SHORT REPORT
Distinct patterns of osteoradionecrosis after photon-based radiotherapy and carbon ion radiotherapy for unresectable adenoid cystic carcinoma of the head and neck: case series from two institutions
Eivind Storaasa , Marta D. Switlykb
, Marta D. Switlykb , Sigrun Dahla
, Sigrun Dahla , Cecilie D. Amdala
, Cecilie D. Amdala , Åse Bratlanda
, Åse Bratlanda , Thuy-Tien M. Huynha,c
, Thuy-Tien M. Huynha,c , Hanne A. Eidea
, Hanne A. Eidea , Barbara Vischionid
, Barbara Vischionid , Ester Orlandid,e
, Ester Orlandid,e and Einar Dalea
and Einar Dalea
aDepartment of Oncology, Oslo University Hospital, Oslo, Norway; bDepartment of Radiology and Nuclear Medicine, Oslo University Hospital, Oslo, Norway; cFaculty of Medicine, University of Oslo, Oslo, Norway; dClinical Department, National Center for Oncological Hadrontherapy (Fondazione CNAO), Pavia, Italy; eDepartment of Clinical, Surgical, Diagnostic, and Pediatric Sciences, University of Pavia, Pavia, Italy
ABSTRACT
Background and purpose: To present the clinical outcomes of two series of patients treated with carbon-ion radiotherapy (CIRT) and definitive photon radiotherapy (RT) for adenoid cystic carcinoma of the head and neck (HN-ACC).
Material and methods: The first cohort of six patients was referred from Oslo University Hospital (OUS) to Centro Nazionale di Adroterapia Oncologica (CNAO, Pavia, Italy) for CIRT in 2014–2017. The second cohort included 18 patients treated with definitive photon RT at OUS in 2005–2017. The primary endpoint was an evaluation of osteoradionecrosis (ORN) in the two cohorts. The secondary endpoints were treatment efficacy by local control (LC), progression-free survival (PFS), and overall survival (OS).
Results: The tumor stage was T4 for all the patients in the CIRT group and 15 (84%) in the photon group. There were three (50%) patients with grade 3 ORN in the CIRT group compared to one (6%) with grade 3 ORN in the photon group (p = 0.05). The 5-year LC (95% CI), PFS, and OS rates in the CIRT group and the photon group were 33% (11–100) and 39% (19–76), 17% (9–100) and 23% (2–59), and 80% (52–100) and 50% (31–82), respectively.
Interpretation: Half of the patients in the CIRT cohort experienced ORN requiring surgical management during the follow-up. Patients with ACC referred for CIRT often have a worse prognosis and more advanced disease than patients treated with photons. When returning from the referring center, these patients need close follow-up often in collaboration with treating centers to manage toxicity that impacts quality of life.
KEYWORDS: Adenoid cystic carcinoma of the head and neck; carbon-ion radiotherapy; osteoradionecrosis
Citation: ACTA ONCOLOGICA 2025, VOL. 64, 63–68. https://doi.org/10.2340/1651-226X.2025.42209.
Copyright: © 2025 The Author(s). Published by MJS Publishing on behalf of Acta Oncologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/).
Received: 9 October 2024; Accepted: 2 January 2025; Published: 15 January 2025
CONTACT Einar Dale eindal@ous-hf.no Department of Oncology, Oslo University Hospital, PO Box 4953 Nydalen N-0424 Oslo, Norway
Supplemental data for this article can be accessed online at https://doi.org/10.2340/1651-226X.2025.42209
Competing interests and funding: The authors report there are no competing interests to declare.
This study was not supported by any sponsor or funder
Introduction
Salivary gland malignancies account for up to 5% of head and neck cancers [1]. Adenoid cystic carcinoma (HN-ACC), a subset of these, is typically slow growing but locally aggressive, often spreading along skull base nerves. Advanced cases may involve vital structures, making surgery unfeasible. Standard treatment for localized disease combines surgery and postoperative photon radiotherapy (RT), achieving 5-year local control (LC) rates of 68–91% [2]. However, unresectable cases treated with photon RT show poor outcomes [3]. Due to its rarity and diagnostic challenges, especially in high-grade cases, clinical research on HN-ACC is limited, and consensus on the management is slowly emerging [4]. In this regard, patients with an expected R2 margin are preferably sent for primary RT and heavy ion therapy [5].
