ORIGINAL ARTICLE
18F-FDG-PET/CT in breast cancer imaging: Restaging and Implications for treatment decisions in a clinical practice setting
Ida Skarping, MD, PhD, Assoc Profa,b*
aDivision of Oncology, Department of Clinical Sciences, Lund University, Skåne University Hospital, Lund, Sweden; bDepartment of Clinical Physiology and Nuclear Medicine, Skane University Hospital, Lund, Sweden
Restaging
Change in clinical managment
18F-FDG-PET/CT in breast cancer imaging: restaging and implications for treatment decisions in a clinical practice setting
ABSTRACT
Background and purpose: Although the diagnostic accuracy of 18F-fluorodeoxyglucose – positron emission tomography/computed tomography (18F-FDG-PET/CT) for breast cancer (BC) has been well studied, few studies have evaluated the impact of 18F-FDG-PET/CT on BC patient care. This study aimed to investigate restaging and 18F-FDG-PET/CT-induced changes in clinical decision-making in patients with BC.
Material and methods: We retrospectively evaluated 18F-FDG-PET/CT-scans performed for BC-related indications in a prospectively collected consecutive cohort of adult patients at Skane University Hospital, Sweden. Patients with all BC stages were included and divided into three groups based on the indication for 18F-FDG-PET/CT: Group A (primary staging), Group B (response evaluation), and Group C (recurrence). The impact of 18F-FDG-PET/CT-scans on clinical management was categorized as no change, minor change (e.g. modification of treatment plans), or major change (e.g. shift from curative to palliative treatment intention).
Results: A total of 376 scans (151 patients) were included: Group A 9.3% (35 of 376 scans), Group B 77.4% (291 of 376 scans), and Group C 13.3% (50 of 376 scans). Significant stage migration, predominantly upstaging, occurred in Group A (45.7%) and Group C (28.0%). Changes in clinical management were observed in 120 scans (31.9%), of which 66 were major and 54 were minor. The largest proportion of 18F-FDG-PET/CT-induced management changes were observed in Group A (57.1%), most commonly a shift from curative to palliative treatment intention due to upstaging.
Interpretation: Our study indicates the clinical utility of 18F-FDG-PET/CT in BC restaging and changes in clinical management; the latter observed in approximately one-third of all cases.
KEYWORDS: Breast cancer; imaging; staging; 18F-FDG; PET/CT; clinical management
Citation: ACTA ONCOLOGICA 2024, VOL. 63, 669–677. https://doi.org/10.2340/1651-226X.2024.40003.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Oncologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 30 January 2024; Accepted: 4 July 2024; Published: 11 August 2024
CONTACT Ida Skarping ida.skarping@med.lu.se Medicon Village 406, Lund University, SE-223 81 Lund, Sweden.
Supplemental data for this article can be accessed online at https://doi.org/10.2340/1651-226X.2024.40003
Competing interests and funding: The author reports there are no competing interests to declare.
This work was supported by grants from the Governmental Funding of Clinical Research within the National Health Services (Yngre-ALF Ida Skarping). The funding resources had no role in the study design, data collection, analyses, data interpretation, writing of the manuscript or the decision to submit the manuscript for publication.
Introduction
Breast cancer (BC) stage is an important prognostic factor for recurrence and overall survival [1]. Imaging is used to guide clinical decision-making and optimize treatment strategies. Therefore, the choice of diagnostic modality is important. While mammography, ultrasound, and magnetic resonance imaging (MRI), are used for imaging of the breast and axilla, contrast-enhanced computed tomography (i.e. CT) is used widely for staging here beyond. When applicable, bone scintigraphy can be used to detect recurrence [2]. However, positron emission tomography (PET) is a valuable instrument for comprehensive whole-body imaging in BC management in both the initial staging and metastatic setting [3–5].
Contrary to visualizing anatomical structures, PET is a functional imaging modality used to visualize the uptake of radioactive substances, for example, 18F-fluorodeoxyglucose (18F-FDG). PET is combined with CT in PET/CT, where the CT findings act as an anatomical reference for the molecular and functional information provided by PET, in addition to attenuation correction [6]. Following 18F-FDG administration, accumulation in tissue is proportional to the degree of glucose metabolism, where high metabolic activity is one of the hallmarks of cancer, including BC [7,8]. Although the uptake mechanism is similar to that of glucose in cells, the radiotracer is intracellularly trapped and thus not fully metabolized [6], which is a prerequisite for 18F-FDG-PET diagnostics.
The diagnostic accuracy of detecting distant BC metastases with 18F-fluorodeoxyglucose – Positron emission tomography/Computed tomography (18F-FDG-PET/CT) is high; in a meta-analysis by Hong et al. [9], the pooled sensitivity was 96% and the negative likelihood ratio was 0.03. As demonstrated by Koolen et al., for regional BC metastatic evaluation, the sensitivity of 18F-FDG-PET/CT is lower at 82%, however, still with a high specificity of 92% [10]. Moreover, accurate BC staging with 18F-FDG-PET/CT varies with tumor characteristics including St Gallen surrogate and histological subtypes [4,11,12]; a lower sensitivity of 18F-FDG-PET is seen in lobular BC due to its more indolent biology and lower 18F-FDG avidity [4,11,12]. Considering another PET radiotracer in BC, the recently updated NCCN guidelines now include 18F-Fluorestradiol (18F-FES) PET for potential use in evaluating estrogen receptor-positive metastatic BC [13].
