ORIGINAL ARTICLE
Real-world data on utilization of neoadjuvant chemotherapy for muscle invasive bladder cancer: impact on surgical complications and oncological efficacy
Hege S. Haugnesa,b  , Håkon Kjævec, Eivind Bjerkaasd, Ragnhild Hellesnesa,b, Line Hjellea,b and Magnus Larsena,e
, Håkon Kjævec, Eivind Bjerkaasd, Ragnhild Hellesnesa,b, Line Hjellea,b and Magnus Larsena,e
aInstitute of Clinical Medicine, UIT - The Arctic University, Tromsø, Norway; bDepartment of Oncology, University Hospital of North Norway, Tromsø, Norway; cGeneral Practice, Storsteinnes, Norway; dUroNord, Urology in North Norway, Tromsø, Norway; eDepartment of Urology, University Hospital of North Norway, Tromsø, Norway
ABSTRACT
Background and purpose: Recommended treatment of urothelial muscle-invasive bladder cancer (MIBC) is cisplatin-based neoadjuvant chemotherapy (NAC) followed by cystectomy, but there are challenges with low utilization of NAC. We aimed to evaluate the utilization of NAC, perioperative complications and oncological efficacy in a real-world setting.
Patients and methods: All patients operated with radical cystectomy at the University Hospital of North Norway during 2011–2021 for MIBC were included. NAC consisted of three cycles of dose-dense methotrexate, vinblastine, doxorubicin and cisplatin (ddMVAC) every second week. Complications after cystectomy (Clavien-Dindo ≥ grade 3 within 30 days), histopathologic NAC response, cancer recurrence, relapse-free survival (RFS), overall survival (OS) and cause of death were reported.
Results: We included 124 patients, median observation time of 4 years. Fifty-nine patients (48%) received NAC. Most common causes for not receiving NAC were age ≥ 75 years (n = 38; 31%), cardiovascular disease (n = 7; 5.6%), and reduced kidney function (n = 6; 4.8%). Overall 34 patients (27%) had a ≥ grade 3 complication. The 5-year actuarial OS rate was higher among patients treated with NAC than those without NAC (67% vs. 45%, p = 0.02). Among NAC-treated patients, 29 (49%) were downstaged to non-muscle invasive stage (≤pT1), and the 5-year actuarial RFS and OS were higher among patients with ≤pT1 in the post-cystectomy specimen than those with ≥ pT2 (92% vs. 35%, and 94% vs. 39%, both p < 0.001).
Interpretation: The utilization of NAC was high in this real-world setting. Treatment with ddMVAC with achieved downstaging to ≤pT1 was associated with considerably improved RFS and OS.
KEYWORDS: Neoadjuvant chemotherapy; cisplatin; cystectomy; bladder cancer; muscle invasive; utilization; complications; efficacy
Citation: ACTA ONCOLOGICA 2025, VOL. 64, 13–21. https://doi.org/10.2340/1651-226X.2025.42052.
Copyright: © 2025 The Author(s). Published by MJS Publishing on behalf of Acta Oncologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
Received: 19 September 2024; Accepted: 30 November 2024; Published: 2 January 2025
CONTACT: Hege S. Haugnes hege.sagstuen.haugnes@uit.no Department of Oncology, University Hospital of North Norway and The Arctic University, N-9038 Tromsø, Norway
Competing interests and funding: H.S. Haugnes has received honoraria from Merck, Janssen-Cilag and Bayer.
R. Hellesnes has received honoraria from Bayer.
M. Larsen has received honoraria from Pfizer, Bayer, Astellas og Coloplast
Otherwise, there are no other conflicts of interest.
Introduction
Recommended treatment of urothelial muscle-invasive bladder cancer (MIBC) is cisplatin-based neoadjuvant chemotherapy (NAC) followed by radical cystectomy and lymph node dissection [1]. Adjuvant nivolumab or pembrolizumab increases disease-free survival for subgroups of patients; however, overall survival (OS) data are lacking [2, 3]. Although the addition of NAC to surgery has led to an 8% absolute improvement in 5-year OS [4], MIBC remains a highly lethal disease with a historically 5-year OS at 53% for patients treated with NAC plus surgery. Recently, updated data from the VESPER trial reported 5-year OS at 66% after up to six cycles of neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin and cisplatin (ddMVAC) [5].
There are promising data on the combination of immunotherapy with NAC in the perioperative setting [6], and recently an OS benefit with this combination was reported in the phase III Niagara trial [7]. Nevertheless, the most important current effort in the handling of patients with MIBC is to increase the utilization of NAC. The addition of NAC to surgery was demonstrated to increase OS in a meta-analysis already in 2005 [8], but there have been challenges with low utilization of NAC worldwide. A recent meta-analysis showed an overall NAC utilization of 17%, with the highest proportion of NAC in Japan at 44% [9].
Previous studies have identified high age and comorbidity as reasons for not receiving NAC [10, 11], and a concern about increased surgical complication rates. An increased rate of 90-day readmissions and more frequent use of blood transfusions after NAC plus cystectomy versus cystectomy alone has been reported [12, 13]. However, there is a lack of data on perioperative complications from population-based studies with a high NAC utilization rate. Smoking is associated with a higher risk of post-cystectomy complications [14], and has a negative impact on treatment efficacy [15]. Other factors possibly related to perioperative complications and survival are body composition, for example sarcopenia and change in body mass index (BMI) [1, 16, 17].
The aims of this study were to evaluate (1) the feasibility and toxicity of NAC among patients treated with radical cystectomy, (2) perioperative complications and oncological efficacy according to NAC status and response to NAC, and with regard to treatment period, smoking status and body composition.
