ORIGINAL REPORT
Sexual distress among men with cancer – a cross-sectional study
Jonas Nahavandipoura  , Christoffer Johansena
, Christoffer Johansena  , Annamaria Giraldib,c
, Annamaria Giraldib,c  , Bolette Skjødt Rafna
, Bolette Skjødt Rafna  and Annika von Heymanna
and Annika von Heymanna 
aDanish Cancer Society National Cancer Survivorship and Late Effects Research Center (CASTLE), Department of Oncology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark; bSexological Clinic, Mental Health Center Copenhagen, Mental Health Services Capital Region, Copenhagen, Denmark; cDepartment of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
ABSTRACT
Background and purpose: Many men with cancer experience that changes created by cancer and its treatment may impair sexual function. However, many studies investigating sexual impairments fail to consider whether such impairments are perceived as distressing, i.e. create sexual distress. We investigated the prevalence of sexual distress, overlap with sexual impairment, and sociodemographic and clinical characteristics and other symptoms associated with sexual distress in a heterogeneous male cancer population.
Patients and methods: Across cancer diagnoses, 2792 men in treatment or follow up at the Department of Oncology, Rigshospitalet, were invited. The Sexual Complaint Screener (SCS) assessed sexual impairments and sexual distress. Regression analyses estimated the association of sexual distress with sociodemographic and tumor-related factors, other symptoms (pain, depression, fatigue, insomnia, fear of recurrence), and health-related quality of life. The number of patients who received help for or were interested in a consultation for sexual problems was calculated.
Results: Six hundred and ninety-six patients, most frequently diagnosed with testicular (26%) or multiple (16%) cancers, completed the SCS. Forty-one per cent experienced sexual distress, 60% sexual impairment, and 34% overlapping sexual distress and impairment. Sexual distress was significantly associated with clinically relevant insomnia (OR:2.15; 95% CI:1.5–3.1) and pain (OR:1.90; 95% CI:1.3–2.9). Two thirds of all patients wished for help, but only one third of these were receiving help.
Interpretation: Sexual distress was widespread in men across different cancer diagnoses and sometimes presented without impairment, demonstrating that assessment of sexual problems must include the personal experience of distress and extend to men across cancer diagnoses.
KEYWORDS: Sexual distress; sexual function; neoplasms; health related quality of life; late effects
Citation: ACTA ONCOLOGICA 2025, VOL. 64, 214–221. https://doi.org/10.2340/1651-226X.2025.42525.
Copyright: © 2025 The Author(s). Published by MJS Publishing on behalf of Acta Oncologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
Received: 19 November 2024; Accepted: 24 January 2025; Published: 9 February 2025
CONTACT: Annika von Heymann Annika.von.heymann@regionh.dk Department of Oncology, Center for Cancer and Organ Disease, Rigshospitalet, Blegdamsvej 9, 2100 København Ø, Denmark
Supplemental data for this article can be accessed online at https://doi.org/10.2340/1651-226X.2025.42525
Competing interests and funding: The authors report there are no competing interests to declare. ECRS 2024 was financially supported by the Acta Oncologica Foundation.
Introduction
Cancer and its treatment can create many complex side and late effects which can lead to impaired sexual function across different areas, such as reduced desire, difficulty achieving erection, ejaculation/orgasm problems or pain related to intercourse [1, 2]. Sexual impairment significantly impacts health-related quality of life (HRQoL) [3–5], and may be more than five times as frequent among male cancer survivors than among healthy individuals [6]. When sexual impairments are perceived by the individual to be distressing, this is termed sexual distress (or bother) [7], and impairment can be present without sexual distress (Figure 1) as demonstrated in populations with prostate cancer (e.g. Bravi et al. [8]). The presence of sexual impairment alone may thus not be a clear indicator of need for support, and some studies have shown that sexual distress may be more strongly associated with HRQoL than impairment [3, 4]. Even though sexual impairment has been documented for men with many different cancer diagnoses [9–11], sexual distress has been very sparingly assessed in men with cancer diagnoses other than prostate cancer [5, 12–15].
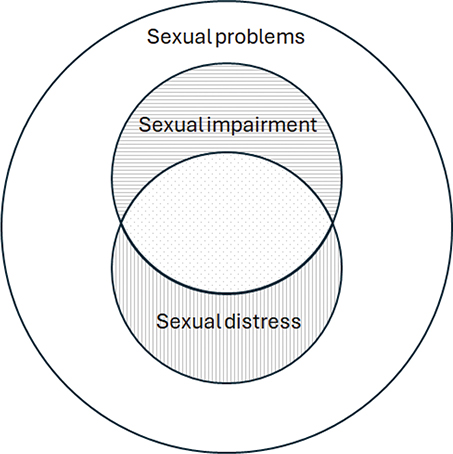
Figure 1. Illustration of sexual problems and the overlap between sexual impairment and sexual distress. Dotted area corresponds to reports of sexual impairments, combined with sexual distress.