18F-FDG-PET/CT is typically indicated only for initial staging in patients with stage IIB-III BC when distant metastases are suspected [13,14]. Additionally, unclear or contradictory findings on conventional imaging or specific symptoms may lead to a referral for 18F-FDG-PET/CT regardless of stage [13,15,16]. For patients with stage I–II and operable stage III (T3, N1), routine use of 18F-FDG-PET/CT is not recommended due to high false-negative rates for small lesions and a low overall probability of metastatic disease [13,15]. 18F-FDG-PET/CT may also be beneficial for treatment evaluation in both neoadjuvant and metastatic settings [13,14], especially for monitoring bone-only/-predominant metastases [17,18] (Supplementary Material 1).
While diagnostic accuracy is of paramount importance, in a clinical context, scan-induced restaging and modification of treatment are also highly relevant. Although the ultimate endpoint is a gain or loss in morbidity and mortality, the direct clinical consequences of diagnostics can be less obvious than those of a therapeutic procedure [19]. The concept of clinical utility emphasizes that a diagnostic test’s health benefit arises from using information to guide management or resolve uncertainty, reducing patients’ emotional burden [19].
This study aimed to investigate stage migration and changes in clinical management following 18F-FDG-PET/CT-scan in a heterogeneous consecutive cohort of clinical BC patients.
Material and methods
Cohort data
Eligible participants in this retrospective analysis included adult patients (aged ≥ 18 years) with a clinical indication for 18F-FDG-PET/CT due to BC at Skane University Hospital, Sweden, between November 2017 and October 2023, who were prospectively included in a larger, overarching institution-based study for validation of PET/CT.
Initially, 325 consecutive scans were identified. Additional 92 18F-FDG-PET/CT scans performed on patients in the cohort, but not previously identified due to missing/inaccurate annotation in the validation study, were also included. A total number of 41 scans were excluded (Figure 1).
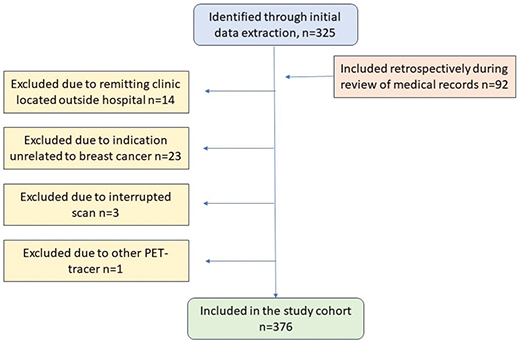
Figure 1. Flow chart of study cohort.
Demographic data, blood glucose level, and reason for medical referral were obtained from the validation study for all patients at each scan. Clinicopathological data, oncological treatment, and Tumor Node Metastasis (TNM) stage before and after the 18F-FDG-PET/CT-scan were verified by reviewing the patient’s digital medical record and picture archiving and communication system.
This study was designed and the manuscript was written in accordance with the STROBE guidelines [20].
Definitions
The TNM stage was determined using the guidelines established by the American Joint Committee on Cancer [21] in cases where the TNM stage was not explicitly stated in the medical records. The TNM stage was determined through the assessment of all available imaging and biopsy results. The classification of ‘no evidence of disease’ was defined as complete remission pathologically/metabolically and structurally for Group B and C.
18F-FDG-PET/CT
18F-FDG-PET/CT scans were performed using Discovery MI or Discovery 690 (GE Healthcare, Milwaukee, USA) PET/CT system. 18F-FDG-radiotracers were prepared according to established techniques and clinical routines. The intravenously administered activity was 4 MBq/kg (maximum 500 MBq). Approximately 60 minutes after injection, the scan was performed in the supine position with arms raised (unless mobility was restricted). As per standard procedure, the scan covered the area from the orbitomeatal line to the upper thigh, with an acquisition time of approximately 1.5 minutes per bed position. Attenuation correction and anatomic correlation were achieved through a low-dose CT or diagnostic CT (with contrast if there were no contraindications).
Clinical management: Group allocation into three categories
The scans were divided into three groups based on the reasons for 18F-FDG-PET/CT referral: Group A, Primary staging (unclear/contradictory findings in conventional imaging scans); Group B, Response evaluation (assessment of treatment response/residual tumor); and Group C, Recurrence (suspected recurrence or proven locoregional recurrence) (Figure 2). All newly diagnosed patients were allocated to Group A. While the patients in Group B and Group C might overlap, those categorized as C had no ongoing treatments and were considered to be in remission or had discovered locoregional recurrence without current knowledge of distant spread.
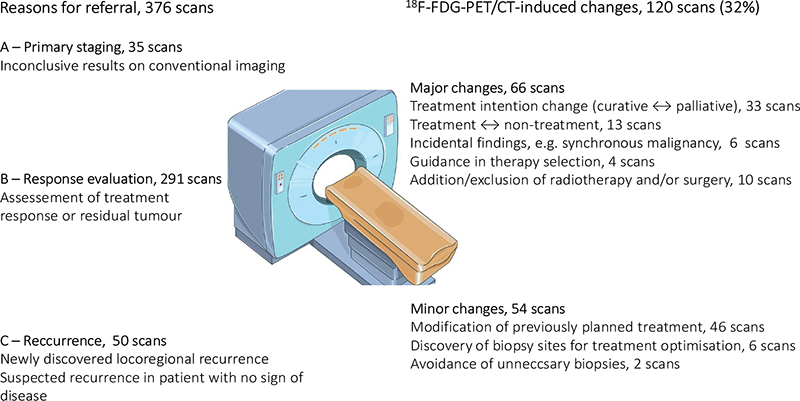
Figure 2. Schematic figure: reason for referral and scan-induced changes in clinical management.
18F-FDG-PET/CT-induced management changes were divided into three prespecified categories: no change, minor change (e.g. modification of treatment), and major changes (e.g. change of treatment) (Figure 2).
Statistical analyses
Categorical variables were summarized as counts/percentages and continuous variables as medians/interquartile ranges (IQRs). Statistics are presented both per-scan and per-patient due to a substantial number of patients having multiple scans. Demographics were analysed using chi-square tests for categorical variables and Kruskal–Wallis test for continuous variables. For assessment of reclassification in Group A, B, and C, respectively, Wilcoxon signed ranks test was used.
A p value < 0.05 was considered significant. No a priori power calculations were performed for this consecutive cohort.
IBM SPSS Statistics version 28 was used for all statistical analyses.
Results
Patient and tumor characteristics: Descriptive results
The patient characteristics of the 376 scans (151 individual patients) are described in Table 1, Supplementary Material 2. The median age was 61 years. A total of 79 patients had multiple scans performed (range 2–18) and 72 patients had single scans performed. The distribution of the tumor characteristics estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2, and histological subtypes were similar in Group A–C, whereas highly proliferative tumors (Ki67) were more common in Group A (Table 1). Invasive BC of no special type (NST) BC was the most common histological subtype in all groups (range 63–76%) followed by ILC (range 12–21%) (Supplementary Material 3). Previous and ongoing treatment per person is outlined in Supplementary Material 4.
| Scans, 376 scans | Group A | Group B | Group C | p | ||||||||||
| n | % | Median | IQR | n | % | Median | IQR | n | % | Median | IQR | |||
| Total scans (%) (NB: Row percent) | 35 | 9.3 | 291 | 77.4 | 50 | 13.3 | ||||||||
| Age at each scan (years) | Median (IQR) | 61 | 48–71 | 61 | 49–71 | 58 | 45–72 | 0.416 | ||||||
| BMI (kg/m2) | Median (IQR) | 25.2 | 22.1–28.5 | 25.8 | 22.1–28.5 | 26.0 | 22.7–29.8 | 0.784 | ||||||
| Missing (n) | 2 | 21 | 3 | |||||||||||
| Blood glucose (mmol/L) | Median (IQR) | 5.6 | 5.1–6.3 | 5.5 | 5.1–6.0 | 5.6 | 5.0–6.1 | 0.701 | ||||||
| Missing (n) | 4 | 26 | 4 | |||||||||||
| Estrogen receptor statusa,b | Positive | 25 | 72.2 | 229 | 79.2 | 32 | 74.4 | 0.614 | ||||||
| Negative | 9 | 26.5 | 60 | 20.8 | 11 | 25.6 | ||||||||
| Missing | 1 | 2 | 7 | |||||||||||
| Progesterone receptor statusa,b | Positive | 24 | 72.7 | 186 | 64.8 | 29 | 67.4 | 0.644 | ||||||
| Negative | 9 | 27.3 | 101 | 35.2 | 14 | 32.6 | ||||||||
| Missing | 2 | 4 | 7 | |||||||||||
| HER2 statusa,c | Positive | 6 | 18.8 | 31 | 10.9 | 39 | 92.9 | 0.277 | ||||||
| Negative | 26 | 81.3 | 254 | 89.1 | 3 | 7.1 | ||||||||
| Missing | 3 | 6 | 8 | |||||||||||
| Ki67a | > 20% (high) | 27 | 84.4 | 127 | 59.6 | 25 | 71.4 | 0.015* | ||||||
| ≤ 20% (low) | 5 | 15.6 | 86 | 40.4 | 10 | 28.6 | ||||||||
| Missing | 3 | 78 | 15 | |||||||||||
| Pre-scan TNM stage | No evidence of disease | 0 | 13 | 4.5 | 43 | 86.0 | ||||||||
| I | 0 | 3 | 1.0 | 0 | ||||||||||
| II | 17 | 48.6 | 8 | 2.7 | 0 | |||||||||
| III | 16 | 45.7 | 84 | 28.9 | 3 | 6.0 | ||||||||
| IV | 2 | 5.7 | 183 | 62.9 | 4 | 8.0 | ||||||||
| Chi-square test for categorical variables and Kruskal Wallis for continuous variables. BMI: body mass index; 18F-FDG-PET/CT: 18F-fluorodeoxyglucose – positron emission tomography/computed tomography; HER2: human epidermal growth factor receptor 2; TNM: tumor node metastases. aTumor characteristics based on biopsy results from the primary tumor at the time of breast cancer diagnosis. In the cases of missing data on the primary tumor, the latest biopsy result was used. bPositive expression of hormone receptors was defined as ≥ 10% for each receptor [15]. cHER2-amplification was defined as 2 + and FISH-positive, or 3 +. *p < 0.05. |
||||||||||||||
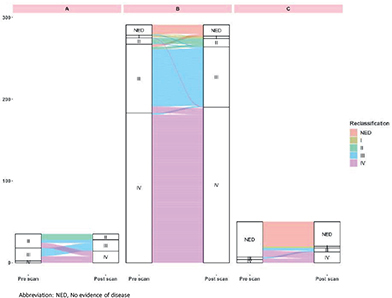
Stage migration
In total, 13.3% (50 of 376) of the scans resulted in restaging, most commonly upstaging (42 of 50, 84%) (Tables 2, 3, and Figure 3). A significant change in stage was observed in Groups A and C but not in Group B (Table 2). All eight scans resulting in downstaging occurred in Group B (response evaluation) (Table 3, Figure 3). In total, 15 of 56 scans (26.8%) categorized as having no evidence of disease pre-scan were restaged. A larger proportion of restaging was observed in the earlier BC stages: 42.9% (12 of 28) in stage I–11, 20.4% (21 of 103) in stage III, and 1.1% (2 of 189) in stage IV (downstaging) (Supplementary Material 5).