Material and methods
Patients and treatment
Overall 244 patients who underwent a cystectomy at The University Hospital of North Norway during January 1, 2011 through December 31, 2021 were identified through procedure codes in the medical record system. After excluding patients not eligible for this study (Figure 1), 124 patients operated with cystectomy as radical treatment for urothelial MIBC were included in the study population. Pre-treatment staging consisted of CT of the chest, abdomen and pelvis.
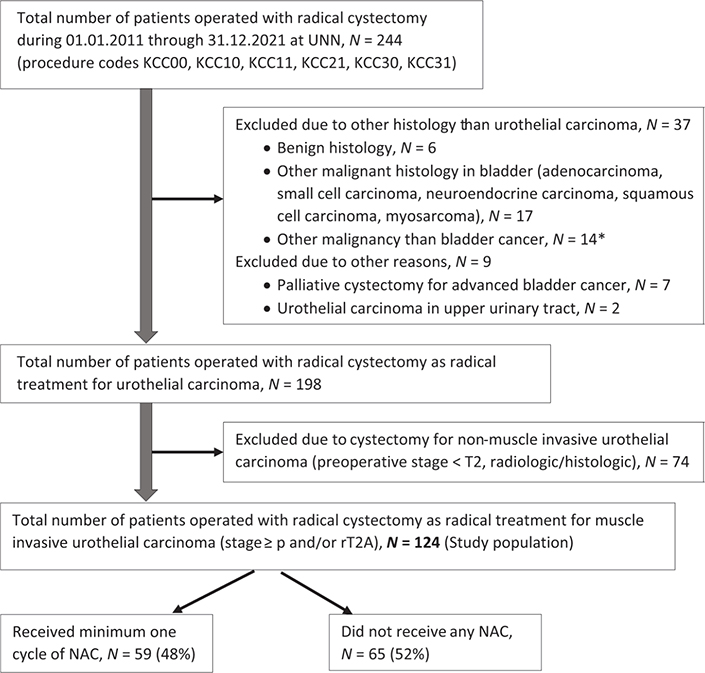
Figure 1. Flow chart for the study population. UNN: University Hospital of North Norway; N: number; NAC: neoadjuvant chemotherapy. *Patients operated with cystectomy as part of treatment for cancer in the prostate (N = 7), cancer in the colon (N = 4) or rectum (N = 3).
Neoadjuvant chemotherapy consisted of three cycles of ddMVAC given every second week if well-tolerated [18, 19]. Each cycle consisted of methotrexate 30 mg/m2 on day 1, and vinblastine 3 mg/m2, doxorubicin 30 mg/m2, and cisplatin 70 mg/m2 on day 2. The patients underwent a radiological evaluation with CT or MRI scan prior to the third cycle. In case of radiological progression, the third cycle was cancelled.
A radical cystectomy included removal of the bladder plus prostate and seminal vesicles in men, removal of the bladder plus uterus and adnexa in women, plus a pelvic lymph node dissection. A urinary diversion was always performed, most commonly as an ileac conduit. Surgery was performed as open surgery until the fall of 2019, after which robotic-assisted laparoscopic surgery was gradually implemented [20].
Ethics declaration
The study was approved by the local data protection officer (project no. 02386).
Assessments
Variables regarding patient characteristics, staging, and treatment of MIBC were collected retrospectively from medical records. Cancer recurrence status, survival, and cause of death was collected until April 2024. Treatment year was dichotomized into two categories (2011–2016 vs. 2017–2021), based on the gradual implementation of the national standardized cancer patient pathways in Norway during 2015 and 2016 [21]. Performance status was evaluated pre-chemotherapy among NAC treated patients according to the Eastern Cooperative Oncology Group (ECOG). Overweight was defined as BMI ≥ 25 kg/m2. Smoking status was dichotomized according to ever versus never smoker (daily or occasionally). Estimated glomerular filtration rate (eGFR) was calculated based on age and serum creatinine [22]. eGFR of 60–89 and 30–59 mL/min/1.73 m2 indicated a slightly or moderately decreased renal function, respectively [23].
Toxicity from chemotherapy was reported as a reason for discontinuing NAC. In addition, change in the proportion of patients with eGFR < 60 mL/min/1.73 m2 and relative weight change from the start of chemotherapy until cystectomy were calculated. Relative weight change was calculated as ((weightcystectomy–weightchemotherapy)/weightchemotherapy) × 100%, as described in a previous publication [24].
Surgical complications were quantified using the Clavien–Dindo classification system [25], a scoring system where complications are categorized from 1 to 5 on a severity scale, where category 5 indicates death. Only grade 3–5 complications within 30 days were reported, apart from infections where all grades were reported.
Statistics
Continuous variables were presented as median (range), while categorical variables were presented as counts (proportion). Differences between groups were compared using Chi square test. Continuous variables (e.g. age) were compared using nonparametric tests (independent samples Median test). The observation time was calculated from the date of cystectomy until death or end of follow-up (as of April 2024).
Cumulative relapse-free survival (RFS) and OS were calculated using the Kaplan–Meier method. Survival curves for RFS and OS are presented according to NAC status, and according to response to NAC (non-muscle invasive; ≤pT1 vs. muscle-invasive; ≥pT2). Cox regression analyses assessed possible associations between different variables and RFS and OS, with time in years from cystectomy until relapse or death as the time scale. The following variables were tested in univariate analyses: age at cystectomy, treatment year, pT stage at cystectomy, treatment with NAC versus no NAC, smoking status, overweight, and kidney function. Variables with p < 0.10 and age at cystectomy were included in the multivariate Cox regression models. Inspection of minus log curves and assessment of Schonfeld residuals confirmed that the proportional hazard assumption was met for both RFS and OS analyses.