We therefore investigated the prevalence of sexual distress and the degree to which it was reported with or without impairment among men with all types of cancer seen in a large oncology department. To characterize patients reporting sexual distress, we investigated the association of demographic and clinical characteristics as well as other symptoms that are potential side or late effects of cancer (depression, fatigue, fear of recurrence, insomnia, and pain) with reporting sexual distress, and to clarify a potential effect of sexual distress on HRQoL, we investigated their association. Finally, we calculated the number of patients who reported that they received help for sexual problems and the number who were interested in a consultation for sexual problems, and their overlap with numbers reporting sexual distress.
Methods
This study utilizes data from a large cross-sectional questionnaire study conducted from February until April 2021 at the Department of Oncology, Copenhagen University Hospital Rigshospitalet, Denmark, approved by the local research legal department (reference 23039798). All patients across diagnoses in active cancer treatment or in a follow-up program, at any time since diagnosis, were invited to complete an online questionnaire through the national secure digital mail ‘e-Boks’. Following informed consent, data was collected and processed according to applicable data protection regulations. In the present analyses, we included only men aged ≥18 and <100 years with access to digital secure mailbox, and complete response to questions on sexual problems.
Participants self-reported sociodemographic and lifestyle characteristics (age, sex, education, cohabitation, employment, alcohol consumption, and tobacco smoking) and clinical characteristics (cancer diagnosis, time since first diagnosis, and treatment received [surgery, radiation, chemo-, immune-, and/or hormone therapy]). Self-reported cancer diagnoses were cross-checked with medical records. Patients with diagnoses reported by fewer than 20 respondents were grouped, as were those reporting multiple cancers.
Instruments
The male version of the Sexual Complaint Screener (SCS-M) is a 10-item patient-reported questionnaire developed by the International Society for Sexual Medicine to screen all major areas of sexual function and distress within the past 6 months [16]. It consists of seven items evaluating sexual impairment, one item evaluating satisfaction with sexual life and one item assessing interest in consultation for sexual problems. Items included here evaluated desire, ability to maintain an erection, need for increased stimulation, experiencing early ejaculation, the lack of orgasm or ejaculation, and pain during or after intercourse. The frequency of experiencing each sexual impairment is reported on a five-point Likert scale (‘Never/almost never’; ‘Rarely’; ‘Sometimes’; ‘Often’; ‘Almost all the time/Always’), unless ‘No sexual activity’ is selected. Each question about sexual impairment is accompanied by a question assessing the severity of distress experienced due to this impairment with responses on a five-point Likert scale (‘Not at all a problem’; ‘A very small problem’; ‘Some problem’; ‘A considerable problem’; ‘A very great problem’). The SCS-M does not have a predefined cut-off to indicate clinical relevance. Therefore, to determine the presence of impairment, regardless of its magnitude, we defined sexual impairment to be present, when it was reported with responses of ‘Sometimes’ or more frequently, and sexual distress as present at responses of ‘Some problem’ or more. Further, we included the item assessing interest in a consultation about sexual problems (Response options: ‘No’, ‘Not now’, and ‘Yes’). The SCS-M was developed in Danish and Swedish and translated to English. It has been preliminarily validated [17], used in studies of male populations with e.g. kidney diseases [18], but not yet validated in Danish. Finally, we used a self-constructed item asking if participants were currently receiving help for a list of issues (yes/no for e.g. depression or fatigue), among these sexual problems.
The remaining questionnaires assessed HRQoL and other symptoms that are potential side or late effects of cancer and its treatment. All questionnaires have previously been used or validated among Danish cancer patients (see Supplement for references).
HRQoL within the last week was assessed using the overall, physical, and emotional subscales of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30). Each sub-score is reported as the transformed score between 0 and 100 with higher scores reflecting better function [19]. To determine clinical relevance, differences of 5–10 were considered small, 10–20 moderate, and >20 large, as suggested by Osoba et al. [20].
Symptoms of depression within the last 2 weeks were measured using the 12-item Major Depression Inventory (MDI) returning scores between 0 and 50. A score of ≥21, indicative of minor depression or worse, was used as a cut-off value for clinically relevant depression [21].
Symptoms of fatigue experienced ‘lately’ were measured with the 20-item Multidimensional Fatigue Inventory-20 (MFI-20) [22]. The general fatigue score, ranging from 4 to 20 with higher scores indicating greater fatigue, was dichotomized at the 75th percentile as a cut-off to indicate clinically relevant fatigue [23].
Fear of recurrence within the last month was measured with the nine item Fear of Cancer Recurrence Inventory (FCRI)-short form. It returns a total score between 0 and 36, with higher scores reflecting worse functioning [24], and a cut-off of ≥22 was used to determine clinically relevant symptoms [25].
The seven item Insomnia Severity Index (ISI) questionnaire assessed symptoms of insomnia with the last month. Possible scores were between 0 and 28, with higher scores indicating greater insomnia [26]. A score of ≥ 8 was used as cut-off for clinically relevant symptoms [27].
Pain during the last 24 hours was assessed using the pain interference (PITS) subscale of the Brief Pain Inventory (BPI) [28]. The PITS score is calculated as a mean of seven items scored on a scale from 0 to 10 measuring interferences with daily activities caused by pain. A cut-off of ≥ 2, indicative of moderate to severe pain, was used [29].
Analysis
Differences in demographic and clinical characteristics between those who completed the SCS-M and those who did not were investigated using logistic regression. Age was divided into quartiles, because of non-linear relationship to the log odds of responding.
To investigate the overall prevalence of sexual distress and its co-occurrence with impairment, the number of participants was calculated who reported sexual distress on one or more SCS-M items (regardless of reporting impairment or not), sexual impairment on one or more items (regardless of reporting distress or not), and distress combined with impairment on one or more items. For each SCS-M item, we calculated the number of participants reporting all possible combinations of distress (yes/no) and impairment (yes/no). Post-hoc analyses were performed for patients in and not in active treatment. Further, patients were grouped by the number of areas in which they experienced distress, and the percentage of participants with interest in consultation for sexual problems was calculated for each group.
To investigate the characteristics associated with experiencing sexual distress, a multivariable logistic regression was performed with distress in any area as the dependent variable, while independent variables were other symptoms, sociodemographic, and clinical characteristics, all mutually adjusted. In post-hoc analyses, we ran this logistic regression separately for subgroups of participants currently receiving and not receiving treatment. To investigate the correlation between HRQoL and sexual distress in any area, linear regressions were performed. For each domain of HRQoL (overall, physical, and emotional), three models were run: (1) crude; (2) adjusted for sociodemographic and clinical characteristics by adding age, cohabitation, employment, cancer site and time since diagnosis; (3) adjusted for sociodemographic, clinical characteristics, and other symptoms, by further adding dichotomized scores for clinically relevant pain, fatigue, depression, fear of recurrence, and insomnia. Because of indication of non-linear relationship, we performed sensitivity analysis with generalized additive models (GAM), with clinically irrelevant differences between estimates in linear models and GAMs (data not shown), substantiating the robustness of the linear analyses. Regression analyses were performed on participants with complete responses to the SCS-M, the EORTC QLQ-C30, MFI-20, FCRI, ISI, and BPI (Figure 2). Outliers and respondents with improbable scores were removed. Analyses were conducted using R version 4.1.0. [30], with the geepack package for linear and logistic analysis, mgcv for GAM-models, and ggplot2 for graphs.
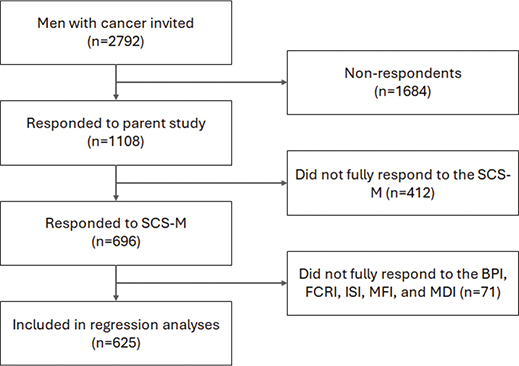
Figure 2. Flowchart for inclusion and exclusion of respondents. BPI: Brief Pain Inventory; FCRI: Fear of Cancer Recurrence Inventory; ISI: Insomnia Severity Index; MDI: Major Depression Inventory; MFI: Multidimensional Fatigue Inventory; SCS-M: Sexual Complaints Screener-Male.
Results
Of 2792 male patients invited to the parent study, 1108 responded (40%) and, of these, 696 (63%) completed the optional SCS-M (Figure 2). Of respondents to the parent study, older patients and patients with esophageal or stomach cancers, brain cancers, or multiple cancers were less likely to respond to the SCS-M, while patients receiving help for sexual problems were more likely to respond (Supplement, Table S1). SCS-M respondents’ mean age was 60 years and 76% were cohabitating (n = 530, Table 1). The most prevalent cancer type was testicular cancer (n = 183, 26%), followed by patients with multiple cancers (n = 108, 16%), or other cancers (n = 75, 11%), with a mean time since first diagnosis of 0.9 years (Table 1). The majority (78%) of patients were not currently receiving antineoplastic treatment.
| Age (years) | |
| Mean (SD) | 60 (15) |
| Min – Max | 19–89 |
| Living alone (n, %) | |
| Yes | 166 (24%) |
| Level of education (n, %) | |
| Primary school | 65 (9%) |
| High school | 33 (5%) |
| Continued education (1–2 years) | 84 (12%) |
| Continued education (3–4 years) | 270 (39%) |
| Higher education (> 4 years) | 204 (29%) |
| Others | 40 (6%) |
| Employment (n, %) | |
| Full-time employment | 243 (35%) |
| Part-time employment | 33 (5%) |
| Self-employed | 53 (8%) |
| Unemployed | 25 (4%) |
| Retired | 258 (37%) |
| On sick leave | 50 (7%) |
| Others* | 32 (5%) |
| Missing | 2 (0.3%) |
| Primary cancer site (n, %) | |
| Testicular | 183 (26%) |
| Head and neck | 69 (10%) |
| Esophageal/stomach | 57 (8%) |
| Lung | 51 (7%) |
| Prostate | 51 (7%) |
| Brain | 41 (6%) |
| Colorectal | 41 (6%) |
| Bladder | 20 (3%) |
| Multiple | 108 (16%) |
| Others | 75 (11%) |
| Cancer treatment (n, %) | |
| Surgery | |
| Yes | 486 (70%) |
| Chemotherapy | |
| Received | 467 (67%) |
| Not received | 223 (32%) |
| Do not know | 5 (0.7%) |
| Missing | 1 (0.1%) |
| Radiotherapy | |
| Received | 307 (44%) |
| Not received | 377 (54%) |
| Do not know | 7 (1%) |
| Missing | 5 (0.7%) |
| Hormone therapy | |
| Received | 72 (10%) |
| Not received | 587 (84%) |
| Do not know | 29 (4%) |
| Missing | 8 (1%) |
| Immunotherapy | |
| Received | 592 (85%) |
| Not received | 42 (6%) |
| Do not know | 57 (8%) |
| Missing | 5 (0.7%) |
| Currently receiving treatment** | |
| Yes | 151 (22%) |
| Time since first cancer diagnosis (years) | |
| Mean (SD) | 0.9 (0.3) |
| Missing (n, %) | 47 (7%) |
| Reported receiving help for sexual dysfunction (n, %) | |
| Yes | 65 (9%) |
| No | 643 (90%) |
| Missing | 2 (0.3%) |
| Presence of other clinically significant potential side or late effects (n, %) | |
| Depression | 91 (13%) |
| Missing | 1 (< 1%) |
| Fatigue | 142 (20.4%) |
| Missing | 9 (1.3%) |
| Pain | 143 (21%) |
| Missing | 5 (< 1%) |
| Insomnia | 336 (48%) |
| Missing | 0 (0%) |
| Fear of recurrence | 162 (23%) |
| Missing | 15 (2.2%) |
| *Other occupations: Students and staying-at-home. **Chemotherapy, immunotherapy and radiotherapy. |
|
Overall, 288 (41%) of the 696 respondents reported sexual distress on one or more items (the vertically lined and dotted areas of Figure 1), 420 (60%) reported sexual impairment on at least one item (horizontally lined and dotted areas), and 234 (34%) reported both sexual distress and sexual impairment on one or more items (dotted area alone). When inspecting each item individually, a sexual impairment that was not experienced as distressing was reported by between 2% (pain during or after intercourse) and 19% (lack of desire) (Figure 3). Between 6% (pain during or after intercourse) and 11% (lack of desire) reported sexual distress without reporting a sexual impairment, and of these (Figure 3), the majority responded that they were not sexually active (60–82% per item). The combination of sexual impairment and distress was reported by between 3% (pain during or after intercourse) and 23% (reduced erection firmness) per item (Figure 3, Supplement Table S, Figure S1). A similar pattern was observed in post-hoc analyses among participants in treatment and not in treatment (See Supplement Figure S2, Figure S3, and Table S3)
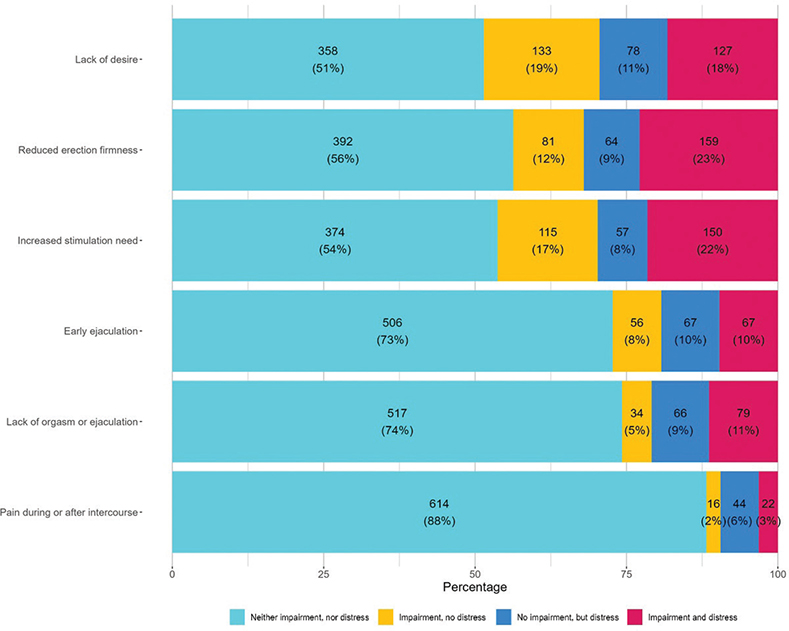
Figure 3. Distribution of sexual distress and impairment among 696 men with cancer at the Department of Oncology, Rigshospitalet.
