REVIEW ARTICLE
The Tumor Immune Microenvironment in Breast Cancer Progression
Marit Otterlei Fjørtofta,b  , Kanutte Husec
, Kanutte Husec  and Inga Hansine Ryea,b
and Inga Hansine Ryea,b 
aDepartment of Cancer Genetics, Institute for Cancer Research, Division of Cancer Medicine, Oslo University Hospital, Radium Hospital, Oslo, Norway; bInstitute of Clinical Medicine, University of Oslo, Oslo, Norway; cDepartment of Cancer Immunology, Institute for Cancer Research, Division of Cancer Medicine, Oslo University Hospital, Radium Hospital, Oslo, Norway; dPrecision Immunotherapy Alliance, University of Oslo, Oslo, Norway
ABSTRACT
Background: The tumor microenvironment significantly influences breast cancer development, progression, and metastasis. Various immune cell populations, including T cells, B cells, NK cells, and myeloid cells exhibit diverse functions in different breast cancer subtypes, contributing to both anti-tumor and pro-tumor activities.
Purpose: This review provides an overview of the predominant immune cell populations in breast cancer subtypes, elucidating their suppressive and prognostic effects. We aim to outline the role of the immune microenvironment from normal breast tissue to invasive cancer and distant metastasis.
Methods: A comprehensive literature review was conducted to analyze the involvement of immune cells throughout breast cancer progression.
Results: In breast cancer, tumors exhibit increased immune cell infiltration compared to normal tissue. Variations exist across subtypes, with higher levels observed in triple-negative and HER2+ tumors are linked to better survival. In contrast, ER+ tumors display lower immune infiltration, associated with poorer outcomes. Furthermore, metastatic sites commonly exhibit a more immunosuppressive microenvironment.
Conclusion: Understanding the complex interaction between tumor and immune cells during breast cancer progression is essential for future research and the development of immune-based strategies. This comprehensive understanding may pave the way for more effective treatment approaches and improved patients outcomes.
KEYWORDS: Breast cancer; tumor immune microenvironment; subtypes; progression
Citation: ACTA ONCOLOGICA 2024, VOL. 63, 359–367. https://doi.org/10.2340/1651-226X.2024.33008.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Oncologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 1 December 2023; Accepted: 17 February 2024; Published: 23 May 2024
CONTACT: Inga Hansine Rye ingrye@rr-research.no Department of Cancer Genetics, Institute for Cancer Research, Oslo University Hospital, Ullernchaussèn 70, NO-0379 OSLO, Norway
Competing interests and funding: The authors report that there are no competing interests to declare.
This paper has been included in the Nordic Precision Cancer Medicine, NPCM, 2023 Symposia Collection
Introduction
The role of the immune system is to eliminate pathogens and aberrant cells through immune surveillance. However, this process becomes unsustainable as tumors gradually change the tumor immune microenvironment (TIME) into an immunosuppressive state, evading the host’s immune defenses. Tumors employ diverse strategies to escape immune detection, including secretion of immunosuppressive cytokines, downregulation of major histocompatibility complex (MHC) class I, and recruitment of tumor promoting immune cells [1]. The balance between pro- and anti-tumor immune cells emerges as a critical determinant influencing the progression of cancer.
The breast is not an immune-cell rich organ, and breast cancer has not traditionally been recognized as an immunogenic cancer. However, emerging evidence reveals varying degrees of immune cell infiltration across the different breast cancer subtypes. Triple negative breast cancer (TNBC), which lacks expression of human epidermal growth factor 2 (HER2) and the hormonal receptors estrogen and progesterone (ER and PR), and HER2+ breast cancer exhibit higher degree of immunogenicity compared to ER+ tumors. The degree of immune infiltration is hypothesized to reflect the tumor mutational burden, which is higher in TNBC and HER2+ tumors due to genomic instability, leading to increased neoantigen presentation [2].
An in-depth knowledge of the TIME is crucial for understanding tumor progression and in the development of novel targeted therapeutic strategies against breast cancer. In this review, we examine the composition of immune cells and their key roles in the molecular subtypes of breast cancer and through progression from normal breast tissue to metastatic disease.
Immune microenvironment in normal breast tissue
The presence of immune cells in normal breast tissue is relatively scarce. Interestingly, higher immune infiltration is observed in healthy individuals with high risk of developing breast cancer, such as BRCA1 mutation carriers [3]. The immune microenvironment in breast tissue primarily consists of CD8+ T cells, CD68+ macrophages, and CD11+ dendritic cells (DCs) [4–7]. These immune cells are predominantly localized in the breast lobular and ductal regions, residing in close proximity to the epithelial cells [4–6]. The CD4+ T cells and CD20+ B cells are less frequent, and often completely absent from the breast [4]. Recently, a comprehensive study by Kumar et al. [7] using single cell RNA sequencing, identified CD8+ and CD4+ T cells and M1 macrophages to be the most prevalent immune cells. The CD8+ T cells expressed RUNX, indicative of a tissue-resident phenotype. B cells were found in lower numbers, and were dominated by Immunoglobulin G (IgG) and Immunoglobulin A (IgA) producing plasma cells [7].
The breast is an organ undergoing constant change throughout life, influenced by hormonal fluctuations during puberty, the menstrual cycle, and pregnancy. The immune microenvironment is also altered by these hormonal fluctuations [8]. Additionally, age-related alterations are observed in the distribution and localization of immune cells, including decreased B and T cell density in peri-epithelial regions and increased M2 macrophages in the intralobular stroma [9]. These observations support the theory of immunosenescence during aging.
Tumor immune microenvironment in breast cancer
In breast cancer, we see an increased presence of immune cells compared to normal breast tissue; this is summarized in Figure 1. Immune cells of both the lymphoid and myeloid lineage contribute to the dynamic changes seen during tumor progression (Table 1).
