, mitoxantrone/methothrexate, and etoposide/mitoguazone, ifosfamide, methotrexate, etoposide), and other heterogeneous chemo-regimen; in third line MINE/MIME, radioimmunotherapy, idealisib, copanlisib, bortezomib, ibrutinib and other heterogeneous chemo-regimen. | |||
| cPercentage of patients treated with induction immunochemotherapy. | |||
| NB: Front-line treatment information was missing for two patients; CI: confidence interval. | |||
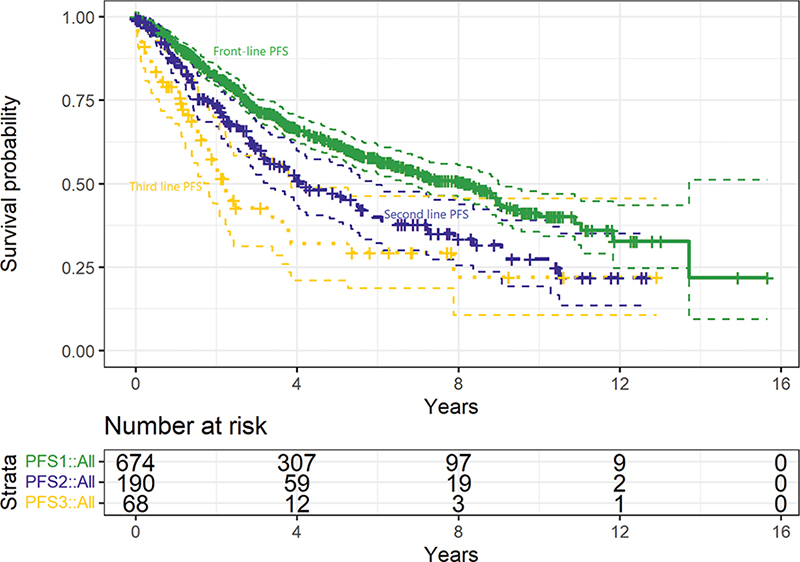
Figure 1. Progression-free survival after front-line (solid line, green, median 8.1 years, 95% CI: 7–9.3 years), second-line (dashed line, purple, median 4.2 years, 95% CI: 2.8–5.6 years), and third-line (dotted line, yellow, median 2.2 years, 95% CI: 1.7–2.8 years) treatment in patients with grade 1–2 FL.
PFS subanalyses can be found in Table 2. Regarding PFS1, we highlight a longer PFS1 for females compared to males, a shorter PFS1 for higher-risk FLIPI categories, a predictable longer PFS1 for standard intensity regimens, for younger age, for immunochemotherapy (over non-immunochemotherapy) and for maintenance rituximab. Regarding PFS2, we find a shorter PFS2 for front-line immunochemotherapy (rather than non-immunochemotherapy) strategies.
| Characteristics | N | PFS1 | p | N | PFS2 | p | N | PFS3 | p | |
| Gender | Female | 333 | 9.0 (7.2–10.8) | 0.004 | 83 | 4.9 (2.6–7.3) | 0.980 | 28 | 2.3 (0.7–4.0) | 0.662 |
| Male | 328 | 6.0 (4.7–7.3) | 99 | 4.0 (2.4–5.5) | 40 | 2.1 (1.2–3.0) | ||||
| Age | <60 | 329 | 7.1 (5.5–8.7) | 0.043 | 108 | 5.1 (3.8–6.4) | 0.224 | 43 | 3.6 (1.0–6.3) | 0.010 |
| 60–69 | 204 | 9.0 (6.7–11.3) | 43 | 3.2 (1.4–4.9) | 16 | 2.2 (1.2–3.3) | ||||
| >70 | 125 | 6.4 (5.0–7.8) | 31 | 2.8 (0.5–5.0) | 9 | 0.6 (0.1–1.0) | ||||
| FLIPI score | 0–1 | 209 | NR (5-year 65% [69–73]) | 0.002 | 53 | 5.1 (3.0–7.3) | 0.107 | 19 | 2.3 (0.7–4.0) | 0.678 |
| 2 | 174 | 8.8 | 37 | 3.2 (2.5–3.8) | 16 | 3.8 (0.3–7.3) | ||||
| 3–5 | 214 | 6.7 (4.5–8.8) | 62 | 3.1 (2.5–3.8) | 23 | 1.7 (0.6–2.1) | ||||
| Front-line induction backbone (Sup. Figure 2) | Anthracycline | 363 | 8.8 (7.5–10.1) | <0.001 | 82 | 4.0 (2.9–5.1) | 0.495 | 34 | 2.0 (1.2–2.8) | 0.985 |
| Bendamustine/fludarabine | 80 | NR (5-year 72% [61–85]) | 10 | 3.9 (0–10.0) | 4 | 0.6 | ||||
| Low intensity regimensa | 111 | 4.2 (3.0–5.5) | 48 | 3.2 (1.8–4.6) | 22 | 2.2 (1.8–2.7) | ||||
| Front-line induction strategy (Sup. Figure 3) | Immunochemot. | 480 | 8.8 (7.4–10.2) | <0.001 | 101 | 3.1 (2.3–3.9) | 0.009 | 41 | 2.0 (1.5–2.5) | 0.581 |
| No immunochemot. | 182 | 5.3 (3.4–7.3) | 81 | 6.1 (3.3–9.0) | 27 | 3.4 (1.0–5.8) | ||||
| Rituximab maintenance after front-line ICT | Yes | 193 | NR (5-year 74% [67–82]) | <0.001 | 28 | 3.0 (2.7–3.2) | 0.589 | 9 | NR (5-year 59% [32–100]) | 0.247 |
| No | 287 | 7.1 (5.1–9.1) | 73 | 3.5 (2.2–4.7) | 32 | 1.9 (1.6–2.2) | ||||
| Front-line response duration | POD24 | 111 | NA | NA | 66b | 3.3 (0.9–5.7) | 0.463 | 29b | 2.2 (0.5–3.9) | 0.581 |