Carbon-ion radiotherapy (CIRT) is emerging as a promising option for inoperable HN-ACC, offering high linear energy transfer (LET) and precise spatial distribution through the Bragg peak. These properties may overcome the radioresistance and hypoxia of HN-ACC [6]. Recent studies suggest that CIRT improves LC, overall survival (OS), and toxicity outcomes, even in R2-resected or inoperable cases [7, 8].
Osteoradionecrosis (ORN), a painful condition from radiation-induced tissue injury, arises when hypoxic, hypovascular, and hypocellular tissue fails to heal [9]. Spontaneous ORN correlates with radiation dose and significantly impacts quality of life [10, 11]. Treatment ranges from improved oral hygiene and antibiotics to surgery and hyperbaric oxygen therapy. Severe ORN (grade ≥ 3) rates are 2–4% with modern photon RT [12, 13] and 4–21% after CIRT [14, 15].
Since 2014, Oslo University Hospital (OUS) has referred younger, inoperable HN-ACC patients for primary CIRT at Centro Nazionale di Adroterapia Oncologica (CNAO) in Italy, which has treated over 300 HN-ACC cases since 2010. This study aimed to report ORN frequency and survival outcomes for Norwegian patients treated with CIRT at CNAO, compared with photon RT at OUS.
Material and methods
This is a retrospective observational study of patients with unresectable HN-ACC who received treatment from 2005 to 2017. There were two groups of patients: the CIRT group treated at CNAO and the photon group treated at OUS.
The patients in the CIRT group (n = 6) were referred to CNAO from 2014 to 2017. These patients received RT with carbon ions to a total dose of 65.6–68.8 Gy (relative biological effectiveness [RBE]) in 4.1–4.3 Gy per fraction (RBE), as described by Vischioni et al. [16].
The patients in the photon group (n = 18) were treated at OUS with primary RT using photons. Planning computed tomography (CT) was performed on an RT-compatible flat table with head support and a fixation mask. Most of the patients were treated with two sequential plans. First, 46 Gy in 2 Gy per fraction to the gross tumor volume (GTV) plus 10 mm margin to the clinical target volume (CTV) including the elective neck volume, followed by 24 Gy in 2 Gy per fraction to the GTV. The planning target volume (PTV) margin to the CTV was 3 mm (Supplementary Table 1).
A complete dental examination was requested prior to treatment in both groups. If clinically indicated, tooth extraction was performed at least 14 days before the start of treatment.
Patient and tumor characteristics were collected from the medical records: age, sex, performance status (Eastern Cooperative Oncology Group [ECOG] status), comorbidity [17], tobacco use, tumor site, TNM stage, and follow-up information. TNM classification was reassessed according to the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) 8th edition. The number of teeth in the PTV was recorded as this parameter has been shown to correlate with the risk of ORN [16].
The patients were followed every 3 months for at least 2 years, thereafter every 6 months for 5 years or more. The institutional practice was to perform imaging (magnetic resonance imaging or CT) 3 months after RT and then as clinically indicated. Grade 2 and 3 ORNs were scored with the available imaging and patient data according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 [18]. Follow-up in this study was terminated on December 6, 2023.
Statistics
Frequencies in contingency tables were compared using Fisher’s exact test. For calculation of survival, the Kaplan–Meier method was used. Time was measured from the start of RT to an event or censoring. Progression-free survival (PFS), LC, regional, and distant failure were counted either by biopsy or by radiological findings if biopsy was not warranted. The median follow-up time was estimated using the reverse Kaplan–Meier method. P-values <0.05 were considered statistically significant. The data were analyzed using RStudio version 2022.2.1.461 with R version 4.1.2 integrated [19].