| Group | Stage | Before 18F-FDG-PET/CT | After 18F-FDG-PET/CT | p | ||
| n | % | n | % | |||
| A, Primary staging (35 scans) | I | 0 | 0 | 0 | 0 | < 0.001* |
| II | 17 | 48.6 | 7 | 20.0 | ||
| III | 16 | 45.7 | 14 | 40.0 | ||
| IV | 2 | 5.7 | 14 | 40.0 | ||
| B, Response evaluation (291 scans) | NED | 13 | 4.5 | 14 | 4.8 | 0.665 |
| I | 3 | 1.0 | 3 | 1.0 | ||
| II | 8 | 2.7 | 10 | 3.5 | ||
| III | 84 | 28.9 | 74 | 25.4 | ||
| IV | 183 | 62.9 | 190 | 65.3 | ||
| C, Recurrence (50 scans) | NED | 43 | 86.0 | 30 | 60.0 | < 0.001* |
| I | 0 | 0 | 2 | 4.0 | ||
| II | 0 | 0 | 0 | 0 | ||
| III | 3 | 6.0 | 5 | 10.0 | ||
| IV | 4 | 8.0 | 13 | 26.0 | ||
| TNM: tumor node metastases; 18F-FDG-PET/CT: 18F-fluorodeoxyglucose – positron emission tomography/computed tomography; NED: no evidence of disease. Wilcoxon Signed Ranks Test, *p < 0.05. |
||||||
| Change in stage (difference between stage pre-scan and post-scan) | -4 | -3 | -2 | -1 | 0 | 1 | 2 | 3 | 4 | Total restaging |
| A, Primary staging (35 scans) | 0 | 0 | 6 | 10 | 19 | 0 | 0 | 0 | 0 | 16 (45.7%) |
| B, Response evaluation (291 scans) | 0 | 2 | 0 | 10 | 271 | 5 | 0 | 3 | 0 | 20 (6.9%) |
| C, Recurrence (50 scans) | 8 | 3 | 0 | 3 | 36 | 0 | 0 | 0 | 0 | 14 (28.0%) |
| Total, 376 scans | 8 | 5 | 6 | 23 | 326 | 5 | 0 | 3 | 0 | 50 (13.3%) |
| p < 0.001* | ||||||||||
| 18F-FDG-PET/CT: 18F-fluorodeoxyglucose – positron emission tomography/computed tomography. *The two-tailed Chi-square test |
||||||||||
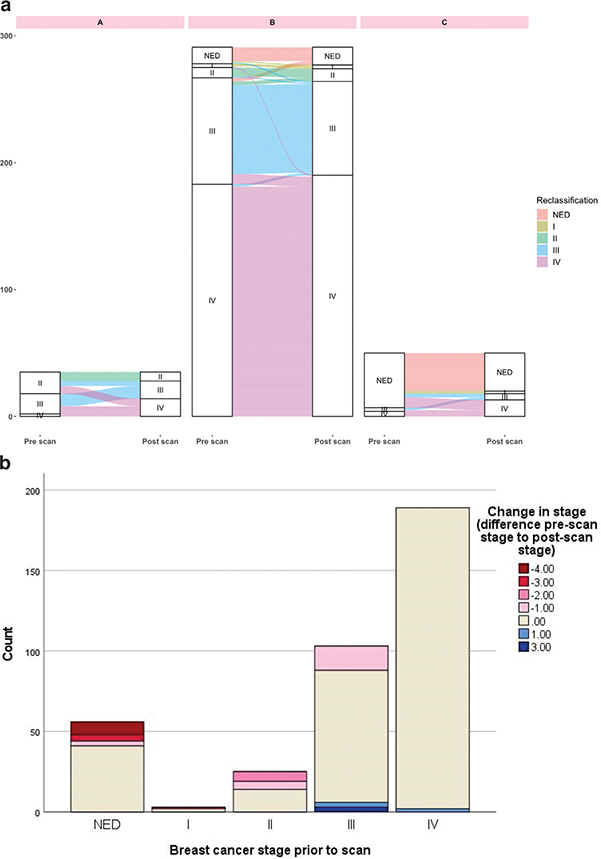
Figure 3. (A) Sankey diagram visualizing changes in breast cancer stage in each Group A–C. The Sankey diagram was made using R version 4.2.2. (B) Restaging according to stage prior to scan. -4 to -1 indicates upstaging, 0 equals no change in stage, and 1–3 indicates downstaging.