| non-POD24 | 486 | NA | 113b | 4.8 (3.5–6.2) | 37b | 2.2 (1.2–3.3) | ||||
| PFS: Progression-free survival; NR: not reached; FLIPI: Follicular Lymphoma International Prognostic Index; ICT: immunochemotherapy; POD24: progression of disease within 24 months of front-line therapy; NA: Not applicable. | ||||||||||
| Data are median (95% confidence interval) and reported in years. Only patients who received treatment are included in these analyses. | ||||||||||
| aAlkylator-based regimens (CVP, RCVP), alkylator monotherapy (cyclophosphamide or chlorambucil) or rituximab monotherapy. Within group survival estimates are in Supplementary Table 2. | ||||||||||
| bSecond-line treatments for non-POD24 patients included: bendamustine-based (n = 34/113, 30%), anthracycline-based (n = 25, 22%), alkylator-based (n = 12, 11%), fludarabine-based (n = 1, 1%), or platinum-based therapy (n = 1, 1%), rituximab monotherapy (n = 19, 17%), radiotherapy (n = 20, 18%), or other (n = 1, 1%). For POD24 patients they included: bendamustine-based (n = 9/66, 14%), anthracycline-based (n = 14, 21%), alkylator-based (n = 7, 11%), fludarabine-based (n = 11, 17%), or platinum-based therapy (n = 7, 11%), rituximab monotherapy (n = 5, 8%), radiotherapy (n = 9, 14%), or other (n = 4, 6%). Stem cell transplant consolidation after second-line induction in 21/113 (19%) patients in the non-POD24 group and 10/66 (15%) in the POD24 group. | ||||||||||
| NB: Bold values indicate statistical significance | ||||||||||
Discussion
In this study of patients with grade 1–2 FL, we: (1) confirmed decreasing PFS after each relapse, (2) confirmed different PFS1 associated with a number of biological and treatment-related variables, (3) found a longer PFS2 for patients treated in the front-line without immunochemotherapy compared to those treated with immunochemotherapy and (4) found either no association or a loose association between PFS2 or PFS3 and biological and treatment-related variables and response to the front line.
We found that PFS in FL decreases after each line of therapy, in line with previous data [12–15]. This is a pattern globally observed in all malignancies and appears to be secondary to clonal selection, as malignant cells are exposed to anticancer agents [16].
Overall, PFS seems satisfactory in the early lines of therapy and translates into prolonged overall survival (OS) – 72.4% at 10 years in this same cohort [4] – but drops substantially particularly after the second line. Indeed, PFS3 is barely over 2 years, despite frequent use of immunochemotherapy, highlighting the need for new strategies. Fortunately, strategies that were not available at the time of this study – patients were diagnosed in 2016 at the latest, to ensure a long enough follow-up – are currently available, including rituximab-lenalidomide, anti-CD3xCD20 bispecific antibodies, or CAR T-cell therapy [2].