Results
Patient characteristics of the CIRT group (n = 6) and the photon group (n = 18) are presented in Table 1. The six patients treated with CIRT were younger (p = 0.001), had larger tumors (p = 0.05), and more often had skull base infiltrating tumors (p = 0.01) compared to the 18 patients treated with photons. There was a tendency toward more bone infiltrating tumors in the CIRT group (p = 0.06).
In the CIRT group, there were three incidents of grade 3 ORN and one in the photon group (Table 2). CIRT patient no. 4 had a parotid gland tumor without bone infiltration and later developed extensive ORN in the temporal bone starting from otitis in the external auditory canal. This patient needed surgical revision of the ORN. Eighteen months prior to the onset of ORN, the multityrosine kinase inhibitor lenvatinib was started because of local recurrence and lung metastases. The medication was discontinued when the ORN was verified. Two other patients in the CIRT group and two patients in the photon group also used lenvatinib without the occurrence of ORN.
| Patient no. | RT modality | Age (years) | Sex | Tumor location | TNM | Bone infiltration | ORN grade 3 | Time of diagnosis/surgery ORN (months after RT) | Number of teeth covered by high dose1 | Overall survival (months after RT) |
| 1 | CIRT | 49 | F | Sinonasal | T4aN0M0 | Yes | ÷ | ÷ | 6 | 52 |
| 2 | CIRT | 45 | F | Sinonasal | T4bN0M0 | Yes | ÷ | ÷ | 7 | 68 |
| 32 | CIRT | 49 | M | Nasopharynx | T4aN0M0 | Yes | Mandible | 34/35 | 1 | Alive (85) |
| 4 | CIRT | 41 | M | Parotid | T4aN0M0 | No | Temporal | 46/46 | 0 | 81 |
| 53 | CIRT | 31 | F | Sinonasal | T4bN0M0 | Yes | Mandible | 18/45 | 7 | Alive (81) |
| 6 | CIRT | 36 | F | Sinonasal | T4bN0M0 | Yes | ÷ | ÷ | 0 | Alive (75) |
| 1 | Photons | 81 | M | Sinonasal | T4bN0M0 | No | ÷ | ÷ | 04 | 2 |
| 2 | Photons | 61 | M | Oropharynx | T3N1M0 | No | ÷ | ÷ | 2 | 26 |
| 3 | Photons | 78 | F | Parotid | T4aN0M0 | Yes | ÷ | ÷ | 0 | 2 |
| 4 | Photons | 76 | M | Parotid | T4aN0M0 | Yes | ÷ | ÷ | 04 | 50 |
| 5 | Photons | 56 | F | Oropharynx | T4aN0M0 | No | ÷ | ÷ | 2 | 110 |
| 6 | Photons | 63 | F | Oral cavity | T4aN0M0 | Yes | ÷ | ÷ | 5 | 111 |
| 7 | Photons | 38 | F | Oral cavity | T4aN0M0 | Yes | ÷ | ÷ | 2 | 63 |
| 8 | Photons | 70 | F | Oropharynx | T4aN0M0 | No | ÷ | ÷ | 04 | Alive (136) |
| 9 | Photons | 71 | F | Sinonasal | T4bN0M0 | Yes | ÷ | ÷ | 04 | 17 |
| 10 | Photons | 54 | M | Oral cavity | T2N1M1 | Yes | Maxilla | 72 | 8 | Alive (125) |
| 11 | Photons | 74 | M | Oral cavity | T4bN0M0 | No | ÷ | ÷ | 04 | Alive (121) |
| 12 | Photons | 53 | M | Parotid | T4aN0M0 | No | ÷ | ÷ | 0 | 105 |
| 13 | Photons | 66 | F | Parotid | T4aN0M0 | No | ÷ | ÷ | 0 | Alive (105) |
