ORIGINAL ARTICLE
Cancer incidence following non-neoplastic medical conditions: a prospective population-based cohort study
Lauri J. Sipiläa,b,c, Tomas Tanskanenc, Sanna Heikkinenc, Karri Seppäc, Mervi Aavikkoa,b,d, Janne Ravanttia,b,e, Lauri A. Aaltonena,b and Janne Pitkäniemic,f,g
aDepartment of Medical and Clinical Genetics, University of Helsinki, Biomedicum Helsinki, Helsinki, Finland; bApplied Tumor Genomics, Research Programs Unit, University of Helsinki, Biomedicum Helsinki, Helsinki, Finland; cFinnish Cancer Registry, Helsinki, Finland; dInstitute for Molecular Medicine Finland (FIMM), HiLIFE, University of Helsinki, Helsinki, Finland; eMolecular and Integrative Biosciences Research Programme, Faculty of Biological and Environmental Sciences, University of Helsinki, Finland; fHealth Sciences Unit, Faculty of Social Sciences (Health Sciences), Tampere University, Tampere, Finland; gDepartment of Public Health, Faculty of Medicine, University of Helsinki, Helsinki, Finland
ABSTRACT
Background and purpose: Many non-neoplastic diseases have been established to be tumorigenic, and cancers are sometimes misdiagnosed as non-neoplastic diseases. We conducted a comprehensive registry-based study of site-specific cancer diagnosis risk following the diagnosis of any preceding medical condition (PMC) encoded by the International Classification of Diseases (ICD)-10 classification.
Material and methods: We analyzed healthcare data and cancer data for a random population-based sample of 2.5 million individuals living in Finland on January 1, 2000. Hazard ratios for each PMC and cancer pair were estimated using piecewise constant hazard regression models. P-values were corrected for multiple testing with the Bonferroni method.
Results: Several lifestyle-related PMCs were associated with the risk of cancer diagnosis, exemplified by chronic obstructive pulmonary disease and subsequent lung cancer diagnosis risk (female hazard ratio [HR] = 9.91, 95% confidence interval [CI]: 9.18–19.7, p-adj. < 0.0001; male HR = 5.69, 95% CI: 5.43–5.96, p-adj. < 0.0001). Diagnosis risk of ill-defined cancers appeared to increase following diagnosis of Alzheimer’s disease (AD). We identified rare PMCs of potential interest.
Interpretation: A considerable proportion of the statistically significant associations were explainable by tobacco smoking and alcohol use. The enrichment of ill-defined cancer diagnoses in persons with AD, together with the overall inverse association between AD and cancer, may reflect underdiagnosis of cancer in this patient population. Our results provide a useful resource for research on the prevention and early detection of cancer.
KEYWORDS: Cancer epidemiology; comorbidity; lifestyle factors; cancer registry; public health
Citation: ACTA ONCOLOGICA 2024, VOL. 63, 841–849. https://doi.org/10.2340/1651-226X.2024.40757.
Copyright: © 2024 The Author(s). Published by MJS Publishing on behalf of Acta Oncologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, with the condition of proper attribution to the original work.
Received: 17 May 2024; Accepted: 11 October 2024; Published: 4 November 2024
CONTACT Janne Pitkäniemi janne.pitkaniemi@cancer.fi Finnish Cancer Registry, Institute for Statistical and Epidemiological Cancer Research, Mäkelänkatu 2, 00500 Helsinki, Finland
Supplemental data for this article can be accessed online at https://doi.org/10.2340/1651-226X.2024.40757
Competing interests and funding: The authors declare no conflict of interest.
This study was supported by the Research Council of Finland (Finnish Centre of Excellence Program grants No. 312041, 335823, and 352814 and Academy Professor grants No. 320149, 345051, 319083) and Cancer Foundation Finland.
Introduction
Cancer incidence is known to be affected by several preceding diseases. Genetic disorders such as Lynch syndrome, Li-Fraumeni syndrome, and neurofibromatosis type 1 (NF1) severely increase the lifetime cancer risk of the patients. Viral and bacterial infections are also major contributors to cancer incidence worldwide [1]. Chronic viral hepatitis and diabetes mellitus are associated with an increased risk of liver cancer [2, 3], exemplifying how disparate diseases may promote the same cancer. The symptoms of cancer may initially be attributed to a benign condition. For example, rectal bleeding due to colorectal cancer may be attributed to hemorrhoids. On the other hand, cancer may be the underlying cause of another disease such as diabetes mellitus or a paraneoplastic syndrome [4]. Environmental agents can cause both cancers and noncancerous diseases, with tobacco smoking being a significant cause of not only cancer but also of various cardiovascular, metabolic, respiratory, and digestive diseases [5]. Preceding medical conditions (PMCs) may also affect the likelihood of being diagnosed with existing cancer. For instance, individuals with schizophrenia are more likely to die of previously undiagnosed cancer compared to the general population [6].