We also report several correlations between PFS1 and patient variables (gender and age), disease-related variables (FLIPI score), treatment selection, and length of remission (POD24). The adverse impact on PFS1 of high-risk FLIPI scores, age, use of low-intensity or non-immunotherapy-containing regimens, a no-maintenance strategy, or POD24 are consistent in published data [17]. The correlation between sex and outcome is more controversial and inconsistent [18–23]. It is possible that male sex has a small negative association with prognosis that reaches significance only in some, but not all, studies, consistent with what has been documented in diffuse large B-cell lymphoma [24, 25].
Perhaps the most novel and interesting datapoints of this analysis are the associations between baseline patient-, disease-, or treatment-related variables and PFS2 and PFS3. Most baseline variables or responses to front-line treatment did not have a strong correlation with PFS2 or PFS3. The one exception is that using non-immunochemotherapy strategies in the front line was associated with a notably longer PFS2 than using immunochemotherapy strategies in the front line. This fits well within the framework of the clonal selection model [16], as these patients can receive in the second-line therapeutic agent/s of a class that they have not previously been exposed to – be it immunotherapy, be it chemotherapy – , unlike those treated in the front line with immunochemotherapy. These results seem to indicate that a shorter PFS1 in the front line can be offset by subsequent lines of therapy, partly challenging the extended notion that obtaining the longer possible front-line PFS1 is essential [26, 27]. This notion is also supported by the long-term survival evidence from patients treated with rituximab monotherapy in the front line [28] or with a no-maintenance approach, which does not impact OS negatively despite notably inferior PFS1 compared to those who receive maintenance [29] and the lack of correlation between PFS1 and OS in FL [30, 31].
Another interesting finding, albeit a negative one, is the lack of an association between PFS2 and POD24 although an adverse impact on PFS2 of small magnitude cannot be ruled out. POD24 is associated with a poor OS in patients treated with immunochemotherapy, a finding that was first described in the mid-00s [32] and that has been replicated [33], including with this very dataset [34]. However, since its first description, other analyses have provided substantial nuance to this finding; replication studies have shown that the prognostic impact is of a smaller magnitude than initially reported, particularly in PET-staged patients [35] and that the poorest OS is likely restricted to primary refractory or very early relapsing (<6 or < 12 months) patients or to those with histological transformation [35–37]. The lack of association between POD24 and PFS2 in our study seems to indicate that POD24 is not always an ominous prognostic sign and that a substantial number of patients with POD24 can be rescued with subsequent lines of therapy, as suggested by a previous analysis [38]. However, patients with POD24, particularly primary refractory with an aggressive clinical course could have died before receiving the second line, and this would not be captured by second-line survival estimates. While this is an uncommon occurrence, it is a catastrophic one that should not be forgotten. Then, patients with POD24 may also be treated more aggressively in the second line, which might partially offset a potential worse prognosis of these patients. While not the object of the present analysis, we did assess whether this might have been the case – to the extent possible, as we could not assess cumulative drug exposure or dose intensity – and we found some differences in the use of rituximab monotherapy (greater in non-POD24) and platinum-based therapy (greater in POD24 patients). However, the differences appeared rather subtle and unlikely to drive the relatively small survival differences between the groups. Of interest, we found no sign of a difference in the use of stem cell transplant between POD24 and non-POD24 patients, as noted in previous cohorts [14].
Regarding PFS3, the analysis is limited by shorter follow-up and a limited number of patients, but the baseline differences we could analyze here have little association with PFS3, likely because of intervening events and treatments.