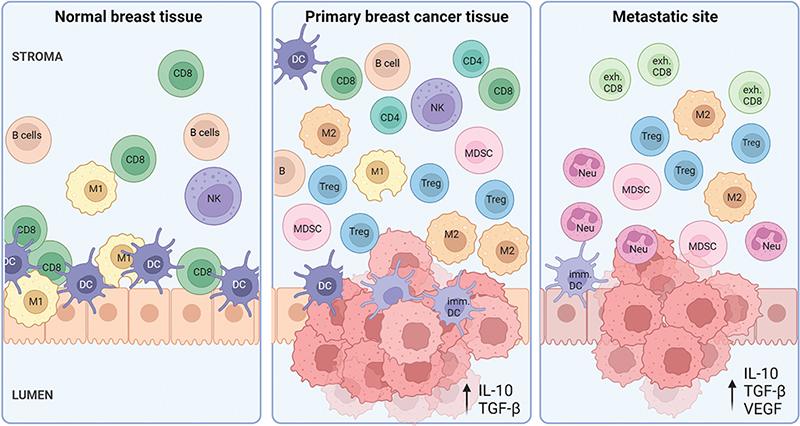
Figure 1. The variation in immune microenvironment from normal breast tissue through the immune escaping invasive cancer to distant metastatic sites. In normal breast tissue, immune cells are located most predominantly within the epithelial regions of the lobules, where CD8 T cells, DCs, NK cells and M1 macrophages are the most dominant cell types. In primary breast tumors, the amount of immune cells increases, where immunosuppressive cells such as Tregs, MDSCs and M2 TAMs aid tumor progression. The TIME in metastatic sites is highly immunosuppressive, including pro-tumor neutrophils, immature DCs and exhausted cytotoxic T cells. CD8=CD8+ T cell, exh.CD8=Exhausted CD8+ T; Treg=Regulatory T cell; DC=Dendritic cell; imm. DC=Immature DC; M1=M1 macrophage; M2=M2 macrophage; NK cell=Natural killer cell; MDSC=Myeloid-derived suppressor cell; Neu=Neutrophil. Created with BioRender.com.
Tumor infiltrating lymphocytes in breast cancer subtypes
Tumor infiltrating lymphocytes (TIL) have migrated from the blood stream to the tumor site. TILs encompass a large group of cells, including T cells, B cells, and NK cells. TILs are recognized for their anti-tumor properties, and it is well-established that high numbers of TILs are correlated with a beneficial prognosis in breast cancer [10–12]. High numbers of TILs are also associated with increased likelihood of response to neoadjuvant chemotherapy in all the molecular subtypes [13]. TILs can be classified as stromal (sTIL) or intratumoral (iTIL). Generally sTILs tend to be of higher prevalence than iTILs, and higher sTILs are associated with longer survival in all subtypes [13].
Within the TIL population, T cells with a memory phenotype emerge as the most abundant, playing a pivotal role in the immune response against tumors [14]. Specifically, CD8+ T cells serve as effector cells engaged in eradication of tumor cells through recognition of tumor-associated antigens and neoantigens presented by MHC class I. Simultaneously, CD4+ T cells provide support to CD8+ T cells by secreting a diverse range of effector cytokines.
B cells represent a minority among the TILs, yet their presence holds significance in relation to the formation of tertiary lymphoid structures (TLS). TLS are aggregates of lymphocytes in non-lymphoid tissue. In breast cancer these are found in the stroma and are associated with high-grade tumors [15]. In the context of triple negative breast cancer, these associations are particularly noteworthy, with TLS identified in higher abundance compared to HER2+ and ER+ subtypes [14]. Tumor infiltrating B cells are associated with an improved clinical outcome in breast cancer [16, 17], although their exact role in anti-tumor activity is not yet fully understood.
Regulatory T cells (Tregs) accumulate in breast cancer tissue compared to normal breast tissue [18], and infiltration of Tregs is correlated with high tumor grade, positive lymph node status and short overall and recurrence-free survival [19]. The prognostic role of Tregs in breast cancer is debated, and some studies have shown opposite results, as reviewed by Saleh and Elkord [19]. Thus, the prognostic effect of Tregs is dependent on the histological grade and molecular subtype.
Natural killer (NK) cells are important cytotoxic cells involved in immune surveillance and direct killing of aberrant cells [20, 21]. In breast cancer, estrogen is well known to have a suppressive effect on NK cells [22, 23]. The presence of NK cells is significantly associated with TILs and Ki-67 index [24]. Because of its killing functions NK cells can be useful in new forms of immunotherapy.
Triple negative breast cancer
Triple negative breast cancer has frequently high infiltration of TILs [25], predominantly CD8+ and CD4+ T cells. B cells [14] and NK cells [24] are also increased in TNBC compared to other subtypes, and the main B cell subpopulation in TNBC is memory B cells, with lower amounts of naïve B cells and plasma cells [26]. Tregs are predominantly found in immune infiltrated TNBC and ER-HER2+ subtypes [27, 28]. TNBC with elevated immune infiltration demonstrates enhanced survival rates and increased pathological complete response (pCR) during neoadjuvant therapy [29]. An increased presence of CD8+ T cells is reported to be associated with ER and PR negativity [28, 30], and has favorable prognostic value in ER- tumors [31]. Surprisingly, while a robust presence of NK cells is associated with a favorable prognosis in ER+ and HER2+ breast cancer patients, a high presence in TNBC correlates with poor prognosis [32]. This can be explained by the dual role of NK cells as they can also exhibit pro-tumor functions. CD56brightCD16dim NK cells in breast and colon cancers have been found to express the pro-angiogenic factor vascular endothelial growth factor (VEGF), which has a major role in tumor vessel growth and development of an immunosuppressive environment [33, 34]. In a suppressive TIME, NK cells can become dysfunctional due to molecular signals produced by tumor cells and environmental factors such as hypoxia and nutrient deprivation [35].