In Group B, analysis of stage migration on a a per-patient basis showed a larger proportion of restaging compared to the per-scan analysis (12 of 76, 15.8% vs. 20 of 291, 6.9%) (Supplementary Material 6–9).
18F-FDG PET/CT-induced change in clinical management
Of the 376 scans, 120 (31.9%) led to changes in the clinical management. A total of 54 of 120 (45.0%) changes were classified as minor changes, and 66 of 120 (55.0%) were classified as major changes (Figure 2, Table 4). In 256 scans, the 18F-FDG-PET/CT-scan did not lead to verified changes in clinical management. The largest proportion of scan-induced changes was observed in Group A (57.1%), followed by Group C (32.0%). In both groups, the specific change was related to the findings of metastatic disease and thus re-evaluation of treatment intention.
In the response evaluation Group B (291 scans), the majority of documented minor changes (50 scans) were in the form of modifications to previously planned treatments (45 scans), whereas in five scans, information on biopsy sites was determined. The major changes observed in this group (34 scans) exhibited a diverse range of alterations.
Analysis on a per-patient basis showed a larger proportion of scan-induced changes in clinical management (61 of 151, 40.4% vs. 120 of 376, 31.9%). Differences in scan-induced changes were observed in Group B (response evaluation) (32.9% vs. 28.9%) and Group C (recurrence) (40.0% vs. 32.0%) (Supplementary Material 10).
Discussion
In this retrospective cohort study involving 376 consecutive clinical 18F-FDG-PET/CT scans performed on BC patients across diverse clinical settings, our findings reveal a high rate of restaging, primarily upstaging. Moreover, one-third of all scans resulted in alterations in clinical management. The most frequent changes involved modifications to the planned systemic treatment, such as adjustments to dosage or switching from one chemotherapy regimen to another, and instances in which the treatment objective was changed from curative to palliative.
Our cohort consisted primarily of patients in late stages (77.7% of the scans BC stage III–IV) prior to undergoing the scan, which aligns with the current national and international guidelines and enhances the validity of our finding [14–18]. The usefulness of 18F-FDG-PET/CT imaging in the late-stage setting was demonstrated by the detection of suspected recurrence in 16 of 50 scans (32.0%) conducted on patients in remission without evidence of disease. However, the highest rate of restaging and scan-induced changes in clinical management was observed in patients undergoing 18F-FDG-PET/CT as part of their initial work-up, who generally had a lower BC stage.
The presented data on the reclassification of staging and alterations in clinical management prompted by 18F-FDG-PET/CT-scans collectively suggest that 18F-FDG-PET/CT could serve as a versatile tool for the clinical management of BC patients across a range of clinical contexts.
Comparison to previous literature
Stage migration
In their meta-analysis, Han et al. [3] reported a change in BC stage in a pooled proportion of 20 and 25% of patients who underwent PET/low-dose CT and PET/diagnostic CT, respectively, during their initial BC work-up, without any specific stage or histological subtype criteria. Groheux et al. [22] conducted a prospective study on 131 patients with stage IIA–IIIA disease and observed changes in stage of 6, 15, and 28% within their stage IIA, IIB, and IIIA groups, respectively. In contrast, our cohort showed 44.0% (11 of 25) of stage II patients and 20.3% (21 of 103) of stage III patients experiencing restaging. However, Groheux et al. did not include patients with an initial stage IV disease, which constituted the majority of our cohort. Yararbas et al. [23] conducted a retrospective study on 234 patients and detected metastases in 64 of them, corresponding to stage migration from IIA–IIIC to stage IV in 28% of the cohort. Vogsen et al. [24] investigated restaging in a Danish study cohort of 103 high-risk primary BC patients with tumor size ≥ 50 mm or ≥ 4 malignant axillary lymph nodes, where 18F-FDG-PET/CT detected previously unknown distant metastases in 23% (24 of 103) of the patients.
The results of our study emphasize findings from previous studies investigating stage migration after 18F-FDG-PET/CT-scans, the results are congruent with the general conclusion that 18F-FDG-PET/CT-scans precedes stage changes across various clinical BC scenarios. Nonetheless, we acknowledge that the clinical significance may be smaller in more advanced stages, such as metastatic stage IV BC. Still, it remains interesting to assess the influence of 18F-FDG-PET/CT scans in stage IV BC patients; for example, in treatment response evaluation, 18F-FDG PET/CT more often discriminates between progression/regression and stable disease compared to conventional imaging techniques [25]. Additionally, 18F-FDG-PET/CT offers distinct advantages over other imaging techniques, such as conventional CT, as it facilitates earlier identification of progression or response during treatment [26–28].