A number of limitations should be acknowledged. The most important one is the observational – and retrospective – nature of this analysis. Several biases, including indication bias, apply and prevent drawing conclusions about the causal link between treatment decisions and PFS estimates. Only 10 patients had documented histological transformation during follow-up. We lacked detailed data on the histological findings and did not have complete data on potential transformations that were not histologically confirmed. While this is a study shortcoming, the small number of transformations, which aligns with the decreasing number of such events in patients treated with immunochemotherapy and staged by PET versus in earlier series [35, 39, 40], as well as with the lower risk of transformation of grade 1–2 FL (compared to grade 3 [40, 41]), suggests that this would not have a major impact on the survival estimates provided here. The inclusion of only grade 1–2 FL should also be kept in mind when evaluating the treatment patterns and survival estimates reported. Despite aiming to make the study representative of patients with grade 1–2 FL, a broad comparison with other studies with different designs [33, 42, 43] shows minor potential differences in selected data points, such as median age and percentage of patients treated with R-single, indicating that elderly patients and those with very indolent disease might be somewhat under-represented in our cohort. Finally, the exploratory nature of the study and the multiple tests ran, raise concerns about false-positive findings. However, most of our results seem to align with previous data and inferences from other analyses, which lowers the likelihood of them being false positives and supports our conclusions. A few findings do not clearly align with previous studies, and these should be more carefully considered. Other limitations include the short follow-up, particularly for PFS2 and PFS3, as well as the small number of patients for the PFS3 analyses, which prevent us from excluding differences of small or, in some instances, moderate magnitude. As a strength of the study, we note the precise estimates that follow a large dataset and the reliability of the results of an unselected patient cohort.
To conclude, in this retrospective analysis of grade 1–2 FL, we confirm a decrease in PFS after each relapse, and we find longer PFS2 for patients treated in the front line without immunochemotherapy but find few other associations between PFS2 or PFS3 and baseline patient- and disease-related variables, front-line treatment strategy, or response.
Acknowledgements
None.
Data availability statement
For legal reasons, individual-level patient data cannot be made available. Access is only allowed to authorized researchers after approval by the appropriate national body.
Ethics declaration
This study was conducted according to the declaration of Helsinki and was approved by the review board of the Northern Ostrobothnia Hospital district.
References
[1] Lackraj T, Goswami R, Kridel R. Pathogenesis of follicular lymphoma. Best Pract Res Clin Haematol. 2018;31:2–14. https://doi.org/10.1016/j.beha.2017.10.006
[2] Carbone A, Roulland S, Gloghini A, Younes A, von Keudell G, Lopez-Guillermo A, et al. Follicular lymphoma. Nat Rev Dis Prim. 2019;5:83.https://doi.org/10.1038/s41572-019-0132-x
[3] Magnano L, Alonso-Alvarez S, Alcoceba M, Rivas-Delgado A, Muntañola A, Nadeu F, et al. Life expectancy of follicular lymphoma patients in complete response at 30 months is similar to that of the Spanish general population. Br J Haematol. 2019;185:480–491. https://doi.org/10.1111/bjh.15805
[4] Rajamäki A, Hujo M, Sund R, Prusila REI, Kuusisto MEL, Kuitunen H, et al. Mortality among patients with low-grade follicular lymphoma: a binational retrospective analysis. Cancer. 2022;128:2474–2482. https://doi.org/10.1002/cncr.34221
[5] Dreyling M, Ghielmini M, Rule S, Salles G, Vitolo U, Ladetto M, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v83–v90. https://doi.org/10.1093/annonc/mdw400
[6] Dreyling M. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:v181–v183. https://doi.org/10.1093/annonc/mdq184
[7] Hiddemann W, Dreyling M. Newly diagnosed follicular lymphoma: ESMO Clinical Recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2007;18:ii63–ii64. https://doi.org/10.1093/annonc/mdm041