HER2+ breast cancer
HER2+ breast cancers are, alongside with TNBC, the subtypes with highest abundance of TILs [28]. The presence of TILs is associated with a favorable prognostic value in both ER-HER2+ and ER+HER2+ tumors [31]. Additionally, in HER2+ breast cancer treated with adjuvant chemotherapy, higher TIL abundance is associated with increased overall survival [13]. An increased presence of CD8+ T cells is associated with favorable prognosis in ER-HER2+ tumors [31]. Conversely, an increased presence of Tregs is associated with HER2 overexpression and decreased overall and progression-free survival [30]. In a spatial context, high CD8+ cell and Treg infiltration in the tumor bed is linked with a decreased survival, while a high CD8+ to Treg ratio in the surrounding area is associated with improved survival [30]. Interestingly, a strong presence of NK cells is associated with positive prognosis in patients with HER2+ subtype, opposite of what is seen in TNBC [24]. Deconvolution methods identified B cell IgG signatures as more strongly associated with pCR and prognosis than TILs in early HER2+ breast cancer [36]. This shows that immune signatures offer valuable insights with potential for predicting treatment responses.
ER+ breast cancer
ER+ tumors exhibit low frequency of TILs. Interestingly, the prognostic impact of TILs is not found to be favorable in this subtype. High TIL infiltration shows adverse prognosis and a shorter overall survival in a neoadjuvant therapy setting [13, 25, 28]. High Treg abundance is linked to lower ER expression [28]. Surprisingly, a high presence of Tregs in ER+ tumors is associated with a better prognosis [30]. NK cells are inversely correlated with ER expression status, and high infiltration is associated with good prognosis in ER+ breast cancers [24].
Tumor infiltrating myeloid cells in breast cancer
Dentritic cells (DCs) are specialized antigen-presenting cells (APC) bridging the innate and adaptive immune responses. There are two distinct types of DCs: plasmacytoid DCs (pDCs) and myeloid DCs (mDCs). pDCs recognize viral infections and produce high levels of interferon type I, whereas mDCs capture, process, and present antigens to T cells [37, 38]. Circulating DCs are more prevalent in breast cancer patients compared to healthy controls [39]. The HER2+ subtype shows the highest amount of circulating pDCs, whereas ER+ subtypes have more circulating mDCs than ER- subtypes [39]. Lower levels of circulating pDCs are found in patients with later stages of breast cancer [40]. Interestingly, while the presence of circulating pDC is associated with better prognosis, the infiltration into the tumor correlates with adverse outcomes [41]. TNBC exhibits high abundance of both intra-tumor and stromal immature pDC, while ER+ and ER+/HER2+ tumors are dominated by functional mature DCs [42]. Although DCs play a crucial role as anti-tumor cells, the tumor can induce a pro-tumorigenic DC phenotype, leading to dysfunctional and poorly activated DCs [37, 43].
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells with immune regulatory and suppressive functions [32, 44–46]. Recent studies have demonstrated that the release of cytokines, including G-CSF, IL-6, and TGF-β by breast cancer cells influences the expansion and activation of MDSCs, establishing a link between MDSCs and breast cancer progression [45]. An increased abundance of MDSCs is found in TNBC tumors [47]. In TNBC, tumor cells expressing the regulating factor ΔNp63 secrete the chemokines CXCL2 and CCL22, shown to attract MDSCs [47]. Elevated levels of MDSCs in the tumor microenvironment and in circulation are strongly associated with tumor progression and worse overall survival [46]. Furthermore, the level of circulating MDCS is higher in metastatic cancer than non-metastatic cancer [48].
Macrophages are terminally differentiated myeloid cells that can be divided into two categories with opposing actions in the TIME: pro-inflammatory M1 and immunosuppressive M2 tumor associated macrophages (TAMs) [28, 29]. The immunosuppressive M2 TAMs are the most abundant in breast cancer [49], and a high presence is associated with higher tumor grade, ER and PR negativity, and a shorter overall survival, especially in HER2+ and TNBC [50–52].
The precise function and composition of the different immune cells within the different breast cancer subtypes remain unclear. This underscores the challenges in interpreting the roles and functions of the cells in the microenvironment, given the highly heterogeneous nature concerning maturation and differentiation steps. The need for further investigation is evident to unravel the complexities surrounding tumor infiltrating lymphocytes and myeloid cells in breast cancer. A simplified summary of the immune composition across the molecular subtypes is given in Figure 2, and the presence and prognostic role of the different cell types are summarized in Table 2.
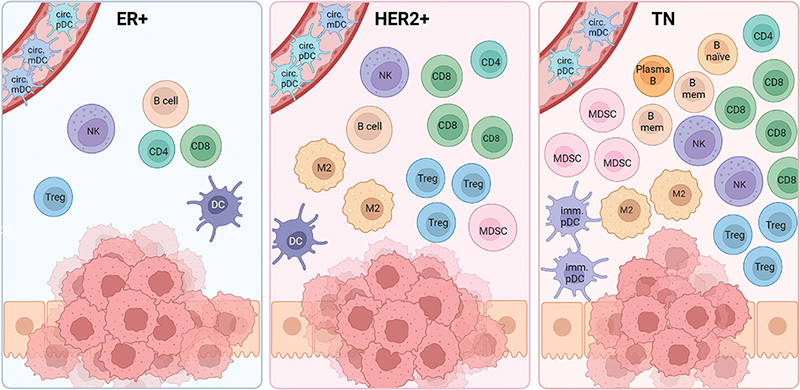
Figure 2. The presence of different immune cells in the molecular subtypes of breast cancer. CD8=CD8 T cell; CD4=CD4 T cell; Treg=Regulatory T cell; DC= Dendritic cell (mature); circ. mDC=Circulating myeloid DC; circ. pDC=Circulating plasmacytoid DC; imm. pDC=Immature pDC; Plasma B=Plasma B cell; B naïve=Naïve B cell; TAM=Tumor-associated macrophage; NK=Natural killer cell; MDSC=Myeloid-derived suppressor cell. Created with BioRender.com.