18F-FDG PET/CT induced change in management
Although 18F-FDG-PET/CT has been repeatedly demonstrated to upstage patients [3], the clinical impact of the scan results is less often evaluated. In this study, we observed that 18F-FDG-PET/CT led to changes in management in 31.9% of the 376 scans performed, with approximately half of these changes considered major (66 of 120, 55.0%), most commonly change from curative to palliative treatment as a result of upstaging. The greatest proportion of scan-induced changes was observed in patients undergoing scans as part of their primary staging workup (57.1%, 20 of 35). In total, 54 changes were minor, mostly modifications to previously planned or ongoing systemic treatment. Notably, in three patients undergoing 18F-FDG-PET/CT as part of restaging, no metabolic activity was detected in previously suspected distant metastases, leading to a lower stage and a shift in treatment from palliative to curative.
Given the different prerequisites in each group, the scan-induced changes in Group A–C should be contextualized. Owing to the diverse nature of the cohort and the reasons for undergoing 18F-FDG-PET/CT scans, the significance of scan-induced changes may vary between the groups. In primary staging, 18F-FDG-PET/CT is often regarded as an alternative to conventional imaging (i.e. CT), due to unclear/contradictory findings. By contrast, 18F-FDG-PET/CT may be the preferred imaging modality for restaging and treatment assessment, with no comparable conventional imaging methods.
Our findings emphasize previous findings from the aforementioned meta-analysis by Han et al. [3], which reported clinical changes after 18F-FDG-PET/CT-scans in a pooled proportion of 17% of cases; our study found a higher proportion of 32%. Furthermore, a study by Vogsen et al. [24] demonstrated a substantial scan-induced impact on clinical management during primary staging in 39% of 103 patients. However, their study was limited to patients undergoing primary staging and was analyzed on a per-patient basis. In contrast, our study included all stage BC and analyzed the scans both on a scan-by-scan and patient-by-patient basis, as nearly half of the patients underwent multiple scans.
We aimed to provide a comprehensive and detailed categorization of the impact of 18F-FDG-PET/CT scans on clinical management, as opposed to the categorization of changes according to intermodality (alteration in the type of management) and intention-to-treat by Han et al. [3]. Vogsen et al. [24] defined changes in management as either changes in treatment or incidental findings with clinical consequences, while Yarabras et al. [23] analyzed changes in management in relation to therapy selection. Our study builds upon these previous analyses by including all previously mentioned changes in management, as well as including the avoidance of unnecessary biopsies and the discovery of biopsy sites for treatment optimization, resulting in a more expansive view of the effects of 18F-FDG-PET/CT.
The majority of scans in our cohort (68.1%) showed no scan-induced changes, as assessed in this study. However, as Bossuyt et al. [19] reported, the clinical utility of a test extends beyond its medical applications to include psychosocial factors. For instance, while the scans in our cohort did not lead to changes in clinical management, they may still have provided reassurance to patients undergoing surveillance for recurrence.
Incidental finding
Recognizing that 18F-FDG-PET/CT can detect incidental findings [24,29], it is essential to consider their clinical impact. In a cohort of high-risk primary BC patients undergoing 18F-FDG-PET as part of initial work-up, up to one-third of the scans revealed incidental findings [24]. In our study, 4.0% (6 out of 151) of first-time 18F-FDG-PET scans (per-patient analysis) resulted in incidental findings. The variation in proportions may be attributed to different BC scenarios and variations in previous imaging procedures. Moreover, what remains uncertain is whether upstaging and the management adjustments prompted by 18F-FDG PET/CT could occasionally result in undertreatment. For example, altering the treatment approach could impact systemic treatment, local surgery, or the extension of radiation treatment fields. Consequently, this may not necessarily be beneficial for every individual patient. Clinicians must balance the advantages of precise staging with the potential risks. Multidisciplinary discussions are imperative to inform optimal, personalized treatment decisions.
Treatment evaluation
Metabolic changes might predict treatment response earlier than structural changes and 18F-FDG-PET/CT in BC holds potential as a useful imaging modality for treatment response evaluation. Importantly, early identification of non-responders [30] enables re-evaluation of unbeneficial treatment regimens. Evaluating metabolic response in patients undergoing neoadjuvant therapy have shown promising results, e.g. in their meta-analysis, Tian et al. [28] included 22 studies evaluating the accuracy of 18F-FDG-PET/CT in assessing treatment response to neoadjuvant chemotherapy, where the results showed a pooled sensitivity of 0.82 for predicting pathological response early on during neoadjuvant chemotherapy. Moreover, other modalities are being compared to 18F-FDG-PET/CT for assessment of treatment response in this setting; in a study by Choi et al. [26], compared to MRI, changes in 18F-FDG metabolism had a higher discriminative performance (responders vs. non-responders), with a sensitivity of 0.83.
In the metastatic BC setting, 18F-FDG-PET/CT has demonstrated higher accuracy for response evaluation than conventional imaging techniques such as CT and bone scintigraphy [31]. Notably, 18F-FDG-PET/CT demonstrates greater sensitivity in detecting both progressive and regressive disease, whereas conventional imaging tends to classify disease as stable more frequently [25]. Moreover, a prospective observational study by Vogsen et al. (N = 87) demonstrated that 18F-FDG-PET/CT was a better predictor of progression-free and disease-specific survival than CT [32].
Treatment evaluation using 18F-FDG-PET/CT in both neoadjuvant and metastatic BC settings would be a clinically important field to explore in future studies, preferably multicenter randomized clinical trials with endpoints including patients’ survival and quality of life.