Large-scale health data is an increasingly important source of information in biomedical research. It allows the assessment of multiple research questions without the time- and cost-intensive process of prospective data collection. For example, in the Prospective Meta-Cohort Study of Cancer Burden in Finland (METCA project), the Finnish Institute for Health and Welfare and Finnish Cancer Registry (FCR) have combined large national health studies to evaluate population attributable fractions for cancer risk factors [7, 8]. In Sweden, the associations of blood group antigens and 1,217 disease endpoints were studied in a sample of 5.1 million individuals by linking a blood donation and transfusion database to national health registries [9]. A sample from The Danish National Patient Register, covering 7.2 million individuals and 1,777 International Classification of Diseases (ICD)-10 disease codes, has been used to generate a browser of disease trajectories [10]. Here, we have linked FCR data with the Finnish Care Register for Health Care and conducted a comprehensive study of associations between non-neoplastic PMCs and the subsequent risk of cancer diagnosis. This is, to our knowledge, the first study using large-scale hospital data and complementary cancer registry data for a thorough scan of population-based cancer risk following almost any condition or event encoded by the ICD, 10th Revision (ICD-10).
Material and methods
The study cohort consisted of a random population-based sample of 2.5 million individuals (Table 1). Study participants were sampled from the Population Information System maintained by the Digital and Population Data Services Agency (https://dvv.fi/en/individuals). All individuals with a permanent address in Finland and alive on 01 January 2000 were eligible for the sampling regardless of age, with the sample representing roughly half of the population of Finland at January 1, 2000. This was also the date of cohort entry for all participants. For each analyzed combination of PMC and cancer site, follow-up started on January 1, 2000 and ended at site-specific cancer diagnosis, death, emigration or December 31, 2017, whichever occurred first (Table 1).
PMC data were acquired from the Care Register for Health Care and included individual-level data on hospital discharges in 1996–2017 and outpatient clinic visits in 1998–2017. Complete records of all diagnoses and procedures for all study participants totaled 137 million entries. Diagnosis codes were based on the international ICD-10 classification with minor national modifications. The most significant difference between the Finnish and the international version of ICD-10 is lesser detail in the classification of external causes of morbidity and mortality (ICD-10 chapter XX) [11]. Cancer diagnoses of all participants in 1953–2017 were obtained from the FCR, and first primary cancers diagnosed after January 1, 2000, were used in the analysis. Cancers were classified into 61 cancer types based on the official cancer classification of the FCR, which is largely equivalent to ICD-10 (Table 2) [12, 13].
PMCs were defined as the exposure variable in our statistical model. We analyzed the data using the second and third hierarchy levels of the national ICD-10 classification, which are generally equivalent to the three-character category codes (ICD-10–3c) and four-character subcategory codes (ICD-10–4c) of the international ICD-10. With both levels combined, a total of 9,998 disease entities were analyzed. Thus, the PMC data consisted of 24,986,722 unique pairs of individuals and diagnoses on the ICD-10-3c level (10,607,379 in men and 14,379,343 in women) and 26,352,387 on the ICD-10-4c level (11,118,517 in men and 15,233,870 in women). Combinations of ICD-10 codes containing information about both an underlying generalized disease and a manifestation in a particular organ or site were considered as separate codes. For example, the code for influenza-related encephalitis G05.1*J09 was split into the codes G05.1 and J09. The outcome variable was diagnosis of a primary cancer during the follow-up period (Table 2).
Exposure and outcome data were linked by the unique personal identity code assigned to all Finnish citizens and permanent residents. There was a total of 96,502 PMC-cancer type combinations on the ICD-10-3c level and 513,376 on the ICD-10-4c level. Individuals with multiple primary cancers or PMCs contributed to all applicable PMC-cancer pair analyses. PMC status was determined by the time elapsed since PMC diagnosis. PMC diagnosis, and thus the beginning of exposure, was recorded even if it occurred before the start of follow-up on January 1, 2000. Individuals with prevalent cancer at the start of follow-up were excluded from the analysis of the same cancer site but permitted to contribute to analyses of different cancer types.
To study the risk of cancer diagnosis following PMCs, hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated using piecewise constant hazard regression models with Poisson likelihood. The estimates were adjusted for attained age (0–4, 5–9, …, 90–94, or ≥ 95 years) and calendar period (2000–2005, 2006–2011, or 2012–2017). PMC status was allowed to vary over time and was categorized into three strata: no diagnosis of PMC, less than 1 year after PMC diagnosis, or at least 1 year after PMC diagnosis. The analyses were performed separately by gender.
To mitigate reverse causality and identify conditions with long-term tumorigenic significance, we report model-based HRs for individuals exposed to PMCs for at least 1 year. We filtered out results for ICD-10 codes of neoplasms (C00–C97, D00–D48) and symptoms, signs, and abnormal clinical and laboratory findings (R00–R99) due to their expected associations with subsequent cancer diagnosis. Lastly, to protect individual participants from being identified from the results, and to limit the reporting of spurious associations, the main results table includes only PMC-cancer diagnosis risk associations in which at least five individuals have developed the relevant cancer. After filtering, the final number of PMC-cancer risk associations was 73,295. P-values were adjusted for all ICD-10-3c and ICD-10-4c level analyses passing filtering criteria (N = 73,295) across both men and women combined, using both Bonferroni correction and the Benjamini-Hochberg procedure with a false discovery rate (FDR) of 0.1. For some PMCs, the Finnish ICD-10 classification did not provide an English translation, and we have left these untranslated.