Tumor immune microenvironment in metastatic breast cancer
Many cancer types metastasize to predefined locations in the body, indicating that the spread is not random [53]. The hypothesis of ‘seed and soil’ was introduced by Paget over a century ago [54], where he proposed that cancer cells (seeds) are thought to thrive and grow in distant sites with favorable conditions (soil), and then ensuring their survival by altering the metastatic environment. The formation of a pre-metastatic niche is created by the primary tumor through several mechanisms including immunosuppression, inflammation, angiogenesis or vascular permeability, lymphangiogenesis, organotropism, and reprogramming [55].
Regional metastasis
Sentinel and the axillary lymph nodes are the lymph nodes located closest to the primary tumor and serve as primary drainage for the breast tissue. Interestingly, the sentinel lymph node, and not the primary tumor, has been suggested to be the first site of tumor–immune interaction [56]. These lymph nodes are the most common sites for metastasis, and approximately 20% of breast cancer patients in Norway have spread to sentinel and regional lymph nodes at the time of diagnosis [57]. Metastatic lymph nodes display a decreased CD4+ to CD8+ T cell ratio [58, 59] and reduced frequency of DCs [59]. Furthermore, various indicators of immunosuppressive environment are noted, including elevated levels of Tregs, MDSCs, and M2 macrophages [60–62]. In metastatic lymph nodes, T cells are discovered to express cytotoxic T-lymphocyte associated protein 4 (CTLA-4), programmed death receptor 1 (PD-1), and T cell immune receptor with Ig and ITIM domains (TIGIT) and exhibit exhaustion by suppressed TCR signaling [58, 61]
Distant metastasis
Distant metastasis involves tumor cells leaving the primary site and settling in distant organs. While early stage breast cancer has an estimated 5-year survival rate of approximately 95% and regional metastasis of 75%, the survival rate drops drastically to 27% for patients with distant metastasis [63]. The bone is the most frequent site for distant breast cancer metastasis for all subtypes, in particular for ER+/HER2- breast cancer, and about 70% of patients with metastatic disease develop bone metastases [64, 65]. The lung and liver are the second most common site of breast cancer metastasis, followed by the brain [66, 67]. The TIME of breast cancer metastasis is highly dependent on the location of the metastasis. By measuring TIL infiltration in secondary lesions from 94 breast cancer patients, Dieci et al. [68] found that TIL levels are generally low (below 5%) in metastatic lesions. In contrast, lung metastases had a median TIL level of approximately 30%.
Bone
Breast cancer is likely predisposed to metastasize to the bone due to the well-vascularized nature of the bone marrow. This quality creates a nutrient-rich environment abundant in growth factors and cytokines [69]. By residing in niches in the bone marrow, tumor cells can stay dormant for decades [70]. In this environment, breast cancer cells can interact with mesenchymal stem cells (MSCs), leading to an increased production of Th2 cytokines, recruitment of Tregs, and secretion of MSC-mediated TGF-β1 [71]. These immune modulatory factors contribute to the creation of an immunosuppressive environment, allowing the cancer cells to evade immune detection and elimination by the immune system. Compared to breast lesions, bone marrow metastases show fewer macrophages and an enrichment of neutrophils, indicating an impaired antigen presentation and increased tumor-promoting cytokine secretion [72].
Lung
TNBC commonly metastasize to the lungs [64, 67]. While interacting with the lung stroma, the cancer cells secrete exosomes that stimulates host fibroblasts to create a pre-metastatic microenvironment, and recruit circulating monocytes that differentiate into pro-tumor macrophages [73]. This results in systemic inflammatory cascades leading to neutrophil-mediated promotion of breast cancer metastasis [74].
Liver
HER2+ breast cancer tends to metastasize to the liver [64]. While the liver is rich in immunoreactive cells, it also possesses an immunotolerant microenvironment [75]. Colonization in the liver is facilitated by the secretion of pro-inflammatory cytokines by breast cancer cells, in addition to modulating hepatocytes to increase metastasis [73]. Resident Kupffer cells, liver-specific macrophages, play a role in promoting metastasis by secreting growth factors and recruiting immunosuppressive cells like neutrophils, macrophages, and MDSCs after extravasation [76].
Brain
Both HER2+ and TNBC metastasize to the brain [77–80]. The brain and central nervous system are immune-privileged sites and are partly separated from the immune system by the blood–brain barrier. The predominant immune cell type in the brain is microglia, capable of differentiating into macrophages. The TIME in brain metastasis is identified as immunosuppressive compared to the primary breast tumor [81], with a decrease in CD8+ T cells and M1 macrophages, and minimal presence of B cells [82, 83]. Conversely, M2 macrophages show an opposite trend [81, 83].
Immune checkpoint inhibitors
Immune checkpoints are regulatory pathways in the immune system, and represent important immunotherapeutic targets. Clinical trials on immunotherapy in breast cancer have increased rapidly after the discovery of immune checkpoint inhibitors, and PD1 and its ligand Programmed death receptor ligand 1 (PD-L1) are currently the most studied targets [84]. The interaction between PD-1, present on T cells, and PD-L1 and PD-L2, expressed by APCs and tumor cells, inhibits the cytotoxic effect of the immune cells, promotes T effector cell exhaustion, and promotes the conversion of T effector cells to Tregs [85]. PD-L1 inhibitors in combination with chemotherapy have demonstrated improved progression-free survival for both PD-L1+ (KEYNOTE-355 [86], KEYNOTE-522 [87], IMPASSION130 [88]) and PD-L1- patients (ALICE [89]). The PD-L1 inhibitor pembrolizumab, in combination with chemotherapy, is approved and used as standard of care in several countries in the treatment of metastatic PD-L1+ TNBC [90–92]. Targeting other immune checkpoint molecules such as T-cell immunoglobulin and mucin domain 3 (TIM-3), TIGIT and Lymphocyte-activation gene 3 (LAG-3) could potentially offer additional novel therapies. Tregs express immune checkpoints, and may be an unintended target for immune checkpoint inhibitors. Blomberg et al. recently discovered that depletion of Tregs in combination with adjuvant checkpoint inhibitors prolonged metastasis-related survival in breast cancer in mice, thus indicating that this could be a potential empowerment of checkpoint therapy [93].
Concluding remarks
The complexity of the tumor infiltrating lymphocytes and myeloid cells, comprising various immune cells, necessitates a deeper exploration of their interplay within the TIME. Technological advancements like single-cell sequencing and multiplexing offer opportunities for more comprehensive analyses, elucidating the dual role of immune cells as both anti-tumor and pro-tumor entities and the interplay between the different cell types. However, numerous aspects remain unknown, emphasizing the need to contextualize immune cell interactions within specific breast cancer subtypes and in various metastatic sites. Integrating emerging technologies and gaining deeper understanding of various immune cell types in breast cancer microenvironment are pivotal for unraveling complexities, refining prognostic and therapeutic strategies tailored to each subtype.
Data availability
Data sharing is not applicable as no new data generated.
Ethical statement
Due to the nature of the manuscript, ethical considerations do not apply.
References
[1] Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Ann Rev Immunol. 2004;22:329–60. https://doi.org/10.1146/annurev.immunol.22.012703.104803
[2] Goff SL, Danforth DN. The role of immune cells in breast tissue and immunotherapy for the treatment of breast cancer. Clin Breast Cancer. 2021;21(1):e63–73. https://doi.org/10.1016/j.clbc.2020.06.011
[3] Ogony J, Hoskin TL, Stallings-Mann M, et al. Immune cells are increased in normal breast tissues of BRCA1/2 mutation carriers. Breast Cancer Res Treat. 2023;197(2):277–85. https://doi.org/10.1007/s10549-022-06786-y
[4] Degnim AC, Brahmbhatt RD, Radisky DC, et al. Immune cell quantitation in normal breast tissue lobules with and without lobulitis. Breast Cancer Res Treat. 2014;144(3):539–49. https://doi.org/10.1007/s10549-014-2896-8
[5] Ferguson DJ. Intraepithelial lymphocytes and macrophages in the normal breast. Virchows Arch A Pathol Anat Histopathol. 1985;407(4):369–78. https://doi.org/10.1007/BF00709984
[6] Azizi E, Carr AJ, Plitas G, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018;174(5):1293–308.e36. https://doi.org/10.1016/j.cell.2018.05.060
[7] Kumar T, Nee K, Wei R, et al. A spatially resolved single-cell genomic atlas of the adult human breast. Nature. 2023;620(7972):181–91. https://doi.org/10.1038/s41586-023-06252-9
[8] Oertelt-Prigione S. Immunology and the menstrual cycle. Autoimmunity Rev. 2012;11(6):A486–92. https://doi.org/10.1016/j.autrev.2011.11.023
[9] Zirbes A, Joseph J, Lopez JC, et al. Changes in immune cell types with age in breast are consistent with a decline in immune surveillance and increased immunosuppression. J Mammary Gland Biol Neoplasia. 2021;26(3):247–61. https://doi.org/10.1007/s10911-021-09495-2
[10] Ménard S, Tomasic G, Casalini P, et al. Lymphoid infiltration as a prognostic variable for early-onset breast carcinomas. Clin Cancer Res. 1997;3(5):817–9.
[11] Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. JCO. 2013;31(7):860–7. https://doi.org/10.1200/JCO.2011.41.0902
[12] Mahmoud SMA, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. JCO. 2011;29(15):1949–55. https://doi.org/10.1200/JCO.2010.30.5037
[13] Denkert C, Von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. https://doi.org/10.1016/S1470-2045(17)30904-X
[14] Buisseret L, Garaud S, de Wind A, et al. Tumor-infiltrating lymphocyte composition, organization and PD-1/ PD-L1 expression are linked in breast cancer. OncoImmunology. 2017;6(1):e1257452. https://doi.org/10.1080/2162402X.2016.1257452
[15] Zhang Q, Wu S. Tertiary lymphoid structures are critical for cancer prognosis and therapeutic response. Front Immunol [Internet]. 2023 [cited 01-12-2023];13. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1063711