Strengths and limitations
The consecutive cohort comprised BC patients who underwent 18F-FDG-PET/CT scans, resulting in a diverse population with varying disease stages, including metastatic disease, and various histopathological subtypes, reflecting the current clinical landscape. This differs from previous studies that focused on a more selected group of patients [3,22–24,33]. The number of scans in this study was relatively large compared to that in previous studies [22,23,33]; although not sufficient for subgroup analyses. Moreover, we presented both per-scan and per-patient data. Our study offers a comprehensive examination of the impact of scans on clinical management, contrasting with previous studies [3,23,24] that have regarded these changes as more general in nature. However, psychosocial factors in addition to health economical aspects were out of scope of this study.
As a retrospective, bi-center, observational study, the very nature of the study presents certain limitations. Ideally, patient outcomes should be evaluated through a randomized, prospective study design. Inclusion bias resulting from the selection of a particular group of patients who undergo 18F-FDG-PET/CT examinations is unavoidable and may have a more significant impact on early-stage patients.
The included patients underwent varying numbers of scans, which could potentially introduce a bias toward an overestimation of scan-induced changes. This is because patients with multiple scans may have more 18F-FDG-avid BC, and thus better visualized using 18F-FDG-PET/CT compared to patients with single scans. However, as demonstrated in the per-patient analyses, considering single scans for each patient led to a higher number of both restaging and changes in clinical management.
The standard practice at both study sites was to perform a diagnostic CT only when none had been done in the preceding 6–8 weeks, we therefore believe that the impact of 18F-FDG-PET on clinical management is consistent regardless of the CT quality.
Future aspects
While 18F-FDG is the most clinically used PET tracer in BC imaging, there are many other radiotracers in clinical use and under evaluation, which would be interesting to investigate. In addition, it would be interesting to explore the clinical consequences of whole-body parametric imaging of 18F-FDG-PET as well as that of PET/MRI. Treatment evaluation using 18F-FDG-PET/CT in both neoadjuvant and metastatic BC settings is a clinically important field to explore in future studies.
Conclusion
With indications for referral mirroring the present clinical landscape of individuals with BC stages I–IV, our research has demonstrated that 18F-FDG-PET/CT is a valuable tool in a wide range of clinical settings. We observed 18F-FDG-PET/CT-induced changes in clinical management in almost one-third of the cases, with the highest rates in patients undergoing initial work-up. However, in the recurrence group, more than every fourth scan led to a change from curative/no treatment to palliative treatment. These findings can help to further explore the use of 18F-FDG-PET/CT in BC. Prospective studies examining the clinical utility and patient outcomes are warranted.
Data availability
The datasets generated and/or analyzed in the current study are not publicly available because of privacy and ethical restrictions. However, should a researcher be interested in the data, they are welcome to contact the corresponding author.
Authors’ contributions, CRediT authorship contribution statement
The author has read and approved the final manuscript.
Acknowledgments
The author would like to thank all study participants. My sincere appreciation is also due to the diligent efforts of medical student Kicki Wong, who contributed significantly to the data extraction and initial analysis.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Regional Ethics Committee in Lund, Sweden (Official records number: LU 2016/417, 2018/753, and 2021-05734-02).
Informed consent
Informed written consent was obtained from all participants included in the study.
References
[1] Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989 Jan 1;63(1):181–7. https://doi.org/10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h
[2] Cook GJ, Azad GK, Goh V. Imaging bone metastases in breast cancer: staging and response assessment. J Nucl Med. 2016 Feb;57(Suppl 1):27S–33S. https://doi.org/10.2967/jnumed.115.157867
[3] Han S, Choi JY. Impact of 18F-FDG PET, PET/CT, and PET/MRI on staging and management as an initial staging modality in breast cancer: a systematic review and meta-analysis. Clin Nucl Med. 2021 Apr 1;46(4):271–82. https://doi.org/10.1097/RLU.0000000000003502
[4] Groheux D, Hindie E. Breast cancer: initial workup and staging with FDG PET/CT. Clin Transl Imaging. 2021;9(3):221–31. https://doi.org/10.1007/s40336-021-00426-z
[5] Pak K, Yoon H-J, Lim W, Kim HY. Impact of 18F-FDG PET on the management of recurrent breast cancer: a meta-analysis. Clin Transl Imaging. 2021;9(3):255–63. https://doi.org/10.1007/s40336-021-00424-1
[6] Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54. https://doi.org/10.1007/s00259-014-2961-x
[7] Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. https://doi.org/10.1016/j.cell.2011.02.013
[8] Miles KA, Williams RE. Warburg revisited: imaging tumour blood flow and metabolism. Cancer Imaging. 2008;8(1):81–6. https://doi.org/10.1102/1470-7330.2008.0011
[9] Hong S, Li J, Wang S. 18FDG PET-CT for diagnosis of distant metastases in breast cancer patients. A meta-analysis. Surg Oncol. 2013;22(2):139–43. https://doi.org/10.1016/j.suronc.2013.03.001
[10] Koolen BB, Valdes Olmos RA, Elkhuizen PH, et al. Locoregional lymph node involvement on 18F-FDG PET/CT in breast cancer patients scheduled for neoadjuvant chemotherapy. Breast Cancer Res Treat. 2012;135(1):231–40. https://doi.org/10.1007/s10549-012-2179-1
[11] Hogan MP, Goldman DA, Dashevsky B, et al. Comparison of 18F-FDG PET/CT for systemic staging of newly diagnosed invasive lobular carcinoma versus invasive ductal carcinoma. J Nucl Med. 2015;56(11):1674–80. https://doi.org/10.2967/jnumed.115.161455
[12] Koo HR, Park JS, Kang KW, et al. 18F-FDG uptake in breast cancer correlates with immunohistochemically defined subtypes. Eur Radiol. 2014;24(3):610–18. https://doi.org/10.1007/s00330-013-3037-1
[13] NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Guideline Breast Cancer V.3.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. [Cited date: June 23, 2024].