To visualize the spectrum of PMC-cancer risk associations, we generated a Manhattan plot of all category-level ICD-10 codes and cancers. A hierarchical cluster analysis of binary logarithms of HR point estimates was conducted using Euclidean distance as the distance metric and Ward linkage as the linkage criterion. Clusters were estimated for a subset of the data consisting of cancer sites with at least 100 ICD-10-3c code associations passing the filtering criteria and ICD-10-3c codes with an HR estimate for at least 30 or 33 cancer sites in men or women, respectively. The thresholds for cancer site numbers were chosen iteratively to maximize the number of data points in the cluster analysis while minimizing missing values. For less common diseases, the spectrum of cancers that passed the filtering criteria was too limited to be meaningfully incorporated into the hierarchical cluster analysis.
We used the R packages data.table version 1.12.2 and popEpi version 0.4.10 to analyze the data, ggplot2 version 3.4.0 to generate the Manhattan plot, and gplots version 3.1.3 to generate the hierarchical clustering plots.
Results
A table of 73,295 associations of PMCs and cancers occurring at least 1 year from PMC diagnosis was produced (Supplementary material). Cancer sites with a strong etiological link to either tobacco smoking or alcohol use showed a larger number of statistically significant associations with PMCs than most other sites in both men and women (Figure 1). The most significant PMC associated with lung cancer risk was chronic obstructive pulmonary disease (ICD-10 J44, female HR = 9.91, 95% CI = 9.18–19.7, Bonferroni-adjusted p < 0.0001; male HR = 5.69, 95% CI: 5.43–5.96, p-adj < 0.0001). For many PMC-cancer combinations, the association appeared to be predominantly explained by tobacco smoking. There was also a high risk of lung cancer following diagnosis of ‘mental and behavioral disorders due to use of tobacco’ (F17, female HR = 9.29, 95% CI: 6.99–12.35, p-adj. < 0.0001; male HR = 5.37, 95% CI: 4.34–6.64, p-adj. < 0.0001). In liver cancer, diseases of the liver (K70–77) formed a peak of consecutive high-significance associations in the Manhattan plots, while esophageal varices were most significantly associated with liver cancer in both men and women (ICD-10 I85, male HR = 56.68, 95% CI: 47.19–68.09, p-adj. < 0.0001; female HR = 85.61, 95% CI: 64.85–113.01, p-adj. < 0.0001). PMCs typically associated with either tobacco or alcohol use had overlapping patterns of cancer risk, with lung cancer clustering together with alcohol-related cancers in men (Figure 2A), while a similar but less pronounced trend was present in women (Figure 2B). Similar associations were also observed following certain injury diagnoses, where fracture of neck of femur (ICD-10 S72.0) increased the diagnosis risk of lung cancer in both genders (male HR = 2.01, 95% CI: 1.76–2.29, p-adj. < 0.0001; female HR = 1.76, 95% CI: 1.52–2.05, p-adj. < 0.0001) and diagnosis risk of pharyngeal cancer in men (HR = 3.88, 95% CI: 2.39–6.29, p-adj. = 0.001; female HR = 3.95, 95% CI: 1.97–7.91, p-adj. = 1). Physical activity-related injuries showed inverse associations; for example, internal derangement of knee (ICD-10 M23) was associated with decreased lung cancer diagnosis risk in men (HR = 0.68, 95% CI: 0.61–0.75, p-adj. < 0.0001; female HR = 0.89, 95% CI: 0.78–1.01, p-adj. = 1), and ‘pedal cyclist injured in transport accident’ (ICD-10 V10–V19) was nonsignificantly associated with decreased risk of any cancer in women (HR = 0.87, 95% CI: 0.8–0.95, p-adj. = 1; male HR = 1.09, 95% CI: 1.02–1.17, p-adj. = 1).