[8] Sorigue M, Prusila REI, Jauhiainen J, Mercadal S, Postila A, Salmi P, et al. Incidence of solid cancer in patients with follicular lymphoma. Acta Oncol (Madr). 2019;58:1564–1569. https://doi.org/10.1080/0284186X.2019.1643918
[9] Zha J, Chen Q, Ye J, Yu H, Yi S, Zheng Z, et al. Differences in clinical characteristics and outcomes between patients with grade 3a and grades 1–2 follicular lymphoma: a real-world multicenter study. Biomark Res. 2023;11:16. https://doi.org/10.1186/s40364-023-00462-z
[10] Strati P, Jain P, Johnson RJ, Forbes S, Feng L, Samaniego F, et al. Long-term follow-up of lenalidomide and rituximab as initial treatment of follicular lymphoma. Blood. 2021;137:1124–1129. https://doi.org/10.1182/blood.2020007994
[11] von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008
[12] Rivas-Delgado A, Magnano L, Moreno-Velázquez M, García O, Nadeu F, Mozas P, et al. Response duration and survival shorten after each relapse in patients with follicular lymphoma treated in the rituximab era. Br J Haematol. 2018;184:753–759. https://doi.org/10.1111/bjh.15708
[13] Batlevi CL, Sha F, Alperovich A, Ni A, Smith K, Ying Z, et al. Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups. Blood Cancer J. 2020;10:74. https://doi.org/10.1038/s41408-020-00340-z
[14] Link BK, Day B, Zhou X, Zelenetz AD, Dawson KL, Cerhan JR, et al. Second-line and subsequent therapy and outcomes for follicular lymphoma in the United States: data from the observational National LymphoCare Study. Br J Haematol. 2019;184:660–663. https://doi.org/10.1111/bjh.15149
[15] Johnson PW, Rohatiner AZ, Whelan JS, Price CG, Love S, Lim J, et al. Patterns of survival in patients with recurrent follicular lymphoma: a 20-year study from a single center. J Clin Oncol. 1995;13:140–147. https://doi.org/10.1200/JCO.1995.13.1.140
[16] Vendramin R, Litchfield K, Swanton C. Cancer evolution: Darwin and beyond.EMBO J. 2021;40:e108389. https://doi.org/10.15252/embj.2021108389
[17] Sorigue M, Sancho JM. Current prognostic and predictive factors in follicular lymphoma. Ann Hematol. 2018;97:209–227. https://doi.org/10.1007/s00277-017-3154-z
[18] Radkiewicz C, Bruchfeld JB, Weibull CE, Jeppesen ML, Frederiksen H, Lambe M, et al. Sex differences in lymphoma incidence and mortality by subtype: a population-based study. Am J Hematol. 2023;98:23–30. https://doi.org/10.1002/ajh.26744
[19] Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, Vitolo U, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27:4555–4562.
[20] Press OW, Unger JM, Rimsza LM, Friedberg JW, LeBlanc M, Czuczman MS, et al. A Comparative Analysis of Prognostic Factor Models for Follicular Lymphoma Based on a Phase III Trial of CHOP-Rituximab versus CHOP + 131Iodine--Tositumomab. Clin Cancer Res. 2013;19:6624–6632. https://doi.org/10.1158/1078-0432.CCR-13-1120
[21] Federico M, Vitolo U, Zinzani PL, Chisesi T, Clò V, Bellesi G, et al. Prognosis of follicular lymphoma: a predictive model based on a retrospective analysis of 987 cases. Intergruppo Italiano Linfomi. Blood. 2000;95:783–789.
[22] Solal-Céligny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. https://doi.org/10.1182/blood-2003-12-4434
[23] Rajamäki A, Sunela K, Prusila REI, Kuusisto MEL, Mercadal S, Selander T, et al. Female patients with follicular lymphoma have a better prognosis if primary remission lasts over 24 months. Leuk Lymphoma. 2021;62:1639–1647. https://doi.org/10.1080/10428194.2021.1872073
[24] Yıldırım M, Kaya V, Demirpençe Ö, Paydaş S. Systematic review/Meta-analysis The role of gender in patients with diffuse large B cell lymphoma treated with rituximab-containing regimens: a meta-analysis. Arch Med Sci. 2015;4:708–714. https://doi.org/10.5114/aoms.2015.53289
[25] Carella AM, de Souza CA, Luminari S, Marcheselli L, Chiappella A, di Rocco A, et al. Prognostic role of gender in diffuse large B-cell lymphoma treated with rituximab containing regimens: a Fondazione Italiana Linfomi/Grupo de Estudos em Moléstias Onco-Hematológicas retrospective study. Leuk Lymphoma. 2013;54:53–57. https://doi.org/10.3109/10428194.2012.691482
[26] Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med. 2017;377:1331–1344. https://doi.org/10.1056/NEJMoa1614598
