LETTER
Blood sampling patterns in primary care change several years before a cancer diagnosis
Mathilde Egelund Christensena,b , Mia Klinten Grandc, Margit Kriegbaumb, Bent Struer Lindb,d, Kirsten Grønbæka, Frederik Perssone, Christoffer Johansenf* and Christen Lykkegaard Andersena,b
, Mia Klinten Grandc, Margit Kriegbaumb, Bent Struer Lindb,d, Kirsten Grønbæka, Frederik Perssone, Christoffer Johansenf* and Christen Lykkegaard Andersena,b
aDepartment of Hematology, Copenhagen University Hospital, Rigshospitalet, Denmark; bCentre for General Practice, Institute for Public Health, University of Copenhagen, Denmark; cDanish Cancer Society, Denmark; dDepartment of Clinical Biochemistry, Copenhagen University Hospital, Hvidovre, Denmark; eSteno Diabetes Center Copenhagen, Herlev, Denmark; fDepartment of Clinical Oncology, Copenhagen University Hospital, Rigshospitalet, Denmark
KEYWORDS: Primary care; early detection of cancer; neoplasms; epidemiologic studies; public reporting of healthcare data; clinical chemistry tests
Citation: ACTA ONCOLOGICA 2024, VOL. 63, 17–22. https://doi.org/10.2340/1651-226X.2024.28559.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Oncologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 23 November 2023; Accepted: 26 November 2023; Published: 13 February 2024
CONTACT Mathilde Egelund Christensen mathilde.egelund.christensen@regionh.dk Department of Haematology, Copenhagen University Hospital, Rigshospitalet, Juliane Maries Vej 6, Building 5, 7th floor, 2100 Copenhagen, Denmark, Phone: + 45 27200219
Supplemental data for this article can be accessed online at https://doi.org/10.2340/1651-226X.2024.28559
*These authors equally contributed.
Competing interests and funding: The authors have no conflict of interest to disclose.
University Hospital Copenhagen, Rigshospitalet, Research Fund; Hasselbalch and Lykkegaard Andersen Research Fund; Department of Hematology, University Hospital of Copenhagen, Rigshospitalet, Research Fund; Miss Amalie Jørgensen Memorial Grant; The Danish Cancer Society. The funding sources had no involvement in the conceptualization, preparation or publishing of this paper.
Background
A sudden increase in blood sample requisitions from primary care physicians may indicate an incentive to investigate certain symptoms further. Patients with slowly progressing malignancies may debut with insidious symptoms and seek medical attention with increasing frequency, which indeed has been demonstrated through different markers of primary care activity in the last 1–2 years prior to a cancer diagnosis [1, 2]. However, within hematological malignancies in particular, early signs of slowly progressing or pre-malignant conditions may in fact be detectable several years before the malignant disease is diagnosed [3–5]. Identifying early signs of malignancy or pre-malignancy is important in order to increase chances of successful treatment and lower morbidity and mortality [6–8]. Even in asymptomatic and otherwise low-risk hematological patients, it has been demonstrated that early detection may enable risk stratification for follow-up of the (pre-)malignant conditions, help provide relevant care and improve early disease detection in the right patients [9]. In this descriptive study we aimed to describe the pre-diagnostic activity in primary care going back 15 years prior to the malignant diagnosis in order to explore if laboratory activity may indicate that early (pre-)malignant conditions register in primary care years before a diagnosis is made. This was done by describing the blood sampling activity patterns in primary care in both cancer patients to-be and controls, looking at both solid tumors and hematological malignancies. The overall aim of the study was to evaluate the potential for earlier diagnosis of certain malignant diseases.
Methods
Population
Cancer cases consisted of patients in the greater Copenhagen area with a malignant diagnosis registered in the exhaustive nationwide Danish Cancer Registry from 2001 to 2015 [10]. We excluded patients under the age of 16 years at the time of diagnosis and patients with previous cancers. Controls were included from the Population Register including all Danish residents with data available from the beginning of 1976 [11]. Cases and controls were matched 1:10 on sex and year of birth, controls being alive and without a cancer diagnosis and living in the greater Copenhagen area as of January 1st of the year of diagnosis for the case, forming our final study population. Cases could serve as controls before their cancer diagnoses and not all controls were unique, however 98% of patients were matched to 10 unique controls.
Data
Malignant diagnoses included all cancers namely, solid tumors as well as hematological malignancies, except non-melanoma skin cancer and certain diagnoses considered to be of pre-malignant or unknown character [12, 13]. Cancer diagnoses were grouped according to organ of origin that is, head and neck, digestive system, respiratory system etc. (Supplementary Table 1).
Laboratory data were extracted from the Copenhagen Primary Care Laboratory (CopLab) Database encompassing the numerical results of all laboratory work-up ordered by primary care physicians and practicing specialists in the greater Copenhagen area (constituting 20% of the Danish population) from 2000 to 2015, n = 112 million results [14]. Nomenclature for properties and units (NPU) codes were selected per unique request and test group including only tests requisitioned on the study population prior to the diagnosis of the case. We excluded test results from the last month leading up to the malignant diagnosis in order to avoid tests possibly relating to diagnostic processes in secondary care. For the purpose of this study, we included only the number of tests performed and not the numerical results of these specific tests.
Statistics
We quantified the number of incident cancer cases within study period as well as per calendar year for men and women separately. In addition, we quantified the number of cancer cases during the study period within each organ system by age at disease onset and sex.
The mean number of tests per person years at risk for cancer cases and their matched controls were reported by year going back a maximum of 15 years prior to the malignant diagnosis for the whole population as well as for each cancer type that is, by organ system (Supplementary Table 1). For the last 6 years prior to diagnosis, we plotted the NPU codes requested for the cases relative to the controls year by year to visualize which samples drove any potential increase in blood sample frequency with impending malignancy. The mean number of tests per person years at risk was calculated as the mean number of tests divided by the number of person-years at risk of having a test performed since not everyone was at risk of having a test in the CopLab database throughout the 15 years period that is, not having lived in the greater Copenhagen area throughout the entire period. As this was a descriptive study on observational data, no statistical tests were applied to assess the significance of mean differences, instead we chose to report and discuss the observations made in the next section [15, 16].
Results
We ultimately included 77,146 patients with primary malignant disease with a median age of 67 years (interquartile range [57;76]) (Table 1). The overall cancer incidence across age groups was comparable for men and women, but the age distribution differed among the sexes with a larger proportion of women among the 30–50-year-old cancer patients, whereas the majority of the 60–80-year-old cancer patients were men (Figure 1). The most frequent malignant diagnoses were prostate cancer for men and breast cancer for women (Figure 1, Supplementary Table 2). Malignant diagnoses were equally distributed across calendar years – although we observed a small peak in incident malignant diagnoses for women in 2008–2009 aligning with the introduction of a nationwide screening program for breast cancer (Supplementary Figure 1).
| Cases | Controls | |||
| Female | Male | Female | Male | |
| n = 39,641 | n = 37,505 | n = 396,410 | n = 375,050 | |
| Age, years | 66 [55;77]a | 68 [59;76] | 66 [55;77] | 68 [59;76] |
| Yearb, n (%) | ||||
| 2001–2005 | 12,739 (32.1) | 11,603 (30.9) | 127,390 (32.1) | 116,030 (30.9) |
| 2006–2010 | 13,398 (33.8) | 12,848 (34.3) | 133,980 (33.8) | 128,480 (34.3) |
| 2011–2015 | 13,504 (34.1) | 13,054 (34.8) | 135,040 (34.1) | 130,540 (34.8) |
| Cohabitation status, n (%) | ||||
| Cohabiting | 17,982 (45.4) | 23,500 (62.7) | 186,184 (47.0) | 238,859 (63.7) |
| Living alone | 21,659 (54.6) | 14,005 (37.3) | 210,226 (53.0) | 136,191 (36.3) |
| Immigration status, n (%) | ||||
| Danish | 36,424 (91.9) | 34,290 (91.4) | 351,773 (88.7) | 333,632 (89.0) |
| Immigrant | 3,065 (7.7) | 3,081 (8.2) | 42,981 (10.8) | 39,998 (10.7) |
| Descendantc | 152 (0.4) | 134 (0.4) | 1,656 (0.4) | 1,420 (0.4) |
| Educational leveld, n (%) | ||||
| Low | 13,336 (36,8) | 10,526 (30.2) | 126,264 (35.3) | 98,141 (28.3) |
| Medium | 13,623 (37.6) | 15,793 (45.4) | 134,486 (37.6) | 153,743 (44.4) |
| High | 9,237 (25.5) | 8,492 (24.4) | 97,247 (27.2) | 94,437 (27.3) |
| Unavailable | 3,445 | 2694 | 38,413 | 28,729 |
| a: Median [IQR], all such numbers; b: Year of diagnosis for the case; c: Descendant of immigrant; d: Educational level was defined according to the International Standard Classification of Education (ISCED): Low: ISCED 0–2 (primary school and high school); Medium: ISCED 3–4 (secondary education); High: ISCED 5–6 (post-secondary education). | ||||
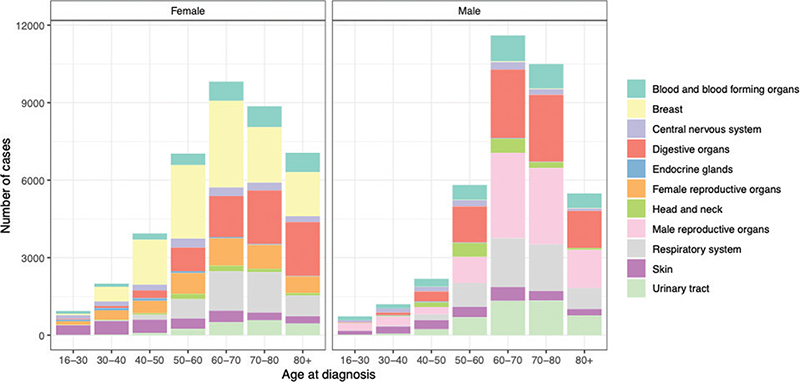
Figure 1. Cancer incidence by age at disease onset and sex.
For most malignancies the mean number of tests per person year at risk in primary care increased within the last year before the cancer diagnosis, with cases having up to 10 additional tests performed per year at risk, relative to the controls (Figure 2). This, however, was not the case for malignant melanoma and breast cancer, where test frequencies appeared similar for cases and controls throughout the follow-up period. In the case of CNS (central nervous system) tumors and hematological malignancies we observed an increase in blood sampling activity several years (>5) before the diagnosis driven by observations in myelodysplastic syndromes and the benign CNS tumors (Supplementary Table 3, Supplementary Figure 2). For endocrine malignancies that is, thyroid cancer a two-peaked pattern was observed with a relatively high number of tests 15 years before diagnosis and again in the last year leading up to the diagnosis.
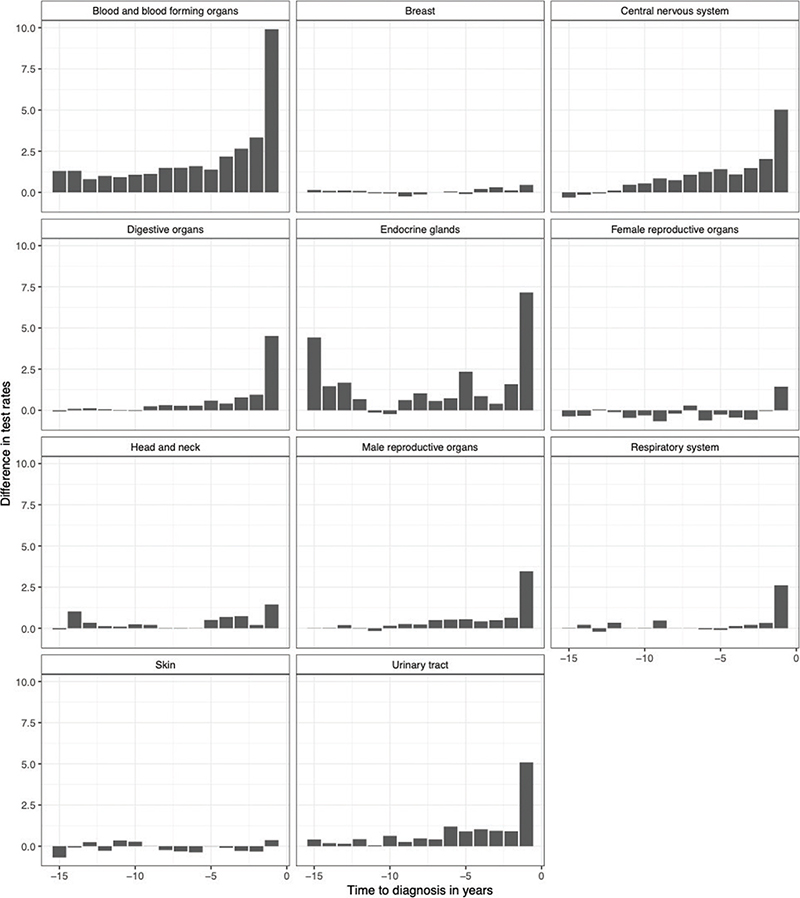
Figure 2. Difference in rate of tests between cases and controls 15 years prior to the diagnosis according to cancer group.
We then analyzed which type of blood samples drove the increased activity among general practitioners on cancer cases-to-be and found that these were largely consisting of standard tests such as complete blood cell counts and kidney function tests. For thyroid cancer, thyroid stimulating hormone (TSH) had the highest rate difference compared with controls throughout the follow-up period.
Discussion
Main findings
This is a descriptive study exploring the number of blood tests performed in cancer patients and healthy controls up to 15 years prior to the malignant diagnosis. We identify prostate cancer as the most frequent cancer type for men and breast cancer for women in concurrence with the literature [17]. We also demonstrate that the frequency of blood tests performed on primary care patients increases within 1 year leading up to most malignant diagnoses, however, hematological malignancies and CNS tumors showed an increased sampling activity as much as 5 years before the diagnosis indicating a potential window for earlier diagnosis in these cases.
Comparison with the literature
Blood sampling frequency and other measures of primary care activity have been described in specific cancer diagnoses before showing increased activity 1–2 years before the diagnosis [1, 18]. This study, however, shows a considerably longer period with increased laboratory work-up for hematological patients and CNS tumors going back more than 5 years prior to the diagnosis. The +5-year latency period in hematological cancers is driven mainly by the myelodysplastic syndromes, which tend to debut with subtle changes in blood hematology, the significance of which can be unclear until the condition develops into more fulminant disease [3, 19]. In some instances the increased blood sample frequency could also reflect the follow-up of comorbidities for example, certain autoimmune diseases with a known association with secondary hematological malignancies, although these secondary malignancies are rare compared with the sporadic cases [20–22].
We observed a similar prolonged period of +5 years with increased attention in primary care before a CNS tumor diagnosis driven by the secreting pituitary adenomas as well as the more slowly developing benign CNS tumors. The endocrine tumors of the brain can be difficult to diagnose and benign CNS tumors may also debut with unspecific symptoms such as dementia possibly prolonging the period from symptom onset to diagnosis [23–25]. In thyroid cancer we noticed an interesting two-peak pattern with sample frequency increasing at -15 years and -1 year before diagnosis, albeit numbers were small. These two peaks were largely comprised by TSH measurements and could indicate a previous benign thyroid disease with chronic inflammation – such as an autoimmune thyroiditis – predating the thyroid cancer by several years [26, 27].
Strengths and limitations
The observations presented in this study are strengthened by the population-based design and the large sample size. The merging of high-quality nation-wide register data with exhaustive primary care laboratory real-world data provides a unique insight into real-life clinical practice predating a malignant diagnosis. We regard the observation of a small peak in incident malignant diagnoses for women in 2008–2009 aligning with the introduction of a nationwide screening program for breast cancer as a sign of data validity. Such validity we believe is further indicated by the lack of increased blood sampling activity before a breast cancer or malignant melanoma diagnosis, since suspicion of these tumors are based on clinical examinations and the patient is referred to a specialized hospital unit usually without delaying with laboratory work-up in primary care. A major limitation to our design is the lack of clinical data from primary care physicians including medical history and the indications and symptoms leading to the blood sample requisition. Cancer patients are known to be more comorbid than their cancer-free peers [28] and the lack of clinical information in our study makes it difficult to separate investigations of possible malignancy from routine follow-up of chronic illnesses. However, if comorbidities were the primary drivers of the observations made we would expect to see the longest pre-diagnostic flair in sample activity in for example, pulmonary cancers or colorectal cancers more thoroughly linked to comorbidities and lifestyle factors than is the case for hematological cancers and CNS tumors [29–34], making it more likely that the blood sampling patterns identified in hematological cancers and CNS tumors could in fact reflect the early stages of development of these diseases. Studies of real-world data are subjected to some level of surveillance bias, individuals with increased health-seeking behavior being more likely to have a slowly progressing malignancy detected. However, the spiked sampling activity observed in myelodysplastic syndrome was not observed to the same extent in chronic lymphocytic leukemia or plasma cell diseases although these are just as often slowly progressing and more frequently occurring [35, 36]. We therefore deem it unlikely that surveillance bias alone drove the observations presented in this study.
This study neither enables us to assess the predictive property of each individual blood test nor does it take the numerical results of the tests into account, which means that we are not able to conclude on the prognostic or diagnostic value of the specific tests in the pre-diagnostic period.
Implications and conclusion
In this descriptive study we confirm previous observations of increasing primary care activity within the last 1–2 years leading up to most malignant diagnoses. In addition, we demonstrate a >5-year period of increased activity for benign CNS tumors and certain hematological diseases, most remarkedly myelodysplastic syndromes, which may be indicative of a potential window characterized by early manifestations of these diseases. Further studies in the pre-diagnostic period of benign CNS tumors and certain hematological cancers may be warranted.
Ethical approval and consent to participate
The CopLab database and its activities are approved by the Danish Data Protection Agency through the joint notification of The Faculty of Health and Medical Sciences at The University of Copenhagen (journal no. 2015-57-0121). According to Danish legislation, no ethical approval or patient consent was required because the patients were not approached at any time during the conduct of the study. No data were reproduced from previous work by the authors or others.
Authors’ contributions
Mathilde Egelund Christensen: Writing – Original Draft; Visualization; Funding acquisition; Mia Klinten Grand: Formal analysis; Visualization; Writing – Review and Editing; Margit Kriegbaum: Validation; Data Curation; Writing – Review and Editing; Bent Struer Lind: Data Curation; Writing – Review and Editing; Kirsten Grønbæk: Writing – Review and Editing; Supervision; Frederik Persson: Writing – Review and Editing; Christoffer Johansen: Conceptualization; Writing – Review & Editing; Supervision; Project administration; Funding acquisition; Christen Lykkegaard Andersen: Conceptualization; Resources; Writing – Review and Editing; Supervision; Project administration; Funding acquisition.
Consent for publication
This manuscript contains no personal data in any form.
Data availability
The Copenhagen Primary Care Laboratory Database (CopLab) is administered by Department of Public Health, University of Copenhagen and access to data is governed by the CopLab steering group. Data cannot be made publicly available as it contains personal information. However, researchers irrespective of nationality and affiliation may submit applications, which will then be assessed by the Steering Group of CopLab for data access. https://publichealth.ku.dk/research/databases-for-collaboration/coplab/
References
[1] Hansen PL, Hjertholm P, Vedsted P. Increased diagnostic activity in general practice during the year preceding colorectal cancer diagnosis. [cited 24-05-2023]. Available from: https://onlinelibrary.wiley.com/doi/10.1002/ijc.29418
[2] Christensen KG, Fenger-Grøn M, Flarup KR, Vedsted P. Use of general practice, diagnostic investigations and hospital services before and after cancer diagnosis – a population-based nationwide registry study of 127,000 incident adult cancer patients. BMC Health Serv Res. 2012 [cited 31-05-2023];12(1):224. Available from: https://bmchealthservres.biomedcentral.com/articles/10.1186/1472-6963-12-224
[3] Hansen JW, Sandholdt H, Siersma V, et al. Anemia is present years before myelodysplastic syndrome diagnosis: results from the pre-diagnostic period. Am J Hematol. 2017 [cited 01-07-2022];92(7):E130–2. Available from: https://pubmed.ncbi.nlm.nih.gov/28383148/
[4] Andersen MA, Grand MK, Brieghel C, Siersma V, Andersen CL, Niemann CU. Pre-diagnostic trajectories of lymphocytosis predict time to treatment and death in patients with chronic lymphocytic leukemia. Commun Med. 2022 [cited 31-08-2022];2(1). Available from: https://pubmed.ncbi.nlm.nih.gov/35603299/
[5] Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–98. https://doi.org/10.1056/NEJMoa1408617
[6] Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92–107. https://doi.org/10.1038/bjc.2015.48
[7] Enblom A, Lindskog E, Hasselbalch H, et al. High rate of abnormal blood values and vascular complications before diagnosis of myeloproliferative neoplasms. Eur J Intern Med. 2015;26(5):344–7. https://doi.org/10.1016/j.ejim.2015.03.009
[8] Koo MM, Swann R, McPhail S, et al. Presenting symptoms of cancer and stage at diagnosis: evidence from a cross-sectional, population-based study. Lancet Oncol. 2020 [cited 06-06-2023];21(1):73. Available from: /pmc/articles/PMC6941215/
[9] Weeks LD, Niroula A, Neuberg DS, et al. Prediction of risk for myeloid malignancy in clonal hematopoiesis. Blood. 2022;140(Suppl 1):2229–31. https://doi.org/10.1182/blood-2022-158960
[10] Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7):42–5. https://doi.org/10.1177/1403494810393562
[11] Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7):22–5. https://doi.org/10.1177/1403494810387965
[12] Nørgaard M, Skriver M, Gregersen H, Pedersen G, Schønheyder H, Sørensen H. The data quality of haematological malignancy ICD-10 diagnoses in a population-based hospital discharge registry. Eur J Cancer Prev. 2005 [cited 28-10-2021];14(3):201–6. Available from: https://pubmed.ncbi.nlm.nih.gov/15901987/
[13] ICD-10 Version:2019. [cited 21-06-2022]. Available from: https://icd.who.int/browse10/2019/en#/
[14] Lykkegaard Andersen C, Kriegbaum M. Copenhagen Primary Care Laboratory Database. 2022 [cited 30-08-2022]. Available from: https://publichealth.ku.dk/research/databases-for-collaboration/coplab/
[15] Greenland S, Senn SJ, Rothman KJ, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31(4):337–50. https://doi.org/10.1007/s10654-016-0149-3
[16] Ludwig DA. Use and misuse of p-values in designed and observational studies: guide for researchers and reviewers. Aviat Sp Environ Med. 2005;76(7 I):675–80.