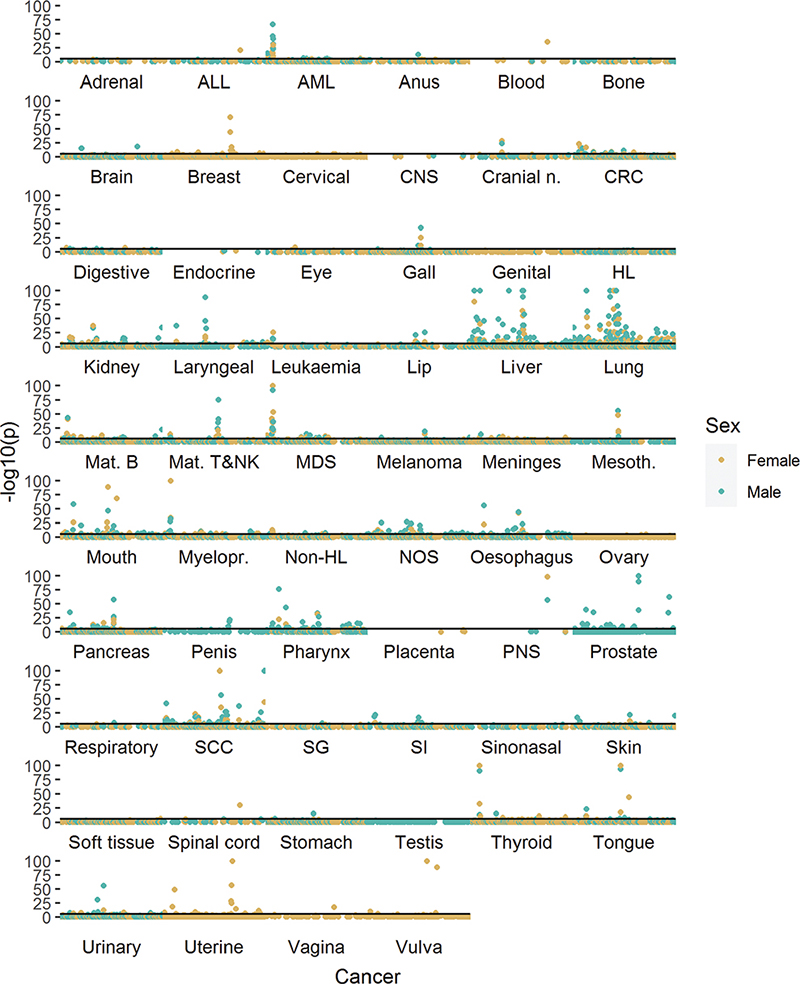
Figure 1. Manhattan plot of preceding medical conditions (PMCs) at the three-character level of ICD-10 and cancer diagnosis risk for each cancer site for women (A) and men (B). Complete results are available in the Supplementary material. Sorted ICD-10-codes (x-axis) and log-transformed p-values (y-axis) are presented, with each point signifying a PMC-cancer pair. Point color alternates between sites. Solid horizontal lines denote a Bonferroni-adjusted p-value of 0.05. Y-axis values are cut at negative log10 value of 100. An abbreviation key is presented in Table 2.
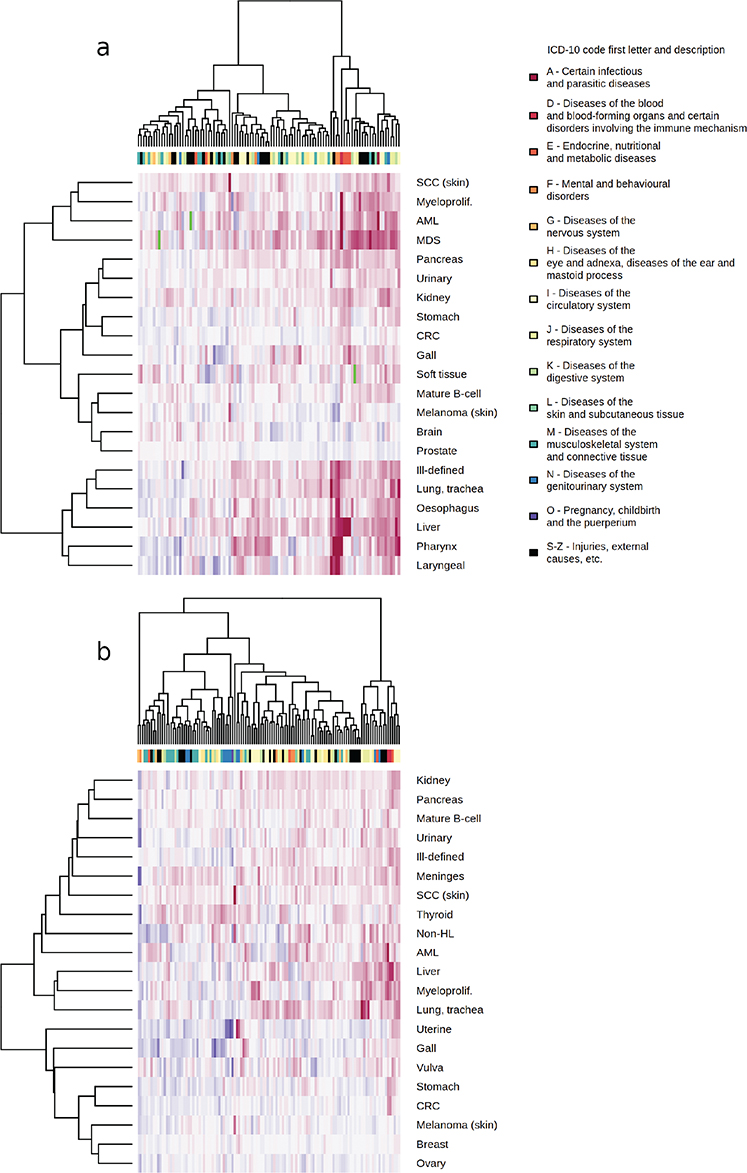
Figure 2. Hierarchical clustering of binary logarithms of common three-character level ICD-10 code (columns) and cancer (rows) hazard ratio estimates, with blue color indicating a decreased cancer diagnosis risk, red indicating an increased cancer diagnosis risk, and green indicating a missing data point. Men in image A, women in image B.