[16] Garaud S, Buisseret L, Solinas C, et al. Tumor-infiltrating B cells signal functional humoral immune responses in breast cancer. JCI Insight [Internet]. 2019 [cited 10-11-2021];4(18). Available from: https://insight.jci.org/articles/view/129641
[17] Harris RJ, Cheung A, Ng JCF, et al. Tumor-infiltrating B lymphocyte profiling identifies IgG-biased, clonally expanded prognostic phenotypes in triple-negative breast cancer. Cancer Res. 2021;81(16):4290–304. https://doi.org/10.1158/0008-5472.CAN-20-3773
[18] Khaja ASS, Toor SM, El Salhat H, et al. Preferential accumulation of regulatory T cells with highly immunosuppressive characteristics in breast tumor microenvironment. Oncotarget. 2017;8(20):33159–71. https://doi.org/10.18632/oncotarget.16565
[19] Saleh R, Elkord E. FoxP3+ T regulatory cells in cancer: prognostic biomarkers and therapeutic targets. Cancer Lett. 2020;490:174–85. https://doi.org/10.1016/j.canlet.2020.07.022
[20] Toffoli EC, Sheikhi A, Höppner YD, et al. Natural killer cells and anti-cancer therapies: reciprocal effects on immune function and therapeutic response. Cancers (Basel). 2021;13(4):711. https://doi.org/10.3390/cancers13040711
[21] Campbell KS, Hasegawa J. Natural killer cell biology: an update and future directions. J Allergy Clin Immunol. 2013;132(3):536–44. https://doi.org/10.1016/j.jaci.2013.07.006
[22] Albrecht AE, Hartmann BW, Scholten C, Huber JC, Kalinowska W, Zielinski CC. Effect of estrogen replacement therapy on natural killer cell activity in postmenopausal women. Maturitas. 1996;25(3): 217–22. https://doi.org/10.1016/S0378-5122(96)01063-8
[23] Scanlan JM, Werner JJ, Legg RL, Laudenslager ML. Natural killer cell activity is reduced in association with oral contraceptive use. Psychoneuroendocrinology. 1995;20(3):281–7. https://doi.org/10.1016/0306-4530(94)00059-J
[24] Bouzidi L, Triki H, Charfi S, et al. Prognostic value of natural killer cells besides tumor-infiltrating lymphocytes in breast cancer tissues. Clin Breast Cancer. 2021;21(6):e738–47. https://doi.org/10.1016/j.clbc.2021.02.003
[25] Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–50. https://doi.org/10.1093/annonc/mdu112
[26] Hu Q, Hong Y, Qi P, et al. Atlas of breast cancer infiltrated B-lymphocytes revealed by paired single-cell RNA-sequencing and antigen receptor profiling. Nat Commun. 2021;12(1):2186. https://doi.org/10.1038/s41467-021-22300-2
[27] Takenaka M, Seki N, Toh U, et al. FOXP3 expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Mol Clin Oncol. 2013;1(4):625–32. https://doi.org/10.3892/mco.2013.107
[28] Glajcar A, Szpor J, Hodorowicz-Zaniewska D, Tyrak KE, Okoń K. The composition of T cell infiltrates varies in primary invasive breast cancer of different molecular subtypes as well as according to tumor size and nodal status. Virchows Arch. 2019;475(1):13–23. https://doi.org/10.1007/s00428-019-02568-y
[29] Ono M, Tsuda H, Shimizu C, et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2012;132(3):793–805. https://doi.org/10.1007/s10549-011-1554-7
[30] Liu F, Lang R, Zhao J, et al. CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130(2):645–55. https://doi.org/10.1007/s10549-011-1647-3
[31] Ali HR, Provenzano E, Dawson SJ, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients. Ann Oncol. 2014;25(8):1536–43. https://doi.org/10.1093/annonc/mdu191
[32] Salemme V, Centonze G, Cavallo F, Defilippi P, Conti L. The crosstalk between tumor cells and the immune microenvironment in breast cancer: implications for immunotherapy. Front Oncol. 2021;11:289. https://doi.org/10.3389/fonc.2021.610303
[33] Levi I, Amsalem H, Nissan A, et al. Characterization of tumor infiltrating natural killer cell subset. Oncotarget. 2015;6(15):13835–43. https://doi.org/10.18632/oncotarget.3453
[34] Lapeyre-Prost A, Terme M, Pernot S, et al. Chapter seven – immunomodulatory activity of VEGF in cancer. In: Galluzzi L, editor. International review of cell and molecular biology [Internet]. Academic Press; 2017 [cited 14-11-2021]. p. 295–342. Available from: https://www.sciencedirect.com/science/article/pii/S1937644816301022
[35] Kiaei SZF, Nouralishahi A, Ghasemirad M, et al. Advances in natural killer cell therapies for breast cancer. Immunol Cell Biol [Internet]. [cited 21-08-2023];n/a(n/a). Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/imcb.12658
[36] Fernandez-Martinez A, Pascual T, Singh B, et al. Prognostic and predictive value of immune-related gene expression signatures vs tumor-infiltrating lymphocytes in early-stage ERBB2/HER2-positive breast cancer: a correlative analysis of the CALGB 40601 and PAMELA trials. JAMA Oncol. 2023;9(4):490–9. https://doi.org/10.1001/jamaoncol.2022.6288
[37] Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7–24. https://doi.org/10.1038/s41577-019-0210-z
[38] Greene TT, Jo Y, Zuniga EI. Infection and cancer suppress pDC derived IFN-I. Curr Opin Immunol. 2020;66:114–22. https://doi.org/10.1016/j.coi.2020.08.001
[39] Paek SH, Kim HG, Lee JW, et al. Circulating plasmacytoid and myeloid dendritic cells in breast cancer patients: a pilot study. J Breast Cancer. 2019;22(1):29–37. https://doi.org/10.4048/jbc.2019.22.e15
[40] Kini Bailur J, Gueckel B, Pawelec G. Prognostic impact of high levels of circulating plasmacytoid dendritic cells in breast cancer. J Transl Med. 2016;14(1):151. https://doi.org/10.1186/s12967-016-0905-x
[41] Treilleux I, Blay JY, Bendriss-Vermare N, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10(22):7466–74. https://doi.org/10.1158/1078-0432.CCR-04-0684
