ORIGINAL REPORT
EFFECTS OF REHABILITATION PROGRAM FOCUSED ON IMPROVING REAL-LIFE DAILY ACTIVITIES OF PATIENTS WITH MILD COGNITIVE IMPAIRMENTS OR DEMENTIA AND THEIR CAREGIVERS
Yohei OTAKA, MD, PhD1, Shin KITAMURA, OTR, MS1,2, Megumi SUZUKI, OTR, PhD2, Akiko MAEDA, OTR, PhD2, Chinami KATO, OTR3, Rena ITO, OTR3, Asuka HIRANO, RPT, MS3, Yuki OKOCHI, OTR3, Koji MIZUTANI, RPT, MS3, Hiroshi YOSHINO, MD, PhD4 and Hajime TAKECHI, MD, PhD4
From the 1Department of Rehabilitation Medicine I, School of Medicine, 2Faculty of Rehabilitation, School of Health Sciences, 3Department of Rehabilitation, Fujita Health University Hospital, Aichi, Japan and 4Department of Geriatrics and Cognitive Disorders, School of Medicine, Fujita Health University, Aichi, Japan
Objective: To evaluate the effectiveness of a dyadic outpatient rehabilitation program focused on improving the real-life daily activities of patients with mild cognitive impairments or dementia and their caregivers.
Design: Retrospective study.
Subjects: Eight patients with mild cognitive impairments or dementia and their caregivers.
Methods: The rehabilitation program comprised eight 1-hour sessions by occupational therapists with patients and his/her caregivers. Patients were assessed for motor function, cognitive function, and quality of life, and their caregivers were assessed for depression and caregiver burden. Participants were assessed at pre-program and post-program, and 3-month follow-up.
Results: The scores of caregiver-assessed Quality of life in Alzheimer’s disease scale in patients significantly improved at post-program (median [interquartile range], 30.0 [7.0]) compared with pre-program (27.0 [2.8], effect size = 0.77, p = 0.029). In caregivers, the Zarit Caregiver Burden Interview scores decreased significantly at post-program (16.5 [13.0]) compared with pre-program (22.0 [17.5], effect size = 0.72, p = 0.042). There were no significant differences in other assessments.
Conclusions: The rehabilitation program focused on real daily activities and demonstrated to improve patients’ quality of life and caregivers’ depression and caring burden through patient-caregiver interaction. Future enhanced follow-up systems are warranted.
LAY ABSTRACT
Although various non-pharmacological interventions have been reported for patients with dementia, few studies have reported interventions focussing on improving activities in the real daily lives of patients and their caregivers. This study evaluated the effectiveness of a dyadic outpatient rehabilitation program focused on the real-life daily activities of patients with mild cognitive impairment or dementia and their caregivers. It consisted of 8 individual 1-hour sessions by occupational therapists to 8 patient-caregiver pairs. Patients were assessed for motor function, cognitive function, and quality of life, and caregivers were assessed for depression and caregiver burden. Participants were assessed before and after the program and at a 3-month follow-up. After the program, patients’ motor and cognitive functions did not change; however, their quality of life improved significantly and caregivers’ sense of caregiver burden decreased significantly. The rehabilitation program demonstrated positive effects on both patients and their caregivers.
Key words: Alzheimer’s disease; behavior therapy; caregivers; dementia; rehabilitation.
Citation: JRM-CC 2023; 6: jrmcc12293. DOI: https://doi.org/10.2340/jrmcc.v6.12293
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Sep 1 2023; Published: Oct 5, 2023
Correspondence address: Yohei Otaka, Department of Rehabilitation Medicine I, School of Medicine, Fujita Health University, 1-98 Dengakugakubo, Kutsukake, Toyoake, Aichi, Japan 470-1192, Japan. E-mail: otaka119@mac.com
Competing interests and funding: The authors have no conflicts of interest to declare.
The prevalence of dementia is increasing rapidly worldwide, with physical, psychological, social, and economic impacts on both patients and their caregivers, family members, and society (1). Japan has the highest aging population globally (2), and the number of patients with dementia is expected to increase remarkably. Dementia has been the leading cause of the need for care in Japan since 2016 (3). Approximately 54.4% of caregivers for patients with dementia living at home were family members residing with patients, whereas 13.6% were family members living separately in Japan in 2019, with family members taking on most caregiving responsibilities (4). Caring for patients with dementia causes psychological, physical, and financial burdens (5–7). Support for both patients with dementia and their caregivers is crucial in Japan, where dementia-related issues are likely to deteriorate.
There are 2 types of treatments for patients with dementia: pharmacological and non-pharmacological. The non-pharmacological interventions have been reported to be as effective as or more effective than pharmacological interventions in affecting the behavior of patients with dementia, without any side effects (8). Various non-pharmacological interventions have been conducted (9–14). These include exercise, for improving functional independence and reducing caregiver burden (10); cognitive stimulation, for delaying or preventing the progression of dementia (14); music therapy, which improves the emotional, psychological, and behavioral symptoms of dementia (13); and psychological treatments, which reduce depression and anxiety (11). Moreover, there are several approaches using activities that are individualized for patients with dementia. In previous studies, interventions as a treatment using individualized occupational activities and pleasant events based on a person’s history, needs, preferences, personality, functioning, and abilities have been reported to improve behavioral and psychological symptoms and quality of life (QOL) in patients with dementia (15–19). However, there are few reports on the effectiveness and development of rehabilitation programs that focus on improving patients’ real-life daily activities. A previous study that intervened in patients’ real-life daily activities demonstrated that these interventions directly improved the activities of patients with dementia (20). Moreover, direct interventions for activities in the patient’s daily life that focus on both caregivers and patients with dementia have also been reported to improve the caregivers’ QOL and reduce their burden of caregiving (21, 22). Since the number of studies on this aspect is small to adequately determine the effectiveness of interventions that focused on real daily activities of patients with dementia and their caregivers, developments of rehabilitation programs and further evaluations of effectiveness are warranted.