PMCs affecting cognitive function decreased cancer diagnosis risk in a wide range of sites. Alzheimer’s disease (AD, ICD-10 G30) and dementia (ICD-10 F00-03) were inversely associated with most of the analyzed cancer types (Supplementary table). Following AD diagnosis, diagnosis risk decreased statistically significantly for colorectal cancer (female HR = 0.67, 95% CI: 0.59–0.77, p-adj. < 0.0001; male HR = 0.6 95% CI: 0.50–0.71, p-adj. = 0.0003), mature B-cell neoplasms (female HR = 0.51 95% CI: 0.41–0.63, p-adj. < 0.0001; male HR = 0.48, 95% CI: 0.36–0.62, p-adj. = 0.004), female lung cancer (HR = 0.51, 95% CI: 0.41–0.63, p-adj. < 0.0001; male HR = 0.77, 95% CI: 0.66–0.89, p-adj. = 1), prostate cancer (HR = 0.50, 95% CI: 0.44–0.55, p-adj. < 0.0001), and female urinary cancer (HR = 0.39, 95% CI: 0.28–0.55, p-adj. = 0.003; male HR = 0.75, 95% CI: 0.62–0.90, p-adj. = 1). Dementia had a largely similar spectrum of statistically significant risk decreases. We observed a reversal of this trend to increased risks of unspecified or ill-defined cancer diagnosis although none of these associations remained statistically significant after correcting for multiple testing. Unspecified digestive organ cancer in women (G30, HR = 1.56, 95% CI: 1.15–2.13, p-adj. = 1; male HR = 1.33, 95% CI: 0.77–2.30, p-adj. = 1) was the most notable example. Schizophrenia (ICD-10 F20) was associated with an increased diagnosis risk of lung cancer (male HR = 2.96, 95%CI: 2.61–3.36, p-adj. < 0.0001; female HR = 2.89, 95% CI: 2.45–3.41, p-adj. < 0.0001) and female breast cancer (HR = 1.37, 95% CI: 1.24–1.51, p-adj. < 0.0001; male HR = 1.67, 95% CI: 0.41–6.76, less than five observations), while prostate cancer diagnosis risk was reduced (HR = 0.50, 95% CI: 0.42–0.59, p-adj. < 0.0001).
The results for rare PMC-cancer pairs and PMCs with weak-to-moderate associations with cancer diagnosis risk may reveal novel insights. For example, diagnosis of Lyme disease (ICD-10 A69.2) was associated with an increased risk of brain cancer diagnosis in women (HR = 3.55, 95% CI: 1.69–7.46, p-adj. = 1; male HR = 1.01, 95% CI: 0.25–4.04, less than five observations) and urinary cancer diagnosis in men (HR = 1.94, 95% CI: 1.24–3.04, p = 0.002, p-adj. = 1; female HR = 0.68, 95% CI: 0.17–2.74, less than five observations). For psoriasis (ICD-10 L40), the highest risk increase was noted for male breast cancer (HR = 3.47, 95% CI: 1.53–7.84, p = 0.001, p-adj. = 1; female HR = 0.99, 95% CI: 0.88–1.1, p-adj. = 1). Diagnosis of ‘other congenital malformations of face and neck’ (ICD-10 Q18) increased diagnosis risk of cervical cancer (HR = 4.33, 95% CI: 1.8–10.42, p-adj. = 1), lung cancer (female HR = 2.46, 95% CI: 1.28–4.73, p-adj. = 1; male HR = 1.61, 95% CI: 0.93–2.77, p-adj. = 1) and female kidney cancer (HR = 3.03, 95% CI: 1.26–7.29, p = 0.006, p-adj. = 1; male HR = 1.15, 95% CI: 0.37–3.58, less than five observations), while its subcategory ‘sinus, fistula, and cyst of branchial cleft’ (ICD-10 Q18.0) increased diagnosis risks for any cancer (male HR = 1.52, 95% CI: 1.14–2.02, p-adj. = 1; female HR = 1.16, 95% CI: 0.84–1.6, p-adj. = 1) and lung cancer (male HR = 2.6, 95% CI: 1.44–4.70, p-adj. = 1; female HR = 2.24, 95% CI: 0.84–5.97, less than five observations). Some combinations produced surprising inverse associations; for example, emphysema (ICD-10 J43) was associated with a decreased risk of female breast cancer diagnosis (HR = 0.44, 95% CI: 0.23–0.85, p = 0.007, p-adj. = 1).
Discussion
We conducted a comprehensive study of population-wide healthcare data and estimated 73,295 gender-specific HRs for cancer diagnosis in persons with pre-existing medical conditions. We used high-quality nationwide health register data [14] from roughly half of the population of Finland. The large sample size allows the quantification of cancer diagnosis risk in common comorbidities and enables the detection of rarer and possibly previously unknown PMC-cancer associations. Knowledge of links between non-neoplastic medical conditions and cancers may propel further research on the prevention and early detection of cancer, potentially leading to better outcomes. Here, we report examples of both previously well-known PMC-cancer pairs and potentially novel associations. Associations presented in the main table of results (Supplementary material) should be viewed critically since despite analyzing high-quality registry data, we lack information on possible confounders beyond age and gender.