| 14 | Photons | 68 | F | Lacrimal duct | T4bN0M1 | No | ÷ | ÷ | 0 | 28 |
| 15 | Photons | 67 | F | Oropharynx | T4aN2bM0 | No | ÷ | ÷ | 7 | 41 |
| 16 | Photons | 64 | M | Oropharynx | T4aN0M0 | No | ÷ | ÷ | 18 | 70 |
| 17 | Photons | 76 | M | Parotid | T2N0M0 | No | ÷ | ÷ | 0 | 52 |
| 18 | Photons | 72 | F | Oropharynx | T4aN0M0 | No | ÷ | ÷ | 0 | Alive (113) |
| RT: radiotherapy. Two rows are shaded because of very short overall survival. 1Definition of ‘High dose’: For CIRT, the number of teeth in the planning target volume (PTV). For photons, the number of teeth covered by 60 Gy isodose. 2CIRT patient no. 3 was also diagnosed with grade 2 ORN in the maxilla 36 months after CIRT. 3CIRT patient no. 5 was also diagnosed with grade 2 ORN in the maxilla 77 months after CIRT. 4Jaw(s) covered by a high dose, but edentulous before radiotherapy (RT). |
||||||||||
The other two CIRT patients with ORN experienced affection of the mandible. One of them, CIRT patient no. 5, had a paranasal sinus tumor with growth along the mandibular nerve, involvement of the mandible, and seven teeth in the PTV (Table 2 and Figure 1). This patient was later diagnosed with an additional grade 2 ORN in the maxilla. CIRT patient no. 3 did not have tumor involving the mandible but experienced ORN related to a tooth extraction (molar) 6 months before the onset of ORN. The tooth was extracted because of serious caries despite that the teeth were in healthy condition before CIRT. This patient also developed grade 2 maxillary ORN following another tooth extraction. Patient nos. 3 and 5 with mandibular ORN needed a hemi-mandibulectomy. In the photon group, one patient with tumor infiltration of the hard palate experienced grade 3 ORN of the maxilla and needed surgical revision. The difference in the number of grade 3 ORNs across the groups was statistically significant (p = 0.05). There was no grade 2 ORN in the photon group. All the grade 3 ORNs in both groups were within the PTV. Only one of the patients with ORN (CIRT patient no. 3) had a history of smoking.
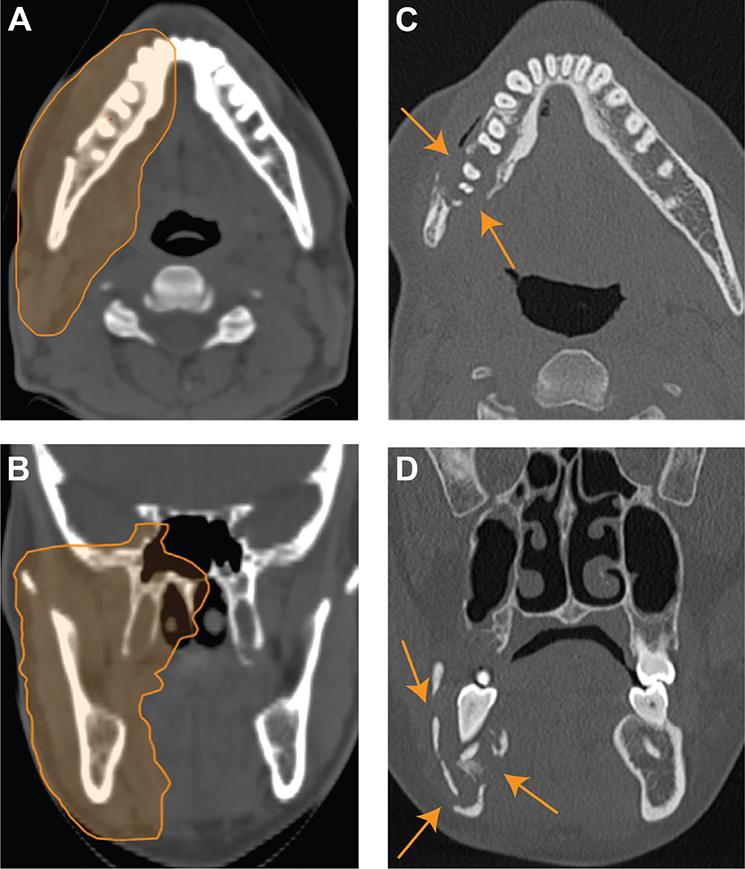
Figure 1A–D. Carbon-ion radiotherapy (CIRT) patient no. 5: Osteoradionecrosis (ORN) development: Axial (A) and coronal (B) views of the treatment plan with the planning target volume (PTV) prescribed 68.8 Gy (relative biological effectiveness [RBE]). Axial (C) and coronal (D) computed tomography (CT) scans show a lucent area with trabecular loss, bicortical erosion, and pathological fracture (arrows). The findings are consistent with grade 3 ORN involving the right mandibular body and angle, which was diagnosed 18 months after CIRT.