When investigating factors associated with reporting sexual distress on at least one item, we found that only clinically relevant insomnia (OR = 2.2; 95% CI:1.48–3.11) and pain (OR = 1.9; 95% CI:1.25–2.91) were significantly associated with experiencing sexual distress (Table 2). This remained unchanged in both post-hoc analyses, while only higher education (1–2 years) among patients in treatment emerged as a new associated factor (see Supplements Table S4 and S5). In fully adjusted analyses, sexual distress was significantly associated with lower overall and emotional HRQoL, but not physical HRQoL. Respondents experiencing distress reported –3.2 (95% CI: –5.6 to –0.8) lower overall HRQoL scores and –3.8 (95% CI: –6.3 to –1.3) lower emotional HRQoL score (Table 3).
| Patient characteristics and symptoms | OR* (95% CI**) | P |
| Age (years) | 1.0 (0.98–1.01) | 0.39 |
| Living alone | ||
| No | Ref | |
| Yes | 0.9 (0.60–1.37) | 0.64 |
| Level of education | ||
| Higher education (> 4 years) | Ref | |
| Higher education (3–4 years) | 1.0 (0.65–1.47) | 0.90 |
| Higher education (1–2 years) | 0.7 (0.39–1.38) | 0.34 |
| High school | 0.7 (0.30–1.78) | 0.49 |
| Primary school | 1.1 (0.56–2.02) | 0.84 |
| Others | 1.5 (0.67–3.38) | 0.33 |
| Primary cancer site | ||
| Testicular | 0.7 (0.29–1.59) | 0.38 |
| Head and neck | 0.5 (0.20–1.18) | 0.11 |
| Esophageal/stomach | 0.9 (0.38–2.18) | 0.84 |
| Lung | 0.5 (0.19–1.13) | 0.15 |
| Prostate | Ref | |
| Brain | 0.8 (0.31–2.02) | 0.63 |
| Colorectal | 0.7 (0.29–1.90) | 0.54 |
| Bladder | 1.3 (0.48–3.74) | 0.58 |
| Multiple | 1.0 (0.46–2.24) | 0.97 |
| Others | 0.5 (0.23–1.25) | 0.15 |
| Time since first cancer diagnosis (years) | 1.0 (0.99–1.07) | 0.21 |
| Pain | ||
| Present | 1.9 (1.25–2.91) | < 0.01 |
| Fatigue | ||
| Present | 0.7 (0.42–1.10) | 0.11 |
| Depression | ||
| Present | 1.5 (0.87–2.71) | 0.18 |
| Fear of recurrence | ||
| Present | 1.3 (0.84–1.98) | 0.25 |
| Insomnia | ||
| Present | 2.2 (1.48–3.11) | < 0.01 |
| *Mutually adjusted, **Confidence intervals. | ||
| Health related quality of life (HRQoL) domain | Coefficients | P |
| Overall (HRQoL) | Beta (95% CI) | P-value |
| 1 Crude Analysis | –8.5 (–11.7 to –5.3) | < 0.01 |
| 2 Adjusted for sociodemographic* and clinical characteristics** | –7.7 (–10.7 to –4.6) | < 0.01 |
| 3 Adjusted for sociodemographic, clinical characteristics and other symptoms*** | –3.2 (–5.6 to –0.8) | 0.01 |
| Emotional (HRQoL) | ||
| 1 Crude Analysis | –8.7 (–12.0 to –5.5) | < 0.01 |
| 2 Adjusted for sociodemographic and clinical characteristics | –8.1 (–11.2 to –5.0) | < 0.01 |
| 3 Adjusted for sociodemographic, clinical characteristics and other symptoms | –3.8 (–6.3 to –1.3) | < 0.01 |
| Physical (HRQoL) | ||
| 1 Crude Analysis | –1.8 (–4.6 to 1.0) | 0.20 |
| 2 Adjusted for sociodemographic and clinical characteristics | –1.3 (–3.8 to 1.2) | 0.32 |
| 3 Adjusted for sociodemographic, clinical characteristics and other symptoms | 1.7 (–0.6 to 3.9) | 0.16 |
| *Sociodemographic characteristics: cohabitation, level of education and age. **Clinical characteristics: cancer site and time since diagnosis. ***Symptoms: Pain, fatigue, depression, fear of recurrence and insomnia. EORTC-QLQ-C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30. |
||
Nine percent (n = 65) of the SCS-M-respondents reported that they received help for sexual problems, 28% (n = 192) were interested in receiving a consultation about sexual problems at the time of response, and 34% (n = 236) at a later point. Interest in consultation for sexual problems was more frequently endorsed by patients experiencing distress in more areas (Supplement, Figure S4).
Discussion
To the best of our knowledge, this is the first study to investigate the prevalence of sexual distress, associated sociodemographic and clinical factors, and the potential impact of sexual distress on HRQoL in a heterogeneous population of men with cancer. In our sample of men with diverse cancers, 60% reported a sexual impairment, 41% experienced sexual distress, and 34% reported the combination of both impairment and distress in at least one area. Sexual distress was significantly associated with clinical levels of pain and insomnia. More than two thirds of participants were interested in a consultation for sexual problems.
We found that sexual distress was prevalent, reported by 41% in at least one area. Sexual distress is most widely documented among men with prostate cancer, and among the largest studies is an Australian study that reported the prevalence of sexual distress between 29 and 43% 12 months after treatment, greatest after radical prostatectomy [31]. Meanwhile, a systematic review of 81 studies found prevalence rates up to 88% [1]. Among other male cancer populations, estimates of sexual distress are scarce. A Japanese study reported the prevalence among patients who had undergone bladder cancer surgery (74% men) to be between 25 and 74% for different sexual areas, highest for ability to have intercourse [12]. A Danish study, conceptualizing sexual distress broadly as negative feelings about sexual problems, e.g. including guilt or feelings of inferiority, found sexual distress in 42% of men with hematological cancers [13]. The prevalence found in the present heterogeneous population falls within the range previously reported for individual cancers, underscoring that sexual distress may be widespread in men across diagnoses. Indeed, cancer type was not found to be associated with sexual distress. Some studies have calculated changes in sexual distress over time, rather than prevalences, most reporting increased distress after treatment [32] and improvement over time [33] in men with prostate cancer, while some report distress scores to be stable [4, 5], including one study in colorectal cancer [5]. Our findings agree with part of this evidence, as time since diagnosis was not found to be associated with distress, although this finding should be interpreted with caution due to the cross-sectional design.
We found that men who reported clinically relevant pain and insomnia were more likely to report sexual distress, which was in turn significantly related to lower HRQoL, although with estimates below clinical relevance when adjusted for other symptoms. Prospective studies in men with prostate cancer have identified e.g. pre-treatment sexual distress, neuroticism, and sexual activity [34], as well as therapy modality [31] to be associated with sexual distress at follow-up 1 year after treatment, and pre-treatment sexual distress has been associated with post-radiotherapy HRQoL [4]. To the best of our knowledge, only two longitudinal studies in male populations other than prostate cancer have been published, both examining patients with colorectal cancer. In one, pre-treatment depression and lower confidence were identified as predictors of a combined measure of sexual distress and low sexual satisfaction 2 years later, and having a stoma predicted distress/satisfaction at 5-year follow-up [2]. The other study found that sexual distress at baseline (on average 2.5 years post-diagnosis) was associated with lower HRQoL, depression, and poorer relationship quality at 6-months follow-up [5]. Our results indicate that a potential effect on HRQoL might also be due to co-occurring symptoms, as differences in HRQoL between men with and without sexual distress were no longer clinically relevant, when co-occurring symptoms were included in the model. Contrary to many other studies, we did not find sexual distress to be associated with depression, but rather with clinically relevant pain and insomnia, which were only assessed in one (i.e. pain) or none (i.e. insomnia) of the previous studies. Our findings may be driven by the high level of clinical insomnia identified in our sample (Table 1). Further, age was not associated with sexual distress in our study, potentially due to mutually adjusted analyses. In contrast, Nørskov et al. in unadjusted numbers, found sexual distress to be most frequently reported among patients aged 40–65 years compared to both younger and older persons [13], arguing that fewer impairments in younger people and a protective effect of lifelong sexual history in older individuals may contribute to lower perceived distress compared to individuals in middle-age. Knowledge about the predictors of sexual distress, beyond retrospective reports (e.g. [12]) of patients who have undergone different types of treatment, as well as its potential downstream effects, is limited, and prospective research in heterogenous male cancer populations is needed.
In our study, 6–11% of respondents per item reported distress without also reporting impairment. Most of these respondents were not sexually active (60–82% per item), potentially because of impairment. Conversely, between 2 and 19% per item reported impairment without distress. This illustrates the complex nature of sexual problems, as an interplay of impairments with the psychological and relational context. For some, sexual impairments may not be distressing, e.g. if the individual has little personal interest in sexual activity, for others, distressing sexual impairments may lead to sexual inactivity [8]. This underscores the importance of nuanced inquiry by healthcare professionals, covering both impairment and distress, when investigating sexual problems.
Almost one-third of respondents expressed a current interest in a consultation about sexual problems, and among these, few were receiving help. These findings are similar to a Danish study including people with various cancer diagnoses [11], in which 52% of patients experienced an unmet need for help with sexual problems. We found that when more areas of distress were reported, more participants had a wish for a consultation. Asking patients about sexual distress, not only impairment, may be important to ensure that patients in need can be referred for sexual health care.
Strengths of this study include the use of a questionnaire screening for both sexual impairment and distress in all areas of sexual functioning, and the calculation of percentages reporting combinations of distress and impairment. By including data on other symptoms, we were able to assess the association of sexual distress with other potential side and late effects and adjust our analyses of the association between sexual distress and HRQoL to provide more accurate estimates. To the best of our knowledge, most previous studies have investigated men with cancer primarily after surgical treatment. We invited all patients associated with an oncology department, both those in active treatment and those in follow-up, and thereby included the full spectrum of male cancer patients, as seen in clinical workflow. Access to medical records let us validate self-reported diagnoses and minimize misclassification risk.
Nonetheless, our study has certain limitations, including the cross-sectional design which precludes the possibility to establish causality. The relatively low participation rate in the parent study (40%) and the lower response rate to the SCS-M among older patients as well as those with certain diagnoses may bias our results. The SCS-M has only been preliminarily validated [17], although it has been used in studies of male populations with somatic diseases [18], and it lacks items related to intimacy, which also play a role in sexual life. The SCS-M is, however, to the best of our knowledge, the only questionnaire that assesses frequency of impairment as well as sexual distress across all areas. Further, estimates from post-hoc analyses may be biased due to small sample size and it was not possible to determine if patients were in treatment for primary cancer, relapse or a new cancer. Finally, respondents to the parent study who reported receiving help for sexual problems were more likely to complete the SCS-M, potentially introducing bias. However, they constituted less than 10% of the overall study population, limiting their influence.