We present Bonferroni-corrected p-values to address the multiple comparisons problem and identify highly significant associations. Due to the conservativeness of the Bonferroni method, the Benjamini-Hochberg procedure may be preferred when studying associations between rare PMCs and cancers. We provide both values in our complete results (Supplementary material).
Various local conditions affect disease incidence. These include the environment, population genetics, society, and culture [15, 16]. Consequently, the statistical power of the study is likely to depend on these local conditions, and the degree of confounding may also depend on the study population. The Finnish Care Register for Health Care includes primary healthcare data only since 2011, and therefore we restricted the PMC records to specialized healthcare [14]. The use of single ICD-10 codes to define PMCs may have led to some degree of exposure misclassification because patients with related diagnostic codes, especially at the ICD-10-4c level, may share similar pathophysiological characteristics. Misclassification may also occur if an individual has diagnoses for a PMC only before the year 1996.
In the cohort study design, HRs measured the risk of subsequent cancer diagnosis following a PMC code, and the observed associations may be explained by various types of phenomena. While the study design is agnostic to the type of association observed, we hypothesize that there may be five common causes of association: (1) an undiagnosed cancer or a precancerous condition causing symptoms; (2) a PMC directly promoting tumorigenesis; (3) an environmental or behavioral factor confounding the analysis by causing both the PMC and cancer; (4) a PMC affecting the likelihood of an existing cancer being detected; and (5) possible competing risks of death.
Some PMCs may represent symptoms of underlying cancer, and the true sequence of events may in fact be the inverse of the observed one [4]. In our results, this is likely exemplified by the high risk of being diagnosed with mature T-cell lymphoma after a diagnosis of parapsoriasis or atopic dermatitis. Both may represent a misdiagnosis of mature T-cell lymphoma [17, 18]. Similarly, dorsopathy codes were associated with a spectrum of cancer diagnoses, and back pain is sometimes the first symptom of cancer [19].
In many instances, a high HR reflects a true risk of a tumorigenic process driven by the PMC, as exemplified by Crohn’s disease and small intestinal cancer, in which the cancer may have different characteristics depending on whether it manifests in the context of Crohn’s disease or de novo [20].
We present selected examples of potentially novel PMC-cancer associations. The association between Lyme disease and brain cancer in women is interesting because of the known neurological manifestations of Lyme disease. We observed an increased risk of male breast cancer diagnosis following a psoriasis diagnosis. An increased prevalence of male breast cancer in patients with psoriasis has been previously reported in a Swedish study and was suspected to result from a multiple comparisons problem [21], whereas a Danish study did not observe any statistically significant difference [22]. Finally, congenital malformations of the face and neck (ICD-10 Q18) were associated with a variety of cancers. This category might include persons with rare congenital branchio-oto-renal or branchio-otic syndromes, for which we are not aware of a previously known role in cancer predisposition. This finding might also be explained by increased frequency of head and neck imaging in individuals with related cancer, leading to an increased rate of branchial cleft cyst diagnosis compared to the general population.
Environmental agents may predispose to both cancer and noncancerous disease. In the hierarchical cluster analysis, we observed a subset of cancers clustering together in men based on their shared spectrum of risk-increasing PMCs. We observed PMCs predominantly related to alcohol use increasing diagnosis risk of lung cancer and PMCs related to tobacco smoking increasing liver cancer diagnosis risk. This indicates potential confounding in which excess cancer cases have been contributed by individuals who have had both exposures concurrently. Such individuals may contribute, for example, to the risk increases of lung cancer diagnosis following the diagnosis of alcoholic liver disease (ICD-10 K70, female HR = 4.78, 95% CI: 3.61–6.34, p-adj. < 0.0001; male HR = 2.29, 95% CI: 1.9–2.76, p-adj. < 0.0001), as investigations focused on the connection of alcohol use and lung cancer have observed only modest [23] or no [24] risk increase.
PMCs potentially affecting the likelihood of cancer diagnosis is exemplified by AD. A decreased cancer risk following AD and dementia has been observed in numerous studies [25], with both statistical and biological phenomena discussed as potential causative factors. The decreased cancer risks in our study are consistent with previously published results on colorectal cancer [26, 27], prostate cancer [26, 27], and lung cancer [26]. Of note, we observed increased HRs for ill-defined cancer diagnoses following diagnosis of AD, which may reflect challenges in diagnosing cancer in patients with AD. However, such increased risks were not statistically significant after adjusting for multiple testing. These observations may be attributable to individuals with AD not being able to consent to and partake in extensive diagnostic processes, leading to both fewer cancer diagnoses overall and an excess of ill-defined diagnoses. In schizophrenia, an increased mortality risk related to breast, colon, and lung cancer has been described elsewhere [28–30], which is in line with our results of increased diagnosis risks for lung and breast cancer. The dramatic increase in lung cancer risk is likely a result of heavy smoking among patients with schizophrenia [31], while the associations with breast and prostate cancer have been suggested to be related to adverse effects of antipsychotic medication [29, 30].