The median follow-up time in the CIRT group was 83 months (range 52–85 months) and 121 months (range 2–136 months) in the photon group. The 5-year LC rates in the CIRT group and the photon group were 33% (95% confidence interval: 11–100%) and 39% (19–76%), respectively. The 5-year PFS rates in the CIRT group and the photon group were 17% (9–100%) and 23% (2–59%), respectively. The OS after 5 years was 80% (52–100%) in the CIRT group and 50% (31–82%) in the photon group.
In the CIRT group, there were four cases of local recurrence. All recurrences were within the PTV except for one patient who experienced local recurrence at the border of the PTV. None of the CIRT patients had metastatic lymph nodes in the neck at the time of diagnosis. One patient developed a regional recurrence 51 months after treatment. The same patient experienced a local recurrence 30 months earlier. None of the CIRT patients received elective neck irradiation.
In the photon RT group, two patients aged 78 and 81 years died of unknown causes only 2 months after RT. Of the remaining 16 patients, 12 had local disease recurrence. All recurrences were in the PTV except for one patient with a parotid gland tumor who experienced perineural recurrence in the skull base at the border of the PTV.
Three patients in the photon group had metastatic lymph nodes in the neck at the time of diagnosis. One of them underwent elective neck dissection following RT with viable tumor tissue in the specimen, with no sign of recurrence thereafter. The other two patients achieved regional control of the disease in the high-dose area. One of these two patients had both local disease recurrence (in the high-dose area) and regional disease recurrence at the border of the elective neck volume 69 months after RT. This was the only patient experiencing regional recurrence of the disease in the photon group. Eleven patients received elective neck irradiation.
Discussion
This study assessed outcomes for Norwegian patients with HN-ACC treated with CIRT at CNAO and photon RT at OUS. In the CIRT group, three of six patients (50%) experienced grade 3 ORN, a higher rate than in prior studies. For example, Koto et al. reported a 4% grade 3 ORN rate among 458 patients with sinonasal malignancies treated with CIRT, likely due to lower mandibular doses in their cohort [20]. Mandibular ORN risk is typically higher than maxillary ORN [21], but the risk is also influenced by tumor site, treatment volume, and high-dose exposure. This seems to be confirmed by Ikawa et al. who found a 12% ORN rate in nonsquamous cell oral cavity cancers [22], while Naganawa et al. reported a 21% rate in oral mucosal melanoma cases [15]. By contrast, photon RT studies report lower ORN rates of 2–4% [12, 13]. Notably, previous CIRT studies excluded cases where ORN was likely linked to tumor bone infiltration since ORN might be an expected result of tumor response [16, 23, 24], whereas such patients were included here.
Dental factors contributed to ORN in two CIRT cases, emphasizing the need for individualized dental extraction protocols. One patient’s ORN may also have been exacerbated by lenvatinib, a VEGF inhibitor linked to impaired bone healing [25–27]. VEGF is crucial for bone repair, and the jaw’s high bone turnover makes it particularly vulnerable to disruptions in osteoblast and osteoclast function. Future studies should clarify the effects of combining CIRT with VEGF inhibitors and optimize treatment timing for systemic disease progression.
CIRT is normally administered at a higher dose per fraction than conventional photon or proton RT, with doses equivalent to 100 Gy (EQD2Gy) [28] exceeding standard photon RT dose constraints for bone [29, 30]. This high dose, combined with advanced disease features, likely contributed to the observed ORN rates.
The 5-year LC rate in the CIRT group was 33%, which is lower than the previously reported rates of 68% in Japanese centers [7], 59% in Heidelberg using a carbon-ion boost [8], and 52% reported by CNAO [31]. Factors such as skull base involvement, bone infiltration, and large tumors likely reduced LC in the present cohort [32–34].
CIRT has emerged as a potential treatment for inoperable HN-ACC, offering a chance of local tumor control for patients with advanced disease. However, the small sample size and retrospective design of this study limit its generalizability. Toxicity grading relied on available data, and differences in patient and tumor characteristics between the CIRT and photon cohorts complicated direct comparisons. Patients referred for CIRT typically presented with more unfavorable disease features, such as larger tumors and skull base involvement, introducing selection bias.
In conclusion, half of the CIRT cohort experienced ORN requiring surgery during follow-up at their home institution. Younger patients with advanced disease and poorer prognoses are often referred to particle therapy centers for CIRT. Close follow-up in collaboration with these centers is crucial to manage treatment-related toxicity. Careful patient selection and thorough risk communication are essential. Despite the limitations of this small, retrospective study, these findings may be of value to other centers when referring patients with inoperable HN-ACC for CIRT abroad.
Statement of ethics
The study was approved by The Regional Ethics Committee (REK) in Norway (approval number 198926) and the Institutional Review Board at OUS. Informed consent was obtained from all living patients. An exemption from obtaining informed consent from the relatives of deceased patients was granted by REK.
Author contributions
This study was conceived and designed by ED, ES, SD, CDA, ÅB, TTMA, and HAE. Data acquisition was performed by ES, MDS, and ED, while data analysis was conducted by ES and ED. All authors contributed to the interpretation of the data. The manuscript was drafted by ES and ED, with all authors reviewing and approving the final version. Furthermore, all authors accept responsibility for the integrity of the work and agree to address any questions regarding its accuracy or validity.
Data availability statement
Data will be made available upon reasonable request.
References
[1] Even C, Baste N, Classe M. New approaches in salivary gland carcinoma. Curr Opin Oncol. 2019;31(3):169–74. https://doi.org/10.1097/CCO.0000000000000527
[2] Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Hinerman RW, Villaret DB. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck. 2004;26(2):154–62. https://doi.org/10.1002/hed.10380
[3] Iseli TA, Karnell LH, Graham SM, Funk GF, Buatti JM, Gupta AK, et al. Role of radiotherapy in adenoid cystic carcinoma of the head and neck. J Laryngol Otol. 2009;123(10):1137–44. https://doi.org/10.1017/S0022215109990338
[4] van Herpen C, Vander Poorten V, Skalova A, Terhaard C, Maroldi R, van Engen A, et al. Salivary gland cancer: ESMO-European Reference Network on Rare Adult Solid Cancers (EURACAN) Clinical Practice Guideline for diagnosis, treatment and follow-up (vol 7, 100602, 2022). ESMO Open. 2023;8(5):1–16. https://doi.org/10.1016/j.esmoop.2022.100602