Furthermore, such programs are yet to be established in Japan, which has the highest number of social issues associated with dementia worldwide. The implementation of intervention methods in different countries is not always effective, owing to cultural differences (23). Therefore, it is necessary to develop and verify the effectiveness of interventions tailored to the Japanese social system and culture. This study aimed to conduct a retrospective analysis of the effectiveness of a dementia rehabilitation program that focused on real-life daily activities. The rehabilitation program was implemented in a Japanese hospital for patients with dementia and their caregivers to gain preliminary insight into the establishment of interventions for community-dwelling patients with dementia and their caregivers.
METHODS
Study design
This retrospective study analyzed the results of a dementia rehabilitation program developed and conducted at Fujita Health University Hospital for patients with mild cognitive impairments (MCI) or dementia and their caregivers between December 2018 and January 2021. The study protocol was approved by the Ethics Review Committee of Fujita Health University (approval number: HM20-233). Although this was a retrospective study, all patients and caregivers were informed of the study, and their consent was obtained.
Participants
Patients were recruited using invitations on the posters placed in the hospital. Moreover, the physicians also introduced the program to outpatients in the Department of Geriatrics and Cognitive Disorders. The inclusion criteria were as follows: (i) older community-dwelling patients attending the Department of Geriatrics and Cognitive Disorders of Fujita Health University Hospital, (ii) patients presenting with mild cognitive impairment or mild-to-moderate dementia, (iii) patients physically or psychologically burdened by dementia, either by themselves or by caregivers, and (iv) patients willing to participate in the dementia rehabilitation program. Pairs of patients and caregivers who did not complete all 8 sessions of the program were excluded from the analyses in this study. During the study period, 9 pairs of patients and caregivers participated in the program; one of the 9 pairs ceased the program because they did not feel that it was effective, while the remaining 8 pairs completed the entire program. Eight pairs of patients and caregivers who completed the program were included (3 Alzheimer’s disease [AD], 1 AD with cerebrovascular disease, 3 MCI due to AD, and 1 semantic dementia). A clinical diagnosis of MCI due to AD or AD was made using the National Institute on Aging-Alzheimer’s Association diagnostic criteria (24, 25). In principle, MCI was defined as a Mini-Mental State Examination (MMSE) score of 24–30 points and a Clinical Dementia Rating (CDR) of 0.5, and AD as an MMSE score of 26 points or less and a CDR of 1.0 or above. In addition to the MMSE and CDR, Wechsler Memory Scale-Revised logical memory test (immediate and delayed), executive function tests, visuospatial cognitive tests, and questions about instrumental activities of daily living were also administered. Diagnoses of semantic dementia were made according to the criteria by Neary et al. (26). Participant characteristics are shown in Table I. Five patients were males aged 71–82 (mean, 75.2) years. All caregivers were the spouses of patients aged 69–85 (mean age 73.4).
| Case | Diagnosis | Age, years, Sex | Disease duration | MoCA | Complication |
| 1 | AD† and CVD‡ | 77, M | 9 months | 18 | Benign prostatic hyperplasia |
| 2 | MCI§ | 82, F | 2 years | 17 | None |
| 3 | SD¶ | 74, F | 8 months | 21 | None |
| 4 | AD | 71 , F | 2 years | 20 | Liver cyst |
| 5 | AD | 76, M | 8 months | 21 | None |
| 6 | MCI | 76, M | 2 years | 22 | Post-gastric cancer |
| 7 | AD | 72, M | 2 years | 16 | None |
| 8 | MCI | 74, M | 6 months | 25 | None |
| †Alzheimer’s disease; ‡cerebrovascular disease; §mild cognitive impairment; and ¶semantic dementia. | |||||
| MCI: mild cognitive impairments; AD: Alzheimer’s disease; MoCA: Montreal Cognitive Assessment. | |||||
Dementia rehabilitation program
The dementia rehabilitation program was developed by a research group comprising physicians, physical therapists, and occupational therapists from the Department of Rehabilitation and Department of Geriatrics and Cognitive Disorders. It comprised eight 1-hour sessions, with either 1 or 2 weeks interval between sessions, depending on the participant’s schedule. The program was a dyadic intervention, in which the patients and caregivers both participated in the program. Patients and caregivers received physician consultations together before, after, and 3 months after completion of the program. At the pre-program consultation, participants received an explanation of the overall content of the program and their willingness to participate was confirmed. In the subsequent consultation, participants received feedback on the results of the assessments conducted within the program. One therapist was assigned to the patient and the other therapist was assigned to the caregiver. The patient and caregiver each participated in one-on-one rehabilitation with the same occupational therapist throughout the program. The program aimed to prompt a change in patient behavior and improve the caregivers’ coping skills by focusing on activities, setting goals, and solving problems for each participant pair, rather than improving cognitive function. The contents of the rehabilitation program are shown in Table II. In the initial session, the therapists conducted functional and psychological assessments and interviews with the pair. During the interview, the patient and caregiver were asked about their actual daily life problems, their work interests, the quality of the patient-caregiver relationship, and their expectations of the program. Based on this information, the therapist and patient or therapist and caregiver discussed and set goals for the program. At the end of each session, the therