LETTER
A national precision cancer medicine implementation initiative for Finland
Katriina J. Jalkanena  , Erika Alanneb
, Erika Alanneb  , Sanna Iivanainenc
, Sanna Iivanainenc  , Okko-Sakari Kääriäinend
, Okko-Sakari Kääriäinend  , Minna Tannere
, Minna Tannere  , Annika Auranenf
, Annika Auranenf  , Jussi Koivuneng
, Jussi Koivuneng  , Timo K. Nykopph
, Timo K. Nykopph  , Pia Vihinenb and Mika Mustoneni
, Pia Vihinenb and Mika Mustoneni 
aComprehensive Cancer Center, Helsinki University Hospital, Helsinki, Finland; bTurku University Hospital and FICAN West Cancer Centre, Turku, Finland; cDepartment of Oncology and Radiotherapy, Oulu University Hospital, Oulu, Finland; dDepartment of Oncology, Kuopio University Hospital, Kuopio, Finland; eTAYS Cancer Center, Tampere University Hospital and Tampere University, Tampere, Finland; fFICAN Mid, Central Finland Cancer Center, Tampere University and Tampere University Hospital, Tampere, Finland; gFICAN North, Northern Finland Cancer Center, Oulu University Hospital and University of Oulu, Oulu, Finland; hFICAN East, Eastern Finland Cancer Center, University of Eastern Finland and Kuopio University Hospital, Kuopio, Finland; iFICAN South, Southern Finland Cancer Center, Helsinki University Hospital and University of Helsinki, Helsinki, Finland
KEYWORDS: FINPROVE; DRUP-Like-Trials; Precision Medicine; PRIME-ROSE; PCM4EU
Citation: ACTA ONCOLOGICA 2024, VOL. 63, 395–397. https://doi.org/10.2340/1651-226X.2024.32661.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Oncologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 29 November 2023; Accepted: 5 April 2024; Published: 23 May 2024
CONTACT: Katriina J. Jalkanen katriina.jalkanen@hus.fi Comprehensive Cancer Center, Helsinki University Hospital, Helsinki, Finland
Competing interests and funding: Participation in the FINPROVE Public–Private Partnership is regulated by a consortium agreement that handles conflicts of interest and regulates interaction with the publicly funded infrastructure FINPROVE consortium (co-ordinating PI KJ) has company contributions from Roche, Novartis, Bayer, Janssen and Eli Lilly. The coordinating author (KJ) reports the following outside of this work, advisory fees: Ipsen, BMS, Roche, MSD, Bayer, Novartis and stock ownership Faron Pharmaceuticals.
This paper has been included in the Nordic Precision Cancer Medicine, NPCM, 2023 Symposia Collection
To the Editor – The adaptation of nationwide genomic profiling and personalized cancer therapy in Finland has been challenging due to the lack of a uniform framework and funding. Meanwhile, increasing evidence demonstrates a clear benefit from precision medicine in cancer care [1, 2] while the need for more efficient therapies in hard-to-treat cancers is evident. Equally a challenge for these cancers remains in the lack of randomized trials.
Work to implement precision cancer medicine at a national level in Finland, began in 2021 led by Helsinki University Hospital (HUS) and FICAN South to meet three goals: (1) to implement genomic profiling and precision cancer medicine as standard of care, (2) establish equal access to molecular diagnostics and clinical trials in precision medicine, (3) to increase the number of precision cancer medicine trials and open a national DRUP (Drug Rediscovery Protocol)-like clinical trial.
A multidisciplinary study team including oncologists, hematologists, gynecological oncologists, pathologists, cancer researchers, molecular and clinical geneticists was formed. This nationwide working group had the common aim to enable access to personalized cancer therapy for all patients in Finland irrespective of their residence. Early discussion amongst this initiative urged the need to equally engage stakeholders for reimbursement and sustained funding. Of equal importance was to explore the possibility of a public-private partnership for drug access and possibilities for shared pay for benefit funding. Political and financial support have revealed to be the most challenging steps within this initiative as consistent public funding is still lacking. Without international coordination with other major precision cancer medicine initiatives, especially the Dutch DRUP trial, the implementation would have been impossible.
Tertiary care for cancer treatment in Finland is centralized to five university hospitals each governing a capture area of 0.7 to 1.7 M inhabitants. Each University hospital has a regional cancer center (FICAN South, West, East, North and Mid) that aims to promote equal access to diagnostics and treatment and, thus, the FICANs have played a major role in the precision cancer medicine initiative. During the past 3 years, we have therefore built and raised funding for an ecosystem that includes four major working groups: molecular diagnostics, data storage and secondary use, reimbursement, and finally the national DRUP like trial. National infrastructure for precision cancer diagnostics requires all five university hospitals across Finland, and these in turn facilitate the use of advanced molecular diagnostics, operate local Molecular Tumor Boards (MTBs) and participate in our national MTB. The national MTB operates digitally (Teams) on a weekly basis evaluating cases that have been pre-screened for FINPROVE by the local MTBs (Figure 1).
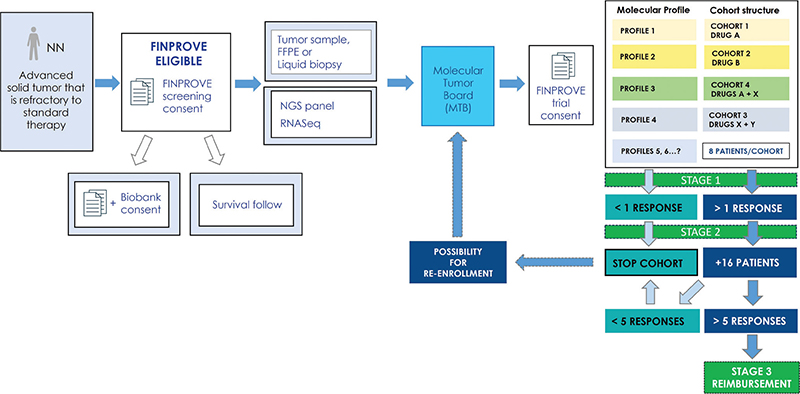
Figure 1. FINPROVE design and patient inclusion.
To facilitate precision cancer medicine implementation and patients’ access to targeted anti-cancer drugs, FINPROVE was launched in August 2021. FINPROVE is a national investigator-initiated interventional phase 2 trial that opened for inclusion in January of 2022 first in Helsinki University Hospital, and now the trial is open in all five University Hospitals. Smaller regional hospitals operate through their governing university hospital to include patients in FINPROVE. The trial is coordinated with and modeled on, the Dutch DRUP trial, which has been successful in the uptake of precision medicine nationwide, due to high inclusion rates resulting in significant patient benefit and exploring new national reimbursement models [3, 4]. FINPROVE is also aligned with similar trials in Denmark (ProTarget), Norway (IMPRESS) and Sweden (FOCUSE). Importantly, EU has funded two large precision cancer initiatives facilitating collaboration within countries working on these trials and precision medicine: PCM4EU and PRIME-ROSE. Both initiatives comprise of projects for research on control cohorts, use of real-world evidence, reimbursement models and health economics, ethics, legal aspects, and data governance. Partly due to this, our national initiative has gained substantial interest in patient organizations, private companies and stakeholders involved in reimbursement strategies for new therapy/drug indications. The majority of funding is covered through the University Hospitals but of equal importance have been private trusts. Additional funding comes from company contributions for drugs, and costs per-patient in FINPROVE.
The FINPROVE ecosystem is currently scaling to include regional hospitals as sites in aiming at nationwide equality for both molecular profiling and inclusion to precision medicine trials. The major challenge for larger uptake of genomic profiling is the lack of public reimbursement for advanced molecular panels and the diversity of genomic analysis used. For this reason, access to expensive panels has not been uniform and requires ongoing discussions with public stakeholders as agnostic indications and approved targeted agents have entered standard-of-care with increasing pace. Actionable targets are confirmed centrally upon trial entry. Yet for screening all University Hospitals use commercial panels (e.g. TruSight Oncology 500, Foundation One Cdx, Oncomine Comprehensive Assay Plus) while validating in-house panels that will hopefully be available for all cancer patients in Finland in the future as national guidance for screening still requires implementation.
By November 2023, the national MTB has evaluated 450 patients with molecular profiling results for inclusion in the FINPROVE trial and other ongoing studies. Of these, more than 100 (25%) patients have been offered treatment within FINPROVE which consists of cohorts defined by a specific molecular profile (eg. mutation, fusion, or amplification) and drug. The trial is a cluster of cohorts that follows a Simon two-stage model with Stage 1 consisting of a cohort of eight patients in a combined umbrella and basket design. Positive cohorts (≥5 of 24 responsive patients, Stage 2) may expand into larger cohorts (Stage 3) from which discussions on drug reimbursement can be based on. Currently reimbursement is only case-based and models for extension of indications are to be explored. When the trial started, eight drugs were offered from Roche, but continuing discussions with other companies have resulted in the inclusion of drugs from Bayer, Novartis, Eli Lilly, and Janssen, adding up to 13 drugs at the end of 2023. By the end of the second quarter in 2024, we foresee to have additional companies, and 18 drugs in the trial, improving patient inclusion and, hopefully, benefits for the patients.
Through this initiative, we now have an ecosystem that has a mutual aim to increase clinical trial access for cancer patients across Finland, harmonize molecular testing, and equalize standard-of-care. Patients with progressive cancer disease can now be referred to their governing University Hospital irrespective of where they live and have the possibility for advanced molecular cancer diagnostics [5]. Through the national MTB, an increasing number of cancer patients now have access to more treatment lines, both for standard-of-care and experimental drugs, beyond previous availability. The whole ecosystem strengthens translational research and innovation through extensive data generation, biobanking and national registries. All University Hospitals have the competence to utilize their data lakes to promote drug efficacy data, data on health economics for future technology assessments, new diagnostic methods and re-enforce international competence through AI.
Future plans for this precision cancer medicine ecosystem include expanding the drugs included in FINPROVE while also allowing drug combinations especially for hematologic malignancies. Moreover, a deeper understanding on the molecular underpinnings of response and resistance of tumors to targeted treatments is urgently needed to enhance precision oncology approaches as it is unknown why some of the patients with the same molecular alteration respond and some fail to respond to the same therapy [6].
FINPROVE PIs Katriina Jalkanen1, Erika Alanne2, Sanna Iivanainen3, Okko Kääriäinen4, Minna Tanner5.
FINPROVE Study Group: Katriina Jalkanen1, Erika Alanne2, Minna Tanner5, Okko Kääriäinen4, Sanna Iivanainen3, Annika Auranen6, Anniina Färkkilä1,11, Heini Lassus1, Peeter Karihtala1, Marjukka Pollari5, Sanni Tulokas1, Siru Mäkelä1, Sari Jäämaa1, Jarkko Ahvonen5, Justiina Kaukola1, Timo Makkonen1, Laura Kohtamäki1, Milla Katajisto1, Emmi Peurala1, Päivi Halonen1, Synnöve Staff13, Satu Karjalainen1, Inga Bauer1, Heidi Burmoi5, Jenna Renko5, Nina Halme2, Riina Sonkkila2, Marjo Väätäinen4, Raija Mikkonen3, Jessica Hannelius5, Kirsi Penttilä5, Tanja Juslin1.
11Research Program in Systems Oncology & Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Hospital; 13Department of Obstetrics and Gynecology, Tampere University Hospital and Faculty of Medicine and Health Technology, Tampere University, Tampere, Finland.
FINPROVE Molecular Tumor Board: Katriina Jalkanen1, Erika Alanne2, Minna Tanner5, Okko Kääriäinen4, Anniina Färkkilä1, Heini Lassus1, Peeter Karihtala1, Ari Ristimäki14, Annukka Pasanen12, Soili Kytölä15, Reetta Vainionpää15, Elina Niemelä15, Elina Kaikkonen15, Iiris Ukkola15, Riikka Nurminen16, Minna Pöyhönen15,17, Nelli Saijets15,17, Mauri Keinänen16, Veli Kairisto18, Eva-Maria Talvitie18, Tanja Juslin1, Satu Karjalainen1.