[5] Locati LD, Ferrarotto R, Licitra L, Benazzo M, Preda L, Farina D, et al. Current management and future challenges in salivary glands cancer. Front Oncol. 2023;13:1264287. https://doi.org/10.3389/fonc.2023.1264287
[6] Loap P, Vischioni B, Bonora M, Ingargiola R, Ronchi S, Vitolo V, et al. Biological rationale and clinical evidence of carbon ion radiation therapy for adenoid cystic carcinoma: a narrative review. Front Oncol. 2021;11:789079. https://doi.org/10.3389/fonc.2021.789079
[7] Sulaiman NS, Demizu Y, Koto M, Saitoh J-I, Suefuji H, Tsuji H, et al. Multicenter study of carbon-ion radiation therapy for adenoid cystic carcinoma of the head and neck: subanalysis of the Japan Carbon-Ion Radiation Oncology Study Group (J-CROS) Study (1402 HN). Int J Radiat Oncol Biol Phys. 2018;100(3):639–46. https://doi.org/10.1016/j.ijrobp.2017.11.010
[8] Jensen AD, Poulakis M, Nikoghosyan AV, Welzel T, Uhl M, Federspil PA, et al. High-LET radiotherapy for adenoid cystic carcinoma of the head and neck: 15 years’ experience with raster-scanned carbon ion therapy. Radiother Oncol. 2016;118(2):272–80. https://doi.org/10.1016/j.radonc.2015.05.010
[9] Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41(5):283–8. https://doi.org/10.1016/0278-2391(83)90294-X
[10] Chieng CY, Davies A, Aziz A, Lowe D, Rogers S. Health related quality of life and patient concerns in patients with osteoradionecrosis. Br J Oral Maxillofac Surg. 2021;59(9):1061–6. https://doi.org/10.1016/j.bjoms.2021.02.011
[11] Rogers SN, D’Souza JJ, Lowe D, Kanatas A. Longitudinal evaluation of health-related quality of life after osteoradionecrosis of the mandible. Br J Oral Maxillofac Surg. 2015;53(9):854–7. https://doi.org/10.1016/j.bjoms.2015.07.008
[12] Studer G, Bredell M, Studer S, Huber G, Glanzmann C. Risk profile for osteoradionecrosis of the mandible in the IMRT era. Strahlenther Onkol. 2016;192(1):32. https://doi.org/10.1007/s00066-015-0875-6
[13] Chen JA, Wang CC, Wong YK, Wang CP, Jiang RS, Lin JC, et al. Osteoradionecrosis of mandible bone in patients with oral cancer – associated factors and treatment outcomes. Head Neck. 2016;38(5):762–8. https://doi.org/10.1002/hed.23949
[14] Koto M, Hasegawa A, Takagi R, Ikawa H, Naganawa K, Mizoe JE, et al. Evaluation of the safety and efficacy of carbon ion radiotherapy for locally advanced adenoid cystic carcinoma of the tongue base. Head Neck. 2016;38 Suppl 1:E2122–6. https://doi.org/10.1002/hed.24397
[15] Naganawa K, Koto M, Takagi R, Hasegawa A, Ikawa H, Shimozato K, et al. Long-term outcomes after carbon-ion radiotherapy for oral mucosal malignant melanoma. J Radiat Res. 2017;58(4):517–22. https://doi.org/10.1093/jrr/rrw117
[16] Vischioni B, Russo S, Meuli M, Bonora M, Ronchi S, Ingargiola R, et al. Dosimetric and clinical risk factors for the development of maxillary osteoradionecrosis in adenoid cystic carcinoma (ACC) patients treated with carbon ion radiotherapy. Front Oncol. 2022;12:829502. https://doi.org/10.3389/fonc.2022.829502
[17] Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. https://doi.org/10.1016/0021-9681(87)90171-8
[18] National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. National Cancer Institute; 2017. [cited Dec 1, 2020] Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
[19] Posit Team. RStudio: Integrated Development Environment for R. Boston, MA: Posit Software, PBC; 2023. Available from: http://www.posit.co/.