[42] Szpor J, Streb J, Glajcar A, et al. Dendritic cells are associated with prognosis and survival in breast cancer. Diagnostics (Basel). 2021;11(4):702. https://doi.org/10.3390/diagnostics11040702
[43] Ma Y, Shurin GV, Peiyuan Z, Shurin MR. Dendritic cells in the cancer microenvironment. J Cancer. 2013;4(1):36–44. https://doi.org/10.7150/jca.5046
[44] Wilson BE, Gorrini C, Cescon DW. Breast cancer immune microenvironment: from pre-clinical models to clinical therapies. Breast Cancer Res Treat. 2021;191:257–267. https://doi.org/10.1007/s10549-021-06431-0
[45] Markowitz J, Wesolowski R, Papenfuss T, Brooks TR, Carson WE. Myeloid-derived suppressor cells in breast cancer. Breast Cancer Res Treat. 2013;140(1):13–21. https://doi.org/10.1007/s10549-013-2618-7
[46] Wang PF, Song SY, Wang TJ, et al. Prognostic role of pretreatment circulating MDSCs in patients with solid malignancies: a meta-analysis of 40 studies. OncoImmunology. 2018;7(10):e1494113. https://doi.org/10.1080/2162402X.2018.1494113
[47] Kumar S, Wilkes DW, Samuel N, et al. ΔNp63-driven recruitment of myeloid-derived suppressor cells promotes metastasis in triple-negative breast cancer. J Clin Invest. 2018;128(11):5095–109. https://doi.org/10.1172/JCI99673
[48] Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. https://doi.org/10.1007/s00262-008-0523-4
[49] Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196(3):254–65. https://doi.org/10.1002/path.1027
[50] Mahmoud SMA, Lee AHS, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2012;65(2):159–63. https://doi.org/10.1136/jclinpath-2011-200355
[51] Zhang Y, Cheng S, Zhang M, et al. High-infiltration of tumor-associated macrophages predicts unfavorable clinical outcome for node-negative breast cancer. PLoS One. 2013;8(9):e76147. https://doi.org/10.1371/journal.pone.0076147
[52] Tiainen S, Tumelius R, Rilla K, et al. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology. 2015;66(6):873–83. https://doi.org/10.1111/his.12607
[53] Cox TR, Gartland A, Erler JT. The pre-metastatic niche: is metastasis random? Bonekey Rep. 2012;1:80. https://doi.org/10.1038/bonekey.2012.80
[54] Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8(2):98–101.
[55] Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 2016;30(5):668–81. https://doi.org/10.1016/j.ccell.2016.09.011
[56] Liao N, Li C, Cao L, et al. Single-cell profile of tumor and immune cells in primary breast cancer, sentinel lymph node, and metastatic lymph node. Breast Cancer. 2023;30(1):77–87. https://doi.org/10.1007/s12282-022-01400-x
[57] Moshina N, Sebuødegård S, Lee CI, et al. Automated volumetric analysis of mammographic density in a screening setting: worse outcomes for women with dense breasts. Radiology. 2018;288(2):343–52. https://doi.org/10.1148/radiol.2018172972
[58] Rye IH, Huse K, Josefsson SE, et al. Breast cancer metastasis: immune profiling of lymph nodes reveals exhaustion of effector T cells and immunosuppression. Mol Oncol. 2022;16(1):88–103. https://doi.org/10.1002/1878-0261.13047
[59] Kohrt HE, Nouri N, Nowels K, Johnson D, Holmes S, Lee PP. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2005;2(9):e284. https://doi.org/10.1371/journal.pmed.0020284
[60] Núñez NG, Tosello Boari J, Ramos RN, et al. Tumor invasion in draining lymph nodes is associated with Treg accumulation in breast cancer patients. Nat Commun. 2020;11:3272. https://doi.org/10.1038/s41467-020-17046-2
[61] van Pul KM, Vuylsteke RJCLM, van de Ven R, et al. Selectively hampered activation of lymph node-resident dendritic cells precedes profound T cell suppression and metastatic spread in the breast cancer sentinel lymph node. J Immunother Cancer. 2019;7:133. https://doi.org/10.1186/s40425-019-0605-1
[62] Mansfield AS, Heikkila P, von Smitten K, Vakkila J, Leidenius M. The presence of sinusoidal CD163+ macrophages in lymph nodes is associated with favorable nodal status in patients with breast cancer. Virchows Arch. 2012;461(6):639–46. https://doi.org/10.1007/s00428-012-1338-4
[63] DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA: A Cancer J Clin. 2019;69(6):438–51. https://doi.org/10.3322/caac.21583
[64] van Uden DJP, van Maaren MC, Strobbe LJA, et al. Metastatic behavior and overall survival according to breast cancer subtypes in stage IV inflammatory breast cancer. Breast Cancer Res. 2019;21(1):113. https://doi.org/10.1186/s13058-019-1201-5
[65] Manders K, van de Poll-Franse LV, Creemers GJ, et al. Clinical management of women with metastatic breast cancer: a descriptive study according to age group. BMC Cancer. 2006;6:179. https://doi.org/10.1186/1471-2407-6-179
[66] Molnár IA, Molnár BÁ, Vízkeleti L, et al. Breast carcinoma subtypes show different patterns of metastatic behavior. Virchows Arch. 2017;470(3):275–83. https://doi.org/10.1007/s00428-017-2065-7
[67] Gerratana L, Fanotto V, Bonotto M, et al. Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis. 2015;32(2):125–33. https://doi.org/10.1007/s10585-015-9697-2
[68] Dieci MV, Tsvetkova V, Orvieto E, et al. Immune characterization of breast cancer metastases: prognostic implications. Breast Cancer Res. 2018;20:62. https://doi.org/10.1186/s13058-018-1003-1
[69] Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7(4):208–18. https://doi.org/10.1038/nrendo.2010.227