[14] American College of Radiology. ACR appropriateness criteria® [Internet]. Reston, VA: American College of Radiology. [Cited date: January 5, 2024] Available from: https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria
[15] Gradishar WJ, Moran MS, Abraham J, et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(6):691–722. https://doi.org/10.6004/jnccn.2022.0030
[16] Regionala cancercentrum i samverkan, Nationellt vårdprogram för bröstcancer version 4.3 [Internet]. Stockholm; 2023. [Cited date: November 22, 2023] Available from: https://kunskapsbanken.cancercentrum.se/diagnoser/brostcancer/vardprogram/
[17] Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. 2019;30(8):1194–220. https://doi.org/10.1093/annonc/mdz173
[18] Gennari A, Andre F, Barrios CH, et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–95. https://doi.org/10.1016/j.annonc.2021.09.019
[19] Bossuyt PM, Reitsma JB, Linnet K, et al. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem. 2012;58(12):1636–43. https://doi.org/10.1373/clinchem.2012.182576
[20] von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. https://doi.org/10.1016/j.jclinepi.2007.11.008
[21] Giuliano AE, Edge SB, Hortobagyi GN. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol. 2018;25(7):1783–5. https://doi.org/10.1245/s10434-018-6486-6
[22] Groheux D, Giacchetti S, Espie M, et al. The yield of 18F-FDG PET/CT in patients with clinical stage IIA, IIB, or IIIA breast cancer: a prospective study. J Nucl Med. 2011;52(10):1526–34. https://doi.org/10.2967/jnumed.111.093864
[23] Yararbas U, Avci NC, Yeniay L, et al. The value of 18F-FDG PET/CT imaging in breast cancer staging. Bosn J Basic Med Sci. 2018;18(1):72–9. https://doi.org/10.17305/bjbms.2017.2179
[24] Vogsen M, Jensen JD, Christensen IY, et al. FDG-PET/CT in high-risk primary breast cancer-a prospective study of stage migration and clinical impact. Breast Cancer Res Treat. 2021;185(1):145–53. https://doi.org/10.1007/s10549-020-05929-3
[25] Naghavi-Behzad M, Oltmann HR, Alamdari TA, et al. Clinical impact of FDG-PET/CT compared with CE-CT in response monitoring of metastatic breast cancer. Cancers (Basel). 2021;13(16):4080. https://doi.org/10.3390/cancers13164080
[26] Choi JH, Kim HA, Kim W, et al. Early prediction of neoadjuvant chemotherapy response for advanced breast cancer using PET/MRI image deep learning. Sci Rep. 2020;10(1):21149. https://doi.org/10.1038/s41598-020-77875-5
[27] Rousseau C, Devillers A, Sagan C, et al. Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2006;24(34):5366–72. https://doi.org/10.1200/JCO.2006.05.7406
[28] Tian F, Shen G, Deng Y, et al. The accuracy of (18)F-FDG PET/CT in predicting the pathological response to neoadjuvant chemotherapy in patients with breast cancer: a meta-analysis and systematic review. Eur Radiol. 2017;27(11):4786–96. https://doi.org/10.1007/s00330-017-4831-y
[29] Vogsen M, Jensen JD, Gerke O, et al. Benefits and harms of implementing [(18)F]FDG-PET/CT for diagnosing recurrent breast cancer: a prospective clinical study. EJNMMI Res. 2021;11(1):93. https://doi.org/10.1186/s13550-021-00833-3
[30] Hadebe B, Harry L, Ebrahim T, et al. The role of PET/CT in breast cancer. Diagnostics (Basel). 2023;13(4):597. https://doi.org/10.3390/diagnostics13040597
[31] Hildebrandt MG, Naghavi-Behzad M, Vogsen M. A role of FDG-PET/CT for response evaluation in metastatic breast cancer? Semin Nucl Med. 2022;52(5):520–30. https://doi.org/10.1053/j.semnuclmed.2022.03.004
[32] Vogsen M, Naghavi-Behzad M, Harbo FG, et al. 2-[(18)F]FDG-PET/CT is a better predictor of survival than conventional CT: a prospective study of response monitoring in metastatic breast cancer. Sci Rep. 2023;13(1):5552. https://doi.org/10.1038/s41598-023-32727-w
[33] Segaert I, Mottaghy F, Ceyssens S, et al. Additional value of PET-CT in staging of clinical stage IIB and III breast cancer. Breast J. 2010;16(6):617–24. https://doi.org/10.1111/j.1524-4741.2010.00987.x