Conclusion
In conclusion, we observed a considerable prevalence of sexual distress as well as of sexual impairment among men with different cancers, with subgroups reporting either impairment without distress or distress without impairment. Sexual distress was associated with experiencing clinically relevant pain and insomnia. Very few respondents reported receiving help for sexual problems, although many expressed an interest in receiving a consultation for sexual problems. A greater focus on sexual health by healthcare professionals, including greater attention to sexual distress in addition to impairment, may be necessary, and this focus should extend to all men with cancer, regardless of diagnosis.
Acknowledgements
The authors would like to thank Jorne Biccler for assistance with statistical analyses, and Kathrine Løppenthin, Lise Bjerrum Thisted and Charlotte Pedersen for assistance with data collection.
Author contributions
Conceptualization: JN, AvH, BSR, AG
Methodology: JN, AvH, BSR
Analysis: JN
Accessed and verified the underlying data: JN, AvH, BSR
Writing (original draft preparation): JN
Writing (review and editing): JN, AvH, BSR, CJ, AG
Funding acquisition: Not applicable
All authors had full access to all the data and have final responsibility for the decision to submit for publication.
Data availability statement
Data is available upon request to the corresponding author.
References
[1] Lee H, Song M-K. State of the science of sexual health among older cancer survivors: an integrative review. J Cancer Surviv. 2024. https://doi.org/10.1007/s11764-024-01541-2
[2] Frankland J, Wheelwright S, Permyakova NV, Wright D, Collaço N, Calman L, et al. Prevalence and predictors of poor sexual well-being over 5 years follo wing treatment for colorectal cancer: results from the ColoREctal Well being (CREW) prospective longitudinal study. BMJ Open. 2020;10:e038953. https://doi.org/10.1136/bmjopen-2020-038953
[3] Benedict C, Traeger L, Dahn JR, Antoni M, Zhou ES, Bustillo N, et al. Sexual bother in men with advanced prostate cancer undergoing androgen deprivation therapy. J Sex Med. 2014;11:2571–80. https://doi.org/10.1111/jsm.12645
[4] Cameron S, Springer C, Fox-Wasylyshyn S, El-Masri MM. A descriptive study of functions, symptoms, and perceived health state after radiotherapy for prostate cancer. Eur J Oncol Nurs Off J Eur N Oncol Nurs Soc. 2012;16:310–14. https://doi.org/10.1016/j.ejon.2011.07.007
[5] Reese JB, Handorf E, Haythornthwaite JA. Sexual quality of life, body image distress, and psychosocial outcomes in colorectal cancer: a longitudinal study. Support Care Cancer. 2018;26:3431–40. https://doi.org/10.1007/s00520-018-4204-3
[6] Bahnsen MK, Graugaard C, Andersson M, Andresen JB, Frisch M. Physical and mental health problems and their associations with inter-personal sexual inactivity and sexual dysfunctions in Denmark: baseline assessment in a national cohort study. J Sex Med. 2022;19:1562–79. https://doi.org/10.1016/j.jsxm.2022.07.004
[7] Derogatis LR, Rosen R, Leiblum S, Burnett A, Heiman J. The Female Sexual Distress Scale (FSDS): initial validation of a stand ardized scale for assessment of sexually related personal distress in women. J Sex Marital Ther. 2002;28:317–30. https://doi.org/10.1080/00926230290001448
[8] Bravi CA, Tin A, Montorsi F, Mulhall JP, Eastham JA, Vickers AJ. Erectile function and sexual satisfaction: the importance of asking about sexual desire. J Sex Med. 2020;17:349–52. https://doi.org/10.1016/j.jsxm.2019.09.024
[9] Den Oudsten BL, Traa MJ, Thong MSY, Martijn H, De Hingh IHJT, Bosscha K, et al. Higher prevalence of sexual dysfunction in colon and rectal cancer sur vivors compared with the normative population: a population-based study. Eur J Cancer Oxf Engl. 2012;48:3161–70. https://doi.org/10.1016/j.ejca.2012.04.004
[10] Ben Charif A, Bouhnik A-D, Courbière B, Rey D, Préau M, Bendiane M-K, et al. Sexual health problems in French cancer survivors 2 years after diagnosis-the national VICAN survey. J Cancer Surviv Res Pract. 2016;10:600–9. https://doi.org/10.1007/s11764-015-0506-3
[11] Bond CB, Jensen PT, Groenvold M, Johnsen AT. Prevalence and possible predictors of sexual dysfunction and self-reported needs related to the sexual life of advanced cancer patients. Acta Oncol. 2019;58:769–75. https://doi.org/10.1080/0284186X.2019.1566774
[12] Osawa T, Wei JT, Abe T, Honda M, Rew KT, Dunn R, et al. Comparison of health-related quality of life between Japanese and American patients with bladder cancer as measured by a newly developed Japanese version of the bladder cancer index. Bladder Cancer. 2021;7:61–9. https://doi.org/10.3233/BLC-200359