We observed some surprising inverse associations in some PMCs, especially with prostate and breast cancer. We presume that at least some of these risk decreases are caused by comparatively more cancer diagnoses being made shortly after PMC diagnosis. In the example of decreased breast cancer diagnosis risk following the diagnosis of emphysema, cancer may be diagnosed during the diagnostic process of the PMC. Thus, there may be an increased risk of breast cancer diagnosis in the first year after emphysema diagnosis and an inverse association thereafter. We cannot rule out the possibility that the competing risk of death may explain the inverse association between emphysema and breast cancer.
In conclusion, we have estimated cancer diagnosis risks following non-neoplastic PMCs by linking FCR data with the Finnish Care Register for Health Care and interpreted both general trends in the data and specific pairs of PMCs and cancers. The presented results are based on modeling in an hypothesis-agnostic framework. Consequently, the associations may lack adjustment for relevant unavailable factors, such as smoking, and may be affected by unmeasured confounding. Despite the large sample size, meaningful associations between rare exposures and rare cancers may have been missed due to the number of observations still being too low. We have shown that the results both agree with and refine existing knowledge and reveal potentially novel associations to be validated in other studies. Fewer or ill-defined cancer diagnoses in patients with AD and dementia underscore a need to evaluate healthcare in these patient populations. Our results provide a useful resource for research on the prevention and early detection of cancer.
Declarations
Author contributions
TT, SH, KS, LAA, and JP conceived and designed the study. LJS and JR procured computing resources. LJS, TT, and KS carried out formal analysis. All authors analyzed the results. LJS prepared visualizations. LJS wrote the initial draft; all other authors reviewed and edited. SH, LAA, and JP supervised the project. All authors reviewed and approved the final manuscript.
Ethics declarations
An ethics committee approval is not required for registry-based studies in Finland according to the Act on Secondary Use of Health and Social Data (552/2019). This study has been approved by the Finnish Institute for Health and Welfare (THL/118/6.02.00/2019) and the Digital and Population Data Services Agency (VRK/4504/2019-2).
Data availability statement
The analyzed raw data cannot be made available by the researchers, due to research permits restricting the sharing of data on ethical and legal grounds. Permissions to use similar administrative health data from various register keepers can be applied from Findata, the Social and Health Data Permit Authority: https://findata.fi/en/.
Acknowledgements
We acknowledge the computational resources provided by CSC-IT Center for Science, Finland. We thank Aapeli Nevala for technical assistance and Alison London for proofreading the manuscript text.
References
[1] Biological Agents. International Agency for Research on Cancer; 2012. Published by the International Agency for Research on Cancer, printed in Lyon, France.
[2] Perz JF, Armstrong GL, Farrington LA, Hutin YJF, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45(4):529–538. https://doi.org/10.1016/j.jhep.2006.05.013
[3] La Vecchia C, Negri E, Decarli A, Franceschi S. Diabetes mellitus and the risk of primary liver cancer. Int J Cancer. 1997;73(2):204–207. https://doi.org/10.1002/(SICI)1097-0215(19971009)73:2<204::AID-IJC7>3.0.CO;2-%23
[4] Gullo L, Pezzilli R, Morselli-Labate AM. Diabetes and the risk of pancreatic cancer. N Engl J Med. 1994;331(2):81–84. https://doi.org/10.1056/NEJM199407143310203
[5] Chan KH, Wright N, Xiao D, et al. Tobacco smoking and risks of more than 470 diseases in China: a prospective cohort study. Lancet Public Health. 2022;7(12):e1014–e1026. https://doi.org/10.1016/S2468-2667(22)00227-4
[6] Crump C, Winkleby MA, Sundquist K, Sundquist J. Comorbidities and mortality in persons with schizophrenia: a Swedish National cohort study. Am J Psychiatry. 2013;170(3):324–333. https://doi.org/10.1176/appi.ajp.2012.12050599
[7] Pitkäniemi J, Heikkinen S, Seppä K, et al. Pooling of Finnish population-based health studies: lifestyle risk factors of colorectal and lung cancer. Acta Oncol. 2020;59(11):1338–1342. https://doi.org/10.1080/0284186X.2020.1789214
[8] Seppä K, Heikkinen S, Ryynänen H, et al. Every tenth malignant solid tumor attributed to overweight and alcohol consumption: a population-based cohort study. Eur J Cancer. 2024;198:113502. https://doi.org/10.1016/j.ejca.2023.113502
[9] Dahlén T, Clements M, Zhao J, Olsson ML, Edgren G. An agnostic study of associations between ABO and RhD blood group and phenome-wide disease risk. eLife. 2021;10:e65658. https://doi.org/10.7554/eLife.65658
[10] Siggaard T, Reguant R, Jørgensen IF, et al. Disease trajectory browser for exploring temporal, population-wide disease progression patterns in 7.2 million Danish patients. Nat Commun. 2020;11(1):4952. https://doi.org/10.1038/s41467-020-18682-4
[11] Komulainen J. Tautiluokitus ICD-10. 3. p. Helsinki: Terveyden ja hyvinvoinnin laitos (THL); 2011.