Statistical analyses were performed using the SPSS (version 29.0 SPSS Inc., Chicago IL) and STATA (version MP 18; STATA, College Station, TX) statistical software. Two-sided p-values <0.05 were considered significant.
Results
Chemotherapy utilization, toxicity and efficacy
Median age at cystectomy was 72 years (range 43–89) and median observation time was 4.0 years (range 0.0–13) (Table 1). Overall 59 patients (48%) received a minimum of one cycle of NAC. Among patients treated during 2011–2016 and 2017–2021, 45 and 51% received NAC, respectively (p = 0.59). The majority of NAC treated patients had ECOG 0 pre-chemotherapy (n = 48, 81%), while 10 (17%) and 1 (1.7%) had ECOG 1 or 2, respectively. Patients who received NAC were younger than those who did not receive NAC (median age 69 years vs. 77 years, p < 0.001). The most common causes for not receiving NAC were age ≥ 75 years (n = 38; 31%), cardiovascular disease (n = 7; 5.6%), and reduced kidney function (n = 6; 4.8%).
| Characteristic | NAC N = 59 | No NAC N = 65 | Total N = 124 |
| Age, years | 69 (42–79) | 77 (47–89) | 72 (43–89) |
| Observation time, years | 5.2 (0.0–11.9) | 3.4 (0.0–13) | 4.0 (0.0–13) |
| Treatment year | |||
| 2011–2016 | 30 (45) | 37 (55) | 67 (54) |
| 2017–2021 | 29 (51) | 28 (49) | 57 (46) |
| Male gender, n (%) | 50 (84.7) | 51 (78.5) | 101 (81.5) |
| Smoking status, n (%) | |||
| Never smoker | 9 (15) | 16 (25) | 25 (20) |
| Ever smoker | 50 (85) | 49 (75) | 99 (51) |
| BMI, kg/m2 | 24.9 (19.2–37.0) | 25.0 (16.7–35.7) | 24.9 (16.7–37.0) |
| BMI < 25 kg/m2, n (%) | 32 (54) | 32 (49) | 64 (52) |
| eGFR, mL/min/1.73 m2,a | 71 (29–110) | 60 (26–107) | 65 (26–110) |
| eGFR, categories, n (%)a | |||
| eGFR < 60 mL/min/1.73 m2 | 16 (27) | 31 (48) | 47 (38) |
| eGFR 60–89 mL/min/1.73 m2 | 35 (59) | 24 (37) | 59 (48) |
| eGFR ≥ 90 mL/min/1.73 m2 | 8 (14) | 10 (15) | 18 (14) |
| Type of surgery, n (%) | |||
| Open cystectomy | 48 (81) | 52 (80) | 100 (81) |
| Robot-assisted | 11 (19) | 13 (20) | 24 (19) |
| Ward time after cystectomy, days | 13 (5–40) | 14 (1–157) | 14 (1–157) |
| cT stage before cystectomy, n (%)b | |||
| T2 | 50 (86) | 57 (88) | 107 (87) |
| T3 | 7 (12) | 5 (8) | 12 (10) |
| T4 | 2 (2) | 3 (5) | 5 (3) |
| rN stage before cystectomy, n (%)c | |||
| rN0 | 54 (92) | 60 (92) | 114 (92) |
| rN1 | 5 (8) | 5 (8) | 10 (8) |
| pT stage after cystectomy, n (%) | |||
| pT0 | 17 (29) | 2 (3) | 19 (15) |
| pTa/PTis | 10 (17) | 2 (3) | 12 (10) |
| pT1 | 2 (3) | 4 (6) | 6 (5) |
| pT2 | 15 (25) | 20 (31) | 35 (28) |
| pT3 | 14 (24) | 28 (43) | 42 (34) |
| pT4 | 1 (2) | 9 (14) | 10 (8) |
| pN stage after cystectomy, n (%) | |||
| pNo | 45 (76) | 29 (45) | 74 (60) |
| pN1 | 5 (8) | 11 (17) | 16 (13) |
| pN2 | 4 (7) | 6 (9) | 10 (8) |
| pNX | 5 (8) | 19 (29) | 24 (19) |
| Data are presented as median (range) unless otherwise specified. There are no missing data. MIBC: muscle-invasive bladder cancer; NAC: neoadjuvant chemotherapy; n, number; BMI: body mass index; eGFR: estimated glomerular filtration rate; c, clinical; r, radiological; p, pathological. aeGFR is calculated based on age and serum creatinine [22]. bcT stage is based on either pathologic or radiologic T stage, where the highest T stage is presented, since this stage gave the indication for NAC. Among patients classified as cT2, 86 (80%) had pT2 stage, while 8 had pT1 stage and were upgraded due to radiologic T2 or T3 tumor. cOne patient treated with NAC had rNX stage. |
|||
Thirty-three patients (56%) received all three cycles of NAC. Twelve patients (20%) discontinued NAC after one cycle due to kidney failure (n = 6), infection (n = 2), and other causes. Additional 14 patients (24%) stopped NAC after two cycles (due to infection, kidney failure, patient decision, severe nausea, radiologic progression). NAC treated patients underwent cystectomy at a median of 4 weeks after day 1 in the last chemotherapy cycle (range 2–8 weeks).
The proportion of NAC treated patients with eGFR < 60 mL/min/1.73 m2 increased from 14% (n = 8) at start chemotherapy to 27% (n = 16) at cystectomy (p = 0.015). Overall 36 patients (61%) had stable weight or small to moderate weight gain. Eight patients (14%) had a small weight loss, while 15 (25%) had a moderate weight loss (> – 5%).