[17] Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends – an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. https://doi.org/10.1158/1055-9965.EPI-15-0578
[18] Pottegård A, Hallas J, Friis S, Verdoodt F, Dehlendorff C, Kjær SK. Use of prescription drugs among women diagnosed with epithelial ovarian cancer in Denmark. Acta Obstetr Gynecol Scand. 2018;97(11):1332–8. https://doi.org/10.1111/aogs.13413
[19] Boennelykke A, Jensen H, Falborg AZ, et al. Diagnostic workup of cancer in patients with new-onset anaemia: a Danish cohort study in general practice. Scand J Prim Health Care. 2021 [cited 01-07-2022];39(4):391–402. Available from: https://pubmed.ncbi.nlm.nih.gov/34463223/
[20] Anderson LA, Pfeiffer RM, Landgren O, Gadalla S, Berndt SI, Engels EA. Risks of myeloid malignancies in patients with autoimmune conditions. Br J Cancer. 2009;100(5):822–8. https://doi.org/10.1038/sj.bjc.6604935
[21] Boddu PC, Zeidan AM. Myeloid disorders after autoimmune disease. Best Pract Res Clin Haematol. 2019 [cited 18-03-2020];32(1):74–88. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30927978
[22] Espinosa G, Font J, Muñoz-Rodríguez FJ, Cervera R, Ingelmo M. Myelodysplastic and myeloproliferative syndromes associated with giant cell arteritis and polymyalgia rheumatica: a coincidental coexistence or a causal relationship? Clin Rheumatol. 2002;21(4):309–13. https://doi.org/10.1007/s100670200081
[23] Buckner JC, Brown PD, O’Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82(10):1271–86. https://doi.org/10.4065/82.10.1271
[24] Nygaard C, Jensen H, Christensen J, Vedsted P. Health care use before a diagnosis of primary intracranial tumor: a Danish nationwide register study. Clin Epidemiol. 2018;10:809–29. https://doi.org/10.2147/CLEP.S147865
[25] Melmed S. Pituitary-tumor endocrinopathies. N Engl J Med. 2020;382(10):937–50. https://doi.org/10.1056/NEJMra1810772
[26] Chen YK, Lin CL, Cheng FTF, Sung FC, Kao CH. Cancer risk in patients with Hashimoto’s thyroiditis: a nationwide cohort study. Br J Cancer. 2013 [cited 01-07-2022];109(9):2496. Available from: /pmc/articles/PMC3817335/
[27] de Paiva CR, Grønhøj C, Feldt-Rasmussen U, von Buchwald C. Association between Hashimoto’s Thyroiditis and Thyroid Cancer in 64,628 Patients. Front Oncol. 2017 [cited 01-07-2022];7(APR):53. Available from: /pmc/articles/PMC5385456/
[28] Hu JX, Helleberg M, Jensen AB, Brunak S, Lundgren J. A large-cohort, longitudinal study determines precancer disease routes across different cancer types. Cancer Res. 2019;79:864–72. https://doi.org/10.1158/0008-5472.CAN-18-1677
[29] O’Sullivan DE, Sutherland RL, Town S, et al. Risk factors for early-onset colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022 [cited 06-06-2023];20(6):1229–40.e5. Available from: https://pubmed.ncbi.nlm.nih.gov/33524598/
[30] Almatrafi A, Thomas O, Callister M, Gabe R, Beeken RJ, Neal R. The prevalence of comorbidity in the lung cancer screening population: a systematic review and meta-analysis. J Med Screen. 2022;30(1):3–13. https://doi.org/10.1177/09691413221117685
[31] Dansk Akut Leukaemi Database & Myelodysplastisk Syndrom Database. [cited 29-11-2021]. Available from: www.leukemia.dk
[32] Deschler B, Lübbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107(9):2099–107. https://doi.org/10.1002/cncr.22233
[33] krabek P, Turner D, Seftel M. Epidemiology of non-Hodgkin lymphoma. Transfus Apher Sci. 2013 [cited 06-06-2023];49(2):133–8. Available from: https://pubmed.ncbi.nlm.nih.gov/23958141/
[34] Anderson LA, McMullin MF. Epidemiology of MPN: what do we know? Curr Hematol Malig Rep. 2014;9(4):340–9. https://doi.org/10.1007/s11899-014-0228-z
[35] Ma X. Epidemiology of myelodysplastic syndromes. Am J Med. 2012;125(7 suppl.):S2. https://doi.org/10.1016/j.amjmed.2012.04.014
[36] Alabrach Y, Mahmoud AA, Abdelhay A et al. Trends of chronic lymphocytic leukemia incidence and mortality in the United States: a population-based study over the last four decades. Expert Rev Hematol. 2023;16(10):785–91. https://doi.org/10.1080/17474086.2023.2243385