[70] Tivari S, Lu H, Dasgupta T, De Lorenzo MS, Wieder R. Reawakening of dormant estrogen-dependent human breast cancer cells by bone marrow stroma secretory senescence. Cell Commun Signa. 2018;16(1):48. https://doi.org/10.1186/s12964-018-0259-5
[71] Walker ND, Patel J, Munoz JL, et al. The bone marrow niche in support of breast cancer dormancy. Cancer Lett. 2016;380(1):263–71. https://doi.org/10.1016/j.canlet.2015.10.033
[72] Lee H, Na KJ, Choi H. Differences in tumor immune microenvironment in metastatic sites of breast cancer. Front Oncol. 2021;11:722. https://doi.org/10.3389/fonc.2021.649004
[73] Terceiro LEL, Edechi CA, Ikeogu NM, et al. The breast tumor microenvironment: a key player in metastatic spread. Cancers. 2021;13(19):4798. https://doi.org/10.3390/cancers13194798
[74] Kersten K, Coffelt SB, Hoogstraat M, et al. Mammary tumor-derived CCL2 enhances pro-metastatic systemic inflammation through upregulation of IL1β in tumor-associated macrophages. OncoImmunology. 2017;6(8):e1334744. https://doi.org/10.1080/2162402X.2017.1334744
[75] Yan CY, Zhao ML, Wei YN, Zhao XH. Mechanisms of drug resistance in breast cancer liver metastases: dilemmas and opportunities. Mol Ther – Oncolytics. 2023;28:212–29. https://doi.org/10.1016/j.omto.2023.02.001
[76] Tsilimigras DI, Brodt P, Clavien PA, et al. Liver metastases. Nat Rev Dis Primers. 2021;7(1):1–23. https://doi.org/10.1038/s41572-021-00261-6
[77] Heitz F, Harter P, Lueck HJ, et al. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer. 2009;45(16):2792–8. https://doi.org/10.1016/j.ejca.2009.06.027
[78] Aversa C, Rossi V, Geuna E, et al. Metastatic breast cancer subtypes and central nervous system metastases. Breast. 2014;23(5):623–8. https://doi.org/10.1016/j.breast.2014.06.009
[79] Kim YJ, Kim JS, Kim IA. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J Cancer Res Clin Oncol. 2018;144(9):1803–16. https://doi.org/10.1007/s00432-018-2697-2
[80] Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. JCO. 2010;28(20):3271–7. https://doi.org/10.1200/JCO.2009.25.9820
[81] Lu W, Xie H, Yuan C, Li J, Li Z, Wu A. Genomic landscape of the immune microenvironments of brain metastases in breast cancer. J Transl Med. 2020;18(1):327. https://doi.org/10.1186/s12967-020-02503-9
[82] Berghoff AS, Lassmann H, Preusser M, Höftberger R. Characterization of the inflammatory response to solid cancer metastases in the human brain. Clin Exp Metastasis. 2013;30(1):69–81. https://doi.org/10.1007/s10585-012-9510-4
[83] Noh MG, Kim SS, Kim YJ, et al. Evolution of the tumor microenvironment toward immune-suppressive seclusion during brain metastasis of breast cancer: implications for targeted therapy. Cancers. 2021;13(19):4895. https://doi.org/10.3390/cancers13194895
[84] Debien V, De Caluwé A, Wang X, et al. Immunotherapy in breast cancer: an overview of current strategies and perspectives. npj Breast Cancer. 2023;9(1):1–10. https://doi.org/10.1038/s41523-023-00508-3
[85] Gaynor N, Crown J, Collins DM. Immune checkpoint inhibitors: key trials and an emerging role in breast cancer. Semin Cancer Biol [Internet]. 2020 [cited 24-11-2021]. Available from: https://www.sciencedirect.com/science/article/pii/S1044579X20301528
[86] Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–28.
[87] Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21. https://doi.org/10.1056/NEJMoa1910549
[88] Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21. https://doi.org/10.1056/NEJMoa1809615
[89] Røssevold AH, Andresen NK, Bjerre CA, et al. Atezolizumab plus anthracycline-based chemotherapy in metastatic triple-negative breast cancer: the randomized, double-blind phase 2b ALICE trial. Nat Med. 2022;28(12):2573–83. https://doi.org/10.1038/s41591-022-02126-1
[90] Merck.com [Internet]. European Commission Approves KEYTRUDA® (pembrolizumab) Plus Chemotherapy as Neoadjuvant Treatment, Then Continued as Adjuvant Monotherapy After Surgery for Locally Advanced or Early-Stage Triple-Negative Breast Cancer at High Risk of Recurrence. [cited 28-11-2023]. Available from: https://www.merck.com/news/european-commission-approves-keytruda-pembrolizumab-plus-chemotherapy-as-neoadjuvant-treatment-then-continued-as-adjuvant-monotherapy-after-surgery-for-locally-advanced-or-early-stage-triple/
[91] Merck.com [Internet]. Merck’s KEYTRUDA® (pembrolizumab) Receives Four New Approvals in Japan, Including in High-Risk Early-Stage Triple-Negative Breast Cancer (TNBC). [cited 28-11-2023]. Available from: https://www.merck.com/news/mercks-keytruda-pembrolizumab-receives-four-new-approvals-in-japan-including-in-high-risk-early-stage-triple-negative-breast-cancer-tnbc/
[92] Merck.com [Internet]. FDA approves KEYTRUDA® (pembrolizumab) for treatment of patients with high-risk early-stage triple-negative breast cancer in combination with chemotherapy as neoadjuvant treatment, then continued as single agent as adjuvant treatment after surgery. [cited 28-11-2023]. Available from: https://www.merck.com/news/fda-approves-keytruda-pembrolizumab-for-treatment-of-patients-with-high-risk-early-stage-triple-negative-breast-cancer-in-combination-with-chemotherapy-as-neoadjuvant-treatment-then-continued/
[93] Blomberg OS, Kos K, Spagnuolo L, et al. Neoadjuvant immune checkpoint blockade triggers persistent and systemic Treg activation which blunts therapeutic efficacy against metastatic spread of breast tumors. Oncoimmunology. 2023;12(1):2201147. https://doi.org/10.1080/2162402X.2023.2201147