Histopathologic evaluation of the cystectomy specimen showed that 29 patients (49%) had a downgrading of pT stage after NAC (Figure 2). Twenty-nine patients (49%) were downstaged to ≤pT1, of whom 17 (29%) were downstaged to pT0.
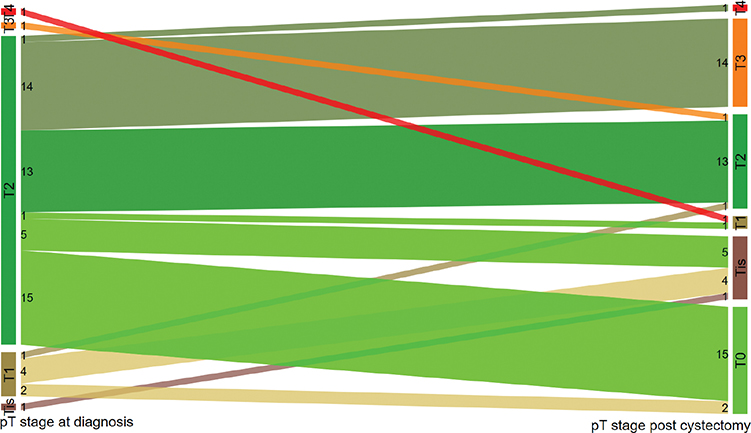
Figure 2. Figure 2 shows the pT stage at diagnosis (before chemotherapy) to the left, and pT stage post cystectomy to the right for NAC-treated patients. Numbers are the actual numbers of patients in each stage category.
Surgical complications
Overall 55 patients (53%) had a 30-day postoperative infection (Table 2). The infection rate was higher among patients aged ≥ 75 years than among those < 75 years (68% vs. 46%, p = 0.02), while there were no differences in infection rates for treatment period (p = 0.21), NAC status (p = 0.15), smoking status (p = 0.82) or BMI (p = 0.75).
| Characteristics | Any grade infections | Grade ≥ 3 all complications | Grade ≥ 3 infections | Grade ≥ 3 gastrointestinal | Grade ≥ 3 heart/circ | Grade ≥ 3 kidney failure | Grade ≥ 3 other |
| All patients, N = 124 | 55 (53) | 34 (27) | 23 (19) | 10 (8.1) | 7 (5.6) | 4 (3.2) | 4 (3.2) |
| Age | |||||||
| < 75 y (N = 83) | 38 (46) | 19 (23) | 14 (17) | 4 (4.8) | 4 (4.8) | 1 (1.2) | 2 (2.4) |
| ≥ 75 y (N = 41) | 28 (68) | 15 (37) | 9 (22) | 6 (15) | 3 (7.3) | 3 (7.3) | 2 (4.9) |
| Treatment period | |||||||
| 2011–2016 | 32 (48) | 14 (21) | 9 (13) | 4 (6.0) | 2 (3.0) | 2 (3.0) | 2 (3.0) |
| 2017–2021 | 34 (60) | 20 (35) | 14 (25) | 6 (10) | 5 (8.8) | 2 (3.5) | 2 (3.5) |
| NAC status | |||||||
| NAC | 27 (46) | 14 (24) | 9 (15) | 3 (5.1) | 2 (3.4) | 1 (1.7) | 2 (3.4) |
| No NAC | 39 (60) | 20 (31) | 14 (22) | 7 (11) | 5 (7.7) | 3 (4.6) | 2 (3.1) |
| Smoking status | |||||||
| Ever smoker | 52 (53) | 25 (25) | 18 (18) | 7 (7.1) | 6 (6.1) | 3 (3.0) | 1 (2.8) |
| Never smoker | 14 (56) | 9 (36) | 5 (20) | 3 (12) | 1 (4.0) | 1 (4.0) | 1 (4.0) |
| BMI | |||||||
| < 25 kg/m2 | 29 (45) | 15 (19) | 12 (19) | 8 (12.5) | 2 (3.1) | 2 (3.1) | 0 |
| ≥ 25 kg/m2 | 37 (62) | 19 (32) | 11 (18) | 2 (3.3) | 5 (8.3) | 2 (3.3) | 4 (6.7) |
| Weight changea | |||||||
| Increase/stable | 15 (42) | 8 (22) | 4 (11) | 1 (2.8) | 1 (2.8) | 0 | 2 (5.6) |
| Decrease | 12 (52) | 6 (26) | 5 (22) | 2 (8.7) | 1 (4.3) | 1 (4.3) | 0 |
| GFR changea | |||||||
| Stable/improved | 15 (50) | 9 (30) | 6 (20) | 3 (10) | 0 | 1 (3.3) | 1 (3.3) |
| Worsened | 12 (41) | 5 (17) | 3 (10) | 0 | 2 (6.9) | 0 | 1 (3.4) |
| Abbreviations: NAC: neoadjuvant chemotherapy; BMI: body mass index; GFR: glomerular filtration rate; N: numbers; y: years. Data are presented as numbers (%). aOnly for patients treated with minimum one cycle of neoadjuvant chemotherapy. |
|||||||
Thirty-four patients (27%) had a ≥ grade 3 complication (Table 2). Infections (19%) and gastrointestinal complications (8.1%) were the most common complications. No statistically significant differences were observed regarding incidence of all ≥ grade 3 complications (age p = 0.14; treatment period p = 0.11; NAC status p = 0.42; smoking status p = 0.32; BMI p = 0.32). Patients aged ≥ 75 years versus < 75 years had a borderline significantly higher incidence of ≥ grade 3 gastrointestinal complications (15% vs. 4.8%, p = 0.08). There were numerically fewer patients with any ≥ grade 3 complications among patients who received NAC than those without NAC, but none were statistically significant. Patients with a weight decrease after NAC had numerically higher incidences of all reported complications compared with patients with stable/increased weight, but none were statistically significant (data not shown).