[27] Dreyling M, Ghielmini M, Rule S, Salles G, Ladetto M, Tonino SH, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:298–308. https://doi.org/10.1016/j.annonc.2020.11.008
[28] Lockmer S, Østenstad B, Hagberg H, Holte H, Johansson A-S, Wahlin BE, et al. Chemotherapy-free initial treatment of advanced indolent lymphoma has durable effect with low toxicity: results from two nordic lymphoma group trials with more than 10 years of follow-up. J Clin Oncol. 2018;36:3315–3323. https://doi.org/10.1200/JCO.18.00262
[29] Bachy E, Seymour JF, Feugier P, Offner F, López-Guillermo A, Belada D, et al. Sustained progression-free survival benefit of rituximab maintenance in patients with follicular lymphoma: long-term results of the PRIMA study. J Clin Oncol. 2019;37:2815–2824. https://doi.org/10.1200/JCO.19.01073
[30] Lee L, Wang L, Crump M. Identification of potential surrogate end points in randomized clinical trials of aggressive and indolent non-Hodgkin’s lymphoma: correlation of complete response, time-to-event and overall survival end points. Ann Oncol. 2011;22:1392–1403. https://doi.org/10.1093/annonc/mdq615
[31] Prasad V, Kim C, Burotto M, Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med. 2015;175:1389–1398. https://doi.org/10.1001/jamainternmed.2015.2829
[32] Casulo C, Byrtek M, Dawson KL, Zhou X, Farber CM, Flowers CR, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol. 2015;33:2516–2522. https://doi.org/10.1200/JCO.2014.59.7534
[33] Weibull CE, Wästerlid T, Wahlin BE, Andersson P-O, Ekberg S, Lockmer S, et al. Survival by first-line treatment type and timing of progression among follicular lymphoma patients: a national population-based study in Sweden. HemaSphere. 2023;7:e838. https://doi.org/10.1097/HS9.0000000000000838
[34] Rajamäki A, Hujo M, Sund R, Prusila REI, Kuusisto MEL, Kuitunen H, et al. Link between disease status at 24 months and mortality in follicular lymphoma. Br J Haematol. 2022;199:458–462. https://doi.org/10.1111/bjh.18423
[35] Batlevi CL, Sha F, Alperovich A, Ni A, Smith K, Ying Z, et al. Positron-emission tomography–based staging reduces the prognostic impact of early disease progression in patients with follicular lymphoma. Eur J Cancer. 2020;126:78–90. https://doi.org/10.1016/j.ejca.2019.12.006
[36] Seymour JF, Marcus R, Davies A, Gallop-Evans E, Grigg A, Haynes A, et al. Association of early disease progression and very poor survival in the GALLIUM study in follicular lymphoma: benefit of obinutuzumab in reducing the rate of early progression. Haematologica. 2019;104:1202–1208. https://doi.org/10.3324/haematol.2018.209015
[37] Maurer MJ, Bachy E, Ghesquières H, Ansell SM, Nowakowski GS, Thompson CA, et al. Early event status informs subsequent outcome in newly diagnosed follicular lymphoma. Am J Hematol. 2016;91:1096–1101. https://doi.org/10.1002/ajh.24492
[38] Mozas P, Sorigué M, Rivas-Delgado A, Rivero A, Correa JG, Castillo C, et al. The interval between frontline treatment and the second relapse (PFS2) predicts survival from the second relapse in follicular lymphoma patients. Eur J Haematol. 2021;106:428–432. https://doi.org/10.1111/ejh.13556
[39] Wagner-Johnston ND, Link BK, Byrtek M, Dawson KL, Hainsworth J, Flowers CR, et al. Outcomes of transformed follicular lymphoma in the modern era: a report from the National LymphoCare Study (NLCS). Blood. 2015;126:851–857. https://doi.org/10.1182/blood-2015-01-621375
[40] Montoto S, Davies AJ, Matthews J, Calaminici M, Norton AJ, Amess J, et al. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol. 2007;25:2426–2433. https://doi.org/10.1200/JCO.2006.09.3260
[41] Gine E, Montoto S, Bosch F, Arenillas L, Mercadal S, Villamor N, et al. The Follicular Lymphoma International Prognostic Index (FLIPI) and the histological subtype are the most important factors to predict histological transformation in follicular lymphoma. Ann Oncol. 2006;17:1539–1545. https://doi.org/10.1093/annonc/mdl162
[42] Friedberg JW, Taylor MD, Cerhan JR, Flowers CR, Dillon H, Farber CM, et al. Follicular lymphoma in the United States: first report of the national LymphoCare study. J Clin Oncol. 2009;27:1202–1208. https://doi.org/10.1200/JCO.2008.18.1495
[43] Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Cancer Stat Facts: NHL — Follicular Lymphoma https://seer.cancer.gov/statfacts/html/follicular.html March 2024