14Department of Pathology, HUSLAB, HUS Diagnostic Center, University of Helsinki and Helsinki University Hospital, Helsinki, Finland; 15HUSLAB, Department of Genetics, HUS Diagnostic Center, Helsinki University Hospital and University of Helsinki, 00029 Helsinki, Finland; 16Department of Genetics, Fimlab Laboratories, Tampere, Finland; 17Department of Medical and Clinical Genetics, University of Helsinki, Helsinki, Finland; 18Department of Genomics, Turku University Hospital, Turku, Finland.
Translational team: Anniina Färkkilä1,11, Soili Kytölä15, Sirpa Leppä19, Ari Ristimäki14, Antti Rannikko20, Anni Virtanen14.
19Applied Tumor Genomics, Research Programs Unit, Faculty of Medicine, University of Helsinki, Helsinki, Finland; Department of Oncology, Helsinki University Hospital Comprehensive Cancer Center, Helsinki, Finland; 20Department of Urology and Research Program in Systems Oncology, University of Helsinki, and Helsinki University Hospital, Helsinki, Finland
Regional FICANs: Annika Auranen6, Jussi Koivunen7, Mika Mustonen10, Timo Nykopp8, Pia Vihinen9
Acknowledgements
This work is supported by public funding to the precision cancer medicine ecosystem from the Regional Cancer Centers for FICAN South, West, Mid and North, and Regional University Hospitals HUS, TYKS, TAYS, KYS and OUS, grants from Cancer Foundation Finland and Eschner Foundation, as well as company contributions received so far from Roche, Novartis, Eli Lilly, Bayer and Janssen.
Author contributions
KJ, EA, SI, OSK and MT from all five university hospitals share equal contribution. Important discussions and contributions to the initiatives were made by all authors. Authors have approved the final version of the text.
The trial is registered both in EudraCT 2021-000689-14 and ClinicalTrials.gov NTC05159245 registries.
Ethics approval
The protocol has been approved by the institutional review boards and independent ethics committees of the participating centers. The study is performed in accordance with the Declaration of Helsinki and is conducted in compliance with the International Conference on Harmonisation on Good Clinical Practice.
Data availability statement
The trial is on progress.
References
[1] Schwaederle M, Zhao M, Lee JJ, Eggermont AM, Schilsky RL, Mendelsohn J, et al. Impact of precision medicine in diverse cancers: a meta-analysis of phase II clinical trials. J Clin Oncol. 2015;33(32):3817–3825. https://doi.org/10.1200/JCO.2015.61.5997
[2] Andre F, Filleron T, Kamal M, Mosele F, Amedos M, Dalenc F, et al. Genomics to select treatment for patients with metastatic breast cancer. Nature. 2022 Oct;610(7931):343–348. https://doi.org/10.1038/s41586-022-05068-3
[3] van der Velden DL, Hoes LR, van der Wijngaart H, van Berge Henegouwen JM, van Werkhoven E, Roepman P, et al. The Drug Rediscovery protocol facilitates the expanded use of existing anticancer drugs. Nature. 2019;574:127–131. https://doi.org/10.1038/s41586-019-1600-x
[4] van der Wijngaart H, Hoes LR, van Berge Henegouwen JM, van der Velden DL, Zeverijn LJ, Roepman P, et al. Patients with biallelic BRCA1/2 inactivation respond to Olaparib treatment across histologic tumor types. Clin Cancer Res. 2021 Nov 15;27(22):6106–6114. https://doi.org/10.1158/1078-0432.CCR-21-1104
[5] Helland Å, Russnes HG, Fagereng GL, Al-Shibli K, Andersson Y, Berg T, et al. Improving public cancer care by implementing precision medicine in Norway: IMPRESS-Norway. J Transl Med. 2022 May 14;20(1):225. https://doi.org/10.1186/s12967-022-03432-5. Erratum in: J Transl Med. 2022 Jul 15;20(1):317.
[6] Aldea M, Friboulet L, Apcher S, Jaulin F, Mosele F, Sourisseau T, et al. Precision medicine in the era of multi-omics: can the data tsunami guide rational treatment decision? ESMO Open. 2023 Oct;8(5):101642. https://doi.org/10.1016/j.esmoop.2023.101642