Five patients died within 30 days after cystectomy (4.0%). One of these patients had received NAC, corresponding to a 30-day mortality rate at 1.7% after NAC, and 4.7% for patients without NAC.
Relapse and survival
At the end of follow-up, 56 patients (45%) were alive and relapse-free (Table 3). The 5-year actuarial RFS was higher among patients treated with NAC than those without NAC (63% vs. 40%, p = 0.003, Figure 3A). Among NAC treated patients, the 5-year RFS was higher among patients who achieved ≤pT1 post-cystectomy compared to those with ≥pT2 (92% vs. 35%, p < 0.001, Figure 3B).
| Characteristic | NAC N = 59 | No NAC N = 65 | Total N = 124 |
| Vital status at end of follow-up | |||
| Alive, relapse-free | 36 (61) | 20 (31) | 56 (45) |
| Alive, with relapse | 1 (1.7) | 6 (9.2) | 7 (5.6) |
| Relapse, overall | 19 (32) | 22 (34) | 41 (33) |
| Dead | 22 (37) | 39 (60) | 61 (49) |
| 5-year RFS, % (95% CI) | 63 (49–75) | 40 (28–52) | 51 (42–60) |
| 5-year OS, % (95% CI) | 67 (53–77) | 45 (32–57) | 55 (46–65) |
| Relapse site | |||
| Regional lymph nodes | 14 (24) | 15 (23) | 29 (23) |
| Lung metastases | 7 (12) | 9 (14) | 16 (13) |
| Liver metastases | 5 (8.5) | 9 (14) | 14 (11) |
| Bone | 8 (14) | 3 (4.6) | 11 (8.9) |
| Othera | 5 (8.5) | 5 (7.7) | 10 (8.1) |
| Cause of death | |||
| Metastatic bladder cancer | 18 (30) | 16 (25) | 34 (56) |
| Treatment-related complications | 1 (1.7) | 8 (12) | 9 (15) |
| Infection, not treatment-related | 2 (3.4) | 7 (11) | 9 (15) |
| Atherosclerotic disease | 1 (1.7) | 2 (3) | 3 (5) |
| Other causesb | 0 | 6 (10) | 6 (15) |
| Data are presented as numbers (n) (%), unless otherwise specified. RFS: relapse-free survival; OS: overall survival; NAC: neoadjuvant chemotherapy; CI: confidence interval. aOther relapse sites: adrenal gland (n = 3), brain (n = 2), carcinomatosis (n = 2), pelvic muscle, spleen, penis. bOther causes of death include kidney failure, prostate cancer, gastrointestinal bleeding, chronic obstructive pulmonary disease, unknown causes. |
|||
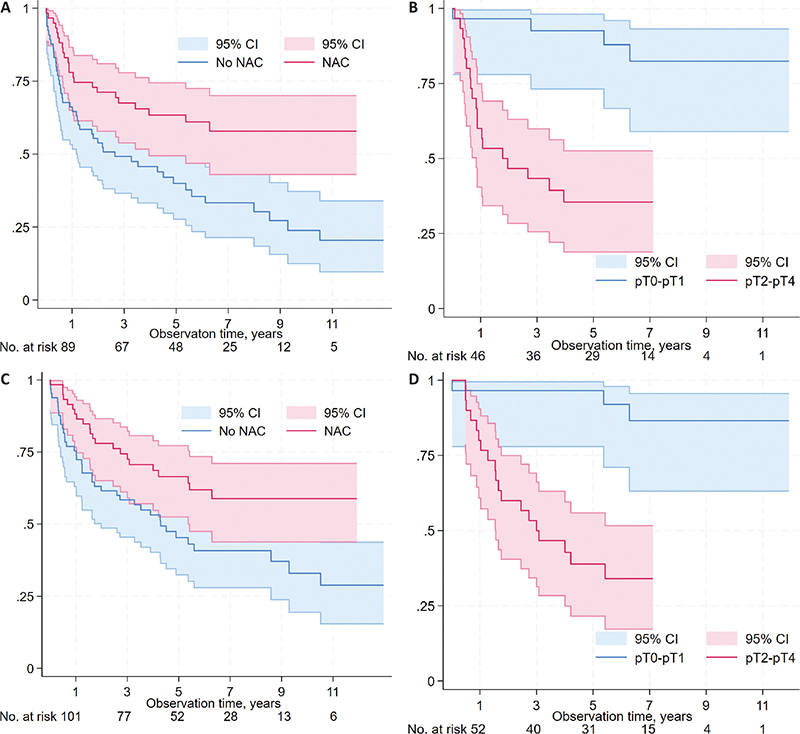
Figure 3. Relapse-free survival (RFS) and overall survival (OS) for all patients according to neoadjuvant chemotherapy (NAC) status (RFS: Figure 3A; OS: Figure 3C), and according to response to NAC (non-muscle invasive; ≤pT1 vs. muscle-invasive; ≥pT2) in the post-cystectomy specimen among NAC-treated patients (RFS: Figure 3B; OS: Figure 3D).
The 5-year actuarial OS rate was higher among patients treated with NAC than those without NAC (67% vs. 45%, p = 0.02, Figure 3C). Among NAC treated patients, the 5-year OS was higher among patients who had ≤pT1 post-cystectomy than those with ≥pT2 (94% vs. 39%, p < 0.001, Figure 3D).