[20] Koto M, Demizu Y, Saitoh J-I, Suefuji H, Tsuji H, Okimoto T, et al. Definitive carbon-ion radiation therapy for locally advanced sinonasal malignant tumors: subgroup analysis of a multicenter study by the Japan Carbon-Ion Radiation Oncology Study Group (J-CROS). Int J Radiat Oncol Biol Phys. 2018;102(2):353–61. https://doi.org/10.1016/j.ijrobp.2018.05.074
[21] Singh A, Huryn JM, Kronstadt KL, Yom SK, Randazzo JR, Estilo CL. Osteoradionecrosis of the jaw: a mini review. Front Oral Health. 2022;3:980786. https://doi.org/10.3389/froh.2022.980786
[22] Ikawa H, Koto M, Demizu Y, Saitoh Ji, Suefuji H, Okimoto T, et al. Multicenter study of carbon‐ion radiation therapy for nonsquamous cell carcinomas of the oral cavity. Cancer Med. 2019;8(12):5482–91. https://doi.org/10.1002/cam4.2408
[23] Sasahara G, Koto M, Ikawa H, Hasegawa A, Takagi R, Okamoto Y, et al. Effects of the dose-volume relationship on and risk factors for maxillary osteoradionecrosis after carbon ion radiotherapy. Radiat Oncol. 2014;9:1–6. https://doi.org/10.1186/1748-717X-9-92
[24] Musha A, Kubo N, Kawamura H, Okano N, Sato H, Okada K, et al. Carbon-ion radiotherapy for inoperable head and neck bone and soft-tissue sarcoma: prospective observational study. Anticancer Res. 2022;42(3):1439–46. https://doi.org/10.21873/anticanres.15614
[25] Lu W, Guo Q, Ma Z, Liu L, Zhao Z. Lenvatinib and osteonecrosis of the jaw: a pharmacovigilance study. Eur J Cancer. 2021;150:211–3. https://doi.org/10.1016/j.ejca.2021.03.046
[26] Mauceri R, Panzarella V, Morreale I, Campisi G. Medication-related osteonecrosis of the jaw in a cancer patient receiving lenvatinib. Int J Oral Maxillofac Surg. 2019;48(12):1530–2. https://doi.org/10.1016/j.ijom.2019.07.010
[27] Monteiro L, Vasconcelos C, Pacheco J-J, Salazar F. Photobiomodulation laser therapy in a lenvatinib-related osteonecrosis of the jaw: a case report. J Clin Exp Dentist. 2021;13(6):e626. https://doi.org/10.4317/jced.58323
[28] Kamada T, Tsujii H, Blakely EA, Debus J, De Neve W, Durante M, et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol. 2015;16(2):e93–100. https://doi.org/10.1016/S1470-2045(14)70412-7
[29] Mohamed AS, Hobbs BP, Hutcheson KA, Murri MS, Garg N, Song J, et al. Dose-volume correlates of mandibular osteoradionecrosis in Oropharynx cancer patients receiving intensity-modulated radiotherapy: results from a case-matched comparison. Radiother Oncol. 2017;124(2):232–9. https://doi.org/10.1016/j.radonc.2017.06.026
[30] Kubota H, Miyawaki D, Mukumoto N, Ishihara T, Matsumura M, Hasegawa T, et al. Risk factors for osteoradionecrosis of the jaw in patients with head and neck squamous cell carcinoma. Radiat Oncol. 2021;16(1):1–11. https://doi.org/10.1186/s13014-020-01701-5
[31] Vischioni B, Bonora M, Ronchi S, Ingargiola R, Camarda AM, Motinetli S, et al. OC-0110 Head and neck adenoid cystic carcinoma treated with raster scanning carbon ion radiotherapy at CNAO. Radiother Oncol. 2023;182:S70–1. https://doi.org/10.1016/S0167-8140(23)08524-9
[32] Jones A, Hamilton J, Rowley H, Husband D, Helliwell T. Adenoid cystic carcinoma of the head and neck. Clin Otolaryngol Allied Sci. 1997;22(5):434–43. https://doi.org/10.1046/j.1365-2273.1997.00041.x
[33] Beckhardt RN, Weber RS, Zane R, Wolf P, Garden AS, Carrillo R, et al. Minor salivary gland tumors of the palate: clinical and pathologic correlates of outcome. Laryngoscope. 1995;105(11):1155–60. https://doi.org/10.1288/00005537-199511000-00003
[34] Lopes MA, Kowalski LP, Cunha Santos G, De Almeida OP. A clinicopathologic study of 196 intraoral minor salivary gland tumours. J Oral Pathol Med. 1999;28(6):264–7. https://doi.org/10.1111/j.1600-0714.1999.tb02036.x