[13] Nørskov KH, Schjoedt I, Tolver A, Jarden M. Sexual health in patients with malignant hematological disease: a Danish cross-sectional study. Sex Med. 2024;12:qfae053. https://doi.org/10.1093/sexmed/qfae053
[14] Gellhaus PT, Cary C, Kaimakliotis HZ, Johnson CS, Weiner M, Koch MO, et al. Long-term health-related quality of life outcomes following radical cystectomy. Urology. 2017;106:82–6. https://doi.org/10.1016/j.urology.2017.03.053
[15] Gilbert SM, Dunn RL, Hollenbeck BK, Montie JE, Lee CT, Wood DP, et al. Development and validation of the bladder cancer index: a comprehensive, disease specific measure of health related quality of life in patients with localized bladder cancer. J Urol. 2010;183:1764–70. https://doi.org/10.1016/j.juro.2010.01.013
[16] Hatzichristou D, Rosen RC, Derogatis LR, Low WY, Meuleman EJH, Sadovsky R, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7:337–48. https://doi.org/10.1111/j.1743-6109.2009.01619.x
[17] Gündüz A, Sertçelik S, Gündoğmuş İ, Zengin Eroğlu M, Kumru Bayazit R, Gönül H, et al. Turkish validity and reliability of the sexual complaints screener for men. Psychiatry Clin Psychopharmacol. 2019;29:597–602. https://doi.org/10.1080/24750573.2018.1505421
[18] Fugl-Meyer KS, Nilsson M, Hylander B, Lehtihet M. Sexual function and testosterone level in men with conservatively treated chronic kidney disease. Am J Mens Health. 2017;11:1069–76. https://doi.org/10.1177/1557988317703207
[19] Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. JNCI J Natl Cancer Inst. 1993;85:365–76. https://doi.org/10.1093/jnci/85.5.365
[20] Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–44. https://doi.org/10.1200/JCO.1998.16.1.139
[21] Olsen LR, Jensen DV, Noerholm V, Martiny K, Bech P. The internal and external validity of the major depression inventory in measuring severity of depressive states. Psychol Med. 2003;33:351–6. https://doi.org/10.1017/S0033291702006724
[22] Smets EMA, Garssen B, Bonke B, De Haes JCJM. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–25. https://doi.org/10.1016/0022-3999(94)00125-O
[23] Thong MSY, Mols F, Poll-Franse LV, Sprangers MAG, Rijt CCD, Barsevick AM, et al. Identifying the subtypes of cancer-related fatigue: results from the p opulation-based PROFILES registry. J Cancer Surviv. 2018;12:38–46. https://doi.org/10.1007/s11764-017-0641-0
[24] Simard S, Savard J. Fear of cancer recurrence inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Support Care Cancer. 2009;17:241–51. https://doi.org/10.1007/s00520-008-0444-y
[25] Fardell JE, Jones G, Smith AB, Lebel S, Thewes B, Costa D, et al. Exploring the screening capacity of the fear of cancer recurrence inventory-short form for clinical levels of fear of cancer recurrence. Psychooncology. 2018;27:492–9. https://doi.org/10.1002/pon.4516
[26] Bastien C. Validation of the insomnia severity index as an outcome measure for in somnia research. Sleep Med. 2001;2:297–307. https://doi.org/10.1016/S1389-9457(00)00065-4
[27] Savard M-H, Savard J, Simard S, Ivers H. Empirical validation of the insomnia severity index in cancer patients: Insomnia Severity Index and Cancer. Psychooncology. 2005;14:429–41. https://doi.org/10.1002/pon.860
[28] Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23:129–38.
[29] Shi Q, Mendoza TR, Dueck AC, Ma H, Zhang J, Qian Y, et al. Determination of mild, moderate, and severe pain interference in patients with cancer. Pain. 2017;158:1108–12. https://doi.org/10.1097/j.pain.0000000000000890
[30] R: the R project for statistical computing. n.d. [cited 2023 May 11]. Available from: https://www.r-project.org/
[31] Tiruye T, O’Callaghan M, Moretti K, Jay A, Higgs B, Santoro K, et al. Patient-reported functional outcome measures and treatment choice for prostate cancer. BMC Urol. 2022;22:169. https://doi.org/10.1186/s12894-022-01117-1
[32] Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425–37. https://doi.org/10.1056/NEJMoa1606221
[33] Yang L, Lee JA, Heer E, Pernar C, Colditz GA, Pakpahan R, et al. One-year urinary and sexual outcome trajectories among prostate cancer patients treated by radical prostatectomy: a prospective study. BMC Urol. 2021;21:81. https://doi.org/10.1186/s12894-021-00845-0
[34] Steinsvik EAS, Axcrona K, Dahl AA, Eri LM, Stensvold A, Fosså SD. Can sexual bother after radical prostatectomy be predicted preoperatively? Findings from a prospective national study of the relation between sexual function, activity and bother. BJU Int. 2012;109:1366–74. https://doi.org/10.1111/j.1464-410X.2011.10598.x