In total 61 patients (49%) died. A larger proportion of patients died due to metastatic disease among patients treated with NAC versus no NAC (30% vs. 25% of all patients), while a larger proportion of patients died from treatment-related complications (12% vs. 1.7%) and causes other than MIBC-related (24% vs. 5%) among patients without NAC (p = 0.002).
Univariate Cox regression analyses showed that treatment during 2017–2021 (vs. 2011–2016) was associated with a lower risk of relapse or death (HR 0.59, 95% CI 0.35–0.99; HR 0.54, 95% CI 0.31–0.95; Table 4). Increasing pT stage at cystectomy was associated with an increased risk of relapse or death (p for trend < 0.001, both). Patients treated with NAC versus no NAC had about half the risk of relapse or death. In multivariate Cox regression, treatment during 2017–2021 was associated with lower risks of relapse and death, while increasing pT stage was associated with higher risks of relapse and death (p for trend < 0.001, both).
Discussion
In this population-based study including urothelial MIBC patients treated with radical cystectomy during 2011–2021, the utilization of NAC was 48%. NAC treatment was not associated with any increased 30-day complication rate. The NAC treated patients who achieved a downstaging to ≤pT1 had impressive 5-year RFS and OS rates of 92% and 94%, respectively.
The ddMVAC regimen used at our institution was well-tolerated and with high rates of pathologic down-staging in two phase II studies [18, 19]. Furthermore, a higher complete response rate from ddMVAC compared to standard NAC was observed in a large retrospective trial among 824 patients 40% of whom received NAC [26]. The VESPER trial has recently reported improved 5-year OS rates with six cycles of ddMVAC compared with four cycles of cisplatin/gemcitabine in the neoadjuvant subgroup (66% vs. 57%) [5], suggesting ddMVAC as the standard NAC regimen.
The proportion receiving NAC herein (48%) is higher than that reported by more recent studies, with rates ranging from 16 to 44% [9, 13, 26–28]. The NAC rates seem to be high in countries with public health care systems providing equal access to treatments which is the case for Sweden and Norway, or with universal health insurance coverages as in Japan, with reported NAC rates up to 42 and 44% [9]. In addition, the NAC utility rate in our most recent treatment period 2017–2021 at 51% is in line with recently published national registry data from Denmark where it was reported that 213/425 (50%) of patients operated with cystectomy for MIBC received NAC [29]. Nonetheless, it is questionable whether the NAC utilization rate can be further increased above 50%, given the toxicity profile from cisplatin-based chemotherapy and the high median age at diagnosis (72 years herein). Supporting other studies, we found that age > 75 years and comorbidities, in particular cardiovascular disease and reduced kidney function, were the most common reasons for not being offered NAC, factors that also influence survival [10, 11].
Concern about increased rates of surgical complications post-cystectomy has been suggested as a possible reason for not offering NAC. However, the 30-day grade ≥ 3 Clavien–Dindo complication rates did not differ according to NAC status in our study, supporting findings from recent retrospective studies [13, 28, 30]. The 30-day mortality rate was low and numerically lower after NAC versus no NAC (1.7% vs. 4.7%). The numerically higher 30-day mortality rate among patients not treated with NAC probably reflects the fact that these patients had more comorbidity. The mortality rates reported herein corroborate numbers reported in the European guidelines [1], as well as data from a large Swedish study (90-day mortality rates at 2.7 and 6.3%, respectively) [13]. In light of concerns regarding surgical complications and loss of bladder function, other treatment options for MIBC are available, that is trimodality treatment with similar outcomes in retrospective analyses [31].
We observed that 49% of the patients treated with NAC achieved pathologic downstaging to ≤pT1, which is in line with the rates of 49–52% reported in the phase II trials [18, 19]. Some of this downstaging may also relate to a complete transurethral resection of the bladder. The complete response rate (pT0) herein was 29%, somewhat lower than numbers reported by three other studies with rates at 38–42% [18, 26, 32]. Two of these studies had, however, a higher number of ddMVAC cycles with 60% of patients treated with six cycles in the VESPER trial [32], and 91% received ≥ three cycles in the Peyton study [26]. In line with these studies, we have recently changed our treatment recommendation to four cycles of ddMVAC.
Our 5-year OS rate at 45% for patients without NAC is similar to the historically 5-year OS rate at 45% for these patients in a large meta-analysis [8], while our 5-year OS rate at 67% for patients treated with NAC is considerably better than previous results from meta-analyses with rates at 50 and 53% [4, 8]. Our 5-year OS rate after NAC, regardless of pathologic response, is in line with results from the VESPER trial, reporting a 5-year OS rate at 66% after ddMVAC [5].
Survival after NAC is strongly influenced by whether a pathologic downstaging is achieved. The observation time after NAC in our study is a median of 5.2 years, considerably longer than in comparable studies. Even so, our 5-year RFS rate among NAC treated patients with downstaging to ≤pT1 at 92% is comparable to the 1-year RFS at 89% and 2-year RFS at 88% reported in the phase II studies [18, 19], and our 5-year OS rate at 94% seems to be higher than the 2-year OS rate at 88% in another study [26]. On the other hand, patients who do not achieve a pathologic downstaging to non-MIBC after NAC seem to have a survival which is similar or even inferior to patients not treated with NAC. Consequently, there is a need for predictive biomarkers that can identify patients who will benefit from NAC, as well as new treatment options for non-responders. A move in the right direction is a recent study reporting that certain molecular subtypes of MIBC were more responsive to NAC [33].