[12] Fritz A, Sankila R, Merikivi M, Mustonen S, Ovaska I. Kansainvälinen syöpäsairauksien luokittelu ICD-O-3. Helsinki: Suomen syöpäyhdistys; 2008.
[13] International statistical classification of diseases and related health problems. 3: Alphabetical index. World Health Organization, editor, 2004. 2nd. ed. Published by World Health Organization, printed in Switzerland.
[14] Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40(6):505–515. https://doi.org/10.1177/1403494812456637
[15] Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360(9343):1347–1360. https://doi.org/10.1016/S0140-6736(02)11403-6
[16] Kim MS, Patel KP, Teng AK, Berens AJ, Lachance J. Genetic disease risks can be misestimated across global populations. Genome Biol. 2018;19(1):179. https://doi.org/10.1186/s13059-018-1561-7
[17] Miyagaki T, Sugaya M. Erythrodermic cutaneous T-cell lymphoma: how to differentiate this rare disease from atopic dermatitis. J Dermatol Sci. 2011;64(1):1–6. https://doi.org/10.1016/j.jdermsci.2011.07.007
[18] Kikuchi A, Naka W, Harada T, Sakuraoka K, Harada R, Nishikawa T. Parapsoriasis en plaques: its potential for progression to malignant lymphoma. J Am Acad Dermatol. 1993;29(3):419–422. https://doi.org/10.1016/0190-9622(93)70204-7
[19] Schiff D, O’Neill BP, Suman VJ. Spinal epidural metastasis as the initial manifestation of malignancy: clinical features and diagnostic approach. Neurology. 1997;49(2):452–456. https://doi.org/10.1212/WNL.49.2.452
[20] Palascak-Juif V, Bouvier AM, Cosnes J, et al. Small bowel adenocarcinoma in patients with Crohn’s disease compared with small bowel adenocarcinoma de novo. Inflamm Bowel Dis. 2005;11(9):828–832. https://doi.org/10.1097/01.mib.0000179211.03650.b6
[21] Lindelöf B, Eklund G, Lidén S, Stern RS. The prevalence of malignant tumors in patients with psoriasis. J Am Acad Dermatol. 1990;22(6):1056–1060. https://doi.org/10.1016/0190-9622(90)70152-8
[22] Jensen P, Egeberg A, Gislason G, Thyssen JP, Skov L. Risk of uncommon cancers in patients with psoriasis: a Danish nationwide cohort study. J Eur Acad Dermatol Venereol. 2018;32(4):601–605. https://doi.org/10.1111/jdv.14610
[23] Troche JR, Mayne ST, Freedman ND, Shebl FM, Abnet CC. The association between alcohol consumption and lung carcinoma by histological subtype. Am J Epidemiol. 2016;183(2):110–112. https://doi.org/10.1093/aje/kwv170
[24] Fehringer G, Brenner DR, Zhang Z, et al. Alcohol and lung cancer risk among never smokers: a pooled analysis from the international lung cancer consortium and the SYNERGY study. Int J Cancer. 2017;140(9):1976–1984. https://doi.org/10.1002/ijc.30618
[25] Shi H, Tang B, Liu Y-W, Wang X-F, Chen G-J. Alzheimer disease and cancer risk: a meta-analysis. J Cancer Res Clin Oncol. 2015;141(3):485–494. https://doi.org/10.1007/s00432-014-1773-5
[26] Lee JE, Kim D, Lee JH. Association between Alzheimer’s Disease and cancer risk in South Korea: an 11-year nationwide population-based study. Dement Neurocognitive Disord. 2018;17(4):137. https://doi.org/10.12779/dnd.2018.17.4.137
[27] Lin H-L, Lin H-C, Tseng Y-F, Chen S-C, Hsu C-Y. Inverse association between cancer and dementia: a population-based registry study in Taiwan. Alzheimer Dis Assoc Disord. 2016;30(2):118–122. https://doi.org/10.1097/WAD.0000000000000116
[28] Ni L, Wu J, Long Y, et al. Mortality of site-specific cancer in patients with schizophrenia: a systematic review and meta-analysis. BMC Psychiatry. 2019;19(1):323. https://doi.org/10.1186/s12888-019-2332-z
[29] Zhuo C, Triplett PT. Association of schizophrenia with the risk of breast cancer incidence: a meta-analysis. JAMA Psychiatry. 2018;75(4):363. https://doi.org/10.1001/jamapsychiatry.2017.4748
[30] Raviv G, Laufer M, Baruch Y, Barak Y. Risk of prostate cancer in patients with schizophrenia. Compr Psychiatry. 2014;55(7):1639–1642. https://doi.org/10.1016/j.comppsych.2014.05.007
[31] Prochaska JJ, Das S, Young-Wolff KC. Smoking, Mental Illness, and Public Health. Annu Rev Public Health. 2017;38(1):165–185. https://doi.org/10.1146/annurev-publhealth-031816-044618