Treatment after 2016 was associated with a 50% reduction of relapse or death compared with the earlier treatment period. The better results after 2016 might be explained by improved diagnostic procedures including standardized waiting times as a part of the national standardized cancer patient pathways [21], as well as improved surgical techniques with the implementation of robotic-assisted cystectomy in 2019 [20]. The improved OS is probably also influenced by better systemic treatment options for patients with relapse after cystectomy [1].
Strengths of this study include the population-based design, where all patients treated with radical cystectomy for urothelial MIBC in our region were included, and with a high degree of data completeness. The same chemotherapy regimen was applied during the whole study period. As a limitation, comorbidity was not systematically registered, but the treatment of NAC versus no NAC was strongly related to comorbidity. The low number of patients with ≥ grade 3 complications in the different categories introduced a possible type II bias, and prevented us from drawing any conclusions regarding risks of complications.
In conclusion, our NAC utility rate at 48% was high, and NAC was not associated with any increased rate of postoperative complications. Survival after NAC is strongly influenced by whether a pathologic downstaging is achieved, and there is a large need for predictive biomarkers to select patients who will benefit from NAC. Survival after a radical cystectomy also depends on the surgical technique applied, with promising results for en bloc radical cystectomy [34].
Data availability statement
The data are not available due to restrictions from the data protection officer and the Regional Committee for Medical and Research Ethics
References
[1] Witjes JA, Bruins H, Carrion A, Cathomas R, Comperat E, Efstathiou JA, et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2023 guidelines. Eur Urol. 2024;85:17–31. https://doi.org/10.1016/j.eururo.2023.08.016
[2] Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384:2102–14. https://doi.org/10.1056/NEJMoa2034442
[3] Apolo AB, Ballman KV, Sonpavde G, Berg S, Kim WY, Parikh R, et al. Adjuvant pembrolizumab versus observation in muscle-invasive urothelial carcinoma. N Engl J Med. 2024 Sep 15. [Online ahead of print].
[4] Yin M, Joshi M, Meijer RP, Glantz M, Holder S, Harvey HA, et al. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: a systematic review and two-step meta-analysis. Oncologist. 2016;21(6):708–15. https://doi.org/10.1634/theoncologist.2015-0440
[5] Pfister C, Gravis G, Flechon A, Chevreau C, Mahammedi H, Laguerre B, et al. Perioperative dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin in muscle-invasive bladder cancer (VESPER): survival endpoints at 5 years in an open-label, randomised, phase 3 study. Lancet Oncol. 2024;25:255–64. https://doi.org/10.1016/S1470-2045(23)00587-9
[6] Cathomas R, Rothschild SI, Hayoz S, Bubendorf L, Ozdemir BC, Kiss B, et al. Perioperative chemoimmunotherapy with durvalumab for muscle-invasive urothelial carcinoma: primary analysis of the single-arm phase II trial SAKK 06/17. J Clin Oncol. 2023;41:5131–9. https://doi.org/10.1200/JCO.23.00363
[7] Powles T, Catto JWF, Galsky MD, Al-Ahmadie H, Meeks JJ, Nishiyama H, et al. Perioperative durvalumab with neoadjuvant chemotherapy in operable bladder cancer. N Engl J Med. 2024; 391(91): 1773-86. https://.doi:10.1056/NEJMoa2408154
[8] Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005; 48:202–5. https://doi.org/10.1016/j.eururo.2005.04.006
[9] Liu W, Tian J, Zhang S, Yang E, Shen H, Li F, et al. The utilization status of neoadjuvant chemotherapy in muscle-invasive bladder cancer: a systematic review and meta-analysis. Minerva Urol Nephrol. 2021;73:144–53. https://doi.org/10.23736/S2724-6051.19.03648-8
[10] McFerrin C, Davaro F, May A, Raza S, Siddiqui S, Hamilton Z. Trends in utilization of neoadjuvant and adjuvant chemotherapy for muscle invasive bladder cancer. Investig Clin Urol. 2020;61:565–72. https://doi.org/10.4111/icu.20200132
[11] Hermans TJN, Fransen van de Putte EE, Horenblas S, Lemmens V, Aben K, van der Heijden MS, et al. Perioperative treatment and radical cystectomy for bladder cancer – a population based trend analysis of 10,338 patients in the Netherlands. Eur J Cancer. 2016;54:18–26. https://doi.org/10.1016/j.ejca.2015.11.006
[12] Aldhaam NA, Elsayed AS, Jing Z, Richstone L, Wagner AA, Rha KH, et al. Neoadjuvant chemotherapy is not associated with adverse perioperative outcomes after Robot-assisted radical cystectomy: a case for increased use from the IRCC. J Urol. 2020;203:57–61. https://doi.org/10.1097/JU.0000000000000445
[13] Jerlstrom T, Chen R, Liedberg F, Andren O, Strock V, Aljabery FAS, et al. No increased risk of short-term complications after radical cystectomy for muscle-invasive bladder cancer among patients treated with preoperative chemotherapy: a nation-wide register-based study. World J Urol. 2020;38:381–8. https://doi.org/10.1007/s00345-019-02770-2
[14] Tellini R, Mari A, Muto G, Cacciamani GE, Ferro M, Stangl-Kremser J, et al. Impact of smoking habit on perioperative morbidity in patients treated with radical cystectomy for urothelial bladder cancer: a systematic review and meta-analysis. Eur Urol Oncol. 2021;4:580–93. https://doi.org/10.1016/j.euo.2020.10.006
[15] Cacciamani GE, Ghodoussipour S, Mari A, Gill KS, Desai M, Artibani W, et al. Association between smoking exposure, neoadjuvant chemotherapy response and survival outcomes following radical cystectomy: systematic review and meta-analysis. J Urol. 2020;204:649–60. https://doi.org/10.1097/JU.0000000000000813
[16] Lyon TD, Frank I, Takahashi N, Boorjian SA, Moynagh MR, Shah PH, et al. Sarcopenia and response to neoadjuvant chemotherapy for muscle-invasive bladder cancer. Clin Genitourin Cancer. 2019;17(3):216–22 e215. https://doi.org/10.1016/j.clgc.2019.03.007
[17] Engelmann SU, Pickl C, Haas M, Kaelble S, Hartmann V, Firsching M, et al. Body composition of patients undergoing radical cystectomy for bladder cancer: sarcopenia, low Psoas Muscle Index, and myosteatosis are independent risk factors for mortality. Cancers. 2023;15(6):1778. https://doi.org/10.3390/cancers15061778
[18] Plimack ER, Hoffman-Censits JH, Viterbo R, Trabulsi EJ, Ross EA, Greenberg RE, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol. 2014;32:1895–901. https://doi.org/10.1200/JCO.2013.53.2465
[19] Choueiri TK, Jacobus S, Bellmunt J, Qu A, Appleman LJ, Tretter C, et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol. 2014;32:1889–94. https://doi.org/10.1200/JCO.2013.52.4785
[20] Catto JWF, Khetrapal P, Ricciardi F, Ambler G, Williams NR, Al-Hammouri T, et al. Effect of Robot-assisted radical cystectomy with intracorporeal urinary diversion vs open radical cystectomy on 90-day morbidity and mortality among patients with bladder cancer: a randomized clinical trial. JAMA. 2022;327:2092–103. https://doi.org/10.1001/jama.2022.7393
[21] The Norwegian Directorate of Health. Cancer patient pathways. [Internet]. Oslo: The Norwegian Directorate of Health; 2023 [cited 2024 October 28]. Available from: https://www.helsenorge.no/en/sykdom/kreft/cancer-patient-pathways/
[22] Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
[23] Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–52. https://doi.org/10.1016/S0140-6736(16)32064-5
[24] Troeschel AN, Hartman TJ, Jacobs EJ, Stevens VL, Gansler T, Flanders W, et al. Postdiagnosis body mass index, weight change, and mortality from prostate cancer, cardiovascular disease, and all causes among survivors of nonmetastatic prostate cancer. J Clin Oncol. 2020;38:2018–27. https://doi.org/10.1200/JCO.19.02185
[25] Mitropoulos D, Artibani W, Graefen M, Remzi M, Roupret M, Truss M. Reporting and grading of complications after urologic surgical procedures: an ad hoc EAU guidelines panel assessment and recommendations. Eur Urol. 2012;61:341–9. https://doi.org/10.1016/j.eururo.2011.10.033
[26] Peyton CC, Tang D, Reich RR, Azizi M, Chipollini J, Pow-Sang JM, et al. Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for muscle-invasive bladder cancer. JAMA Oncol. 2018;4:1535–42. https://doi.org/10.1001/jamaoncol.2018.3542
[27] Salminen AP, Koskinen I, Perez IM, Hurme S, Murtola TJ, Vaarala MH, et al. Neoadjuvant chemotherapy does not increase the morbidity of radical cystectomy: a 10-year retrospective nationwide study. Eur Urol Oncol. 2018;1:525–30. https://doi.org/10.1016/j.euo.2018.06.014
[28] Riveros C, Elshafei A, Chalfant V, Alam U, Balaji KC. Complications after neoadjuvant chemotherapy and radical cystectomy for patients with bladder cancer: a propensity score matched analysis. Can Urol Assoc J. 2023;17(3):57–66. https://doi.org/10.5489/cuaj.7952
[29] Korner SK, Dreyer T, Carus A, Dohn LH, Joensen UN, Lam GW, et al. DaBlaCa-17: nationwide observational study in Denmark on survival before and after implementation of neoadjuvant chemotherapy prior to cystectomy for muscle-invasive bladder cancer. Scand J Urol. 2024;59:39–46. https://doi.org/10.2340/sju.v59.24024
[30] Arora A, Zugail AS, Pugliesi F, Cathelineau X, Macek P, Barbe Y, et al. Neoadjuvant chemotherapy does not increase peri-operative morbidity following radical cystectomy. World J Urol. 2022;40:1697–705. https://doi.org/10.1007/s00345-022-04012-4
[31] Zlotta AR, Ballas LK, Niemierko A, Lajkosz K, Kuk C, Miranda G, et al. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. Lancet Oncol. 2023;24:669–81. https://doi.org/10.1016/S1470-2045(23)00170-5
[32] Pfister C, Gravis G, Flechon A, Chevreau C, Mahammedi H, Laguerre B, et al. Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin or gemcitabine and cisplatin as perioperative chemotherapy for patients with nonmetastatic muscle-invasive bladder cancer: results of the GETUG-AFU V05 VESPER trial. J Clin Oncol. 2022;40:2013–22. https://doi.org/10.1200/JCO.21.02051
[33] Sjodahl G, Abrahamsson J, Holmsten K, Bernardo C, Chebil G, Eriksson P, et al. Different responses to neoadjuvant chemotherapy in urothelial carcinoma molecular subtypes. Eur Urol. 2022;81:523–32. https://doi.org/10.1016/j.eururo.2021.10.035
[34] Kjobli E, Salvesen O, Langorgen S, Storkersen O, Wibe A, Arum CJ. En bloc radical cystectomy: an overview of the technique and oncological results. BJUI Compass. 2023;4:195–205. https://doi.org/10.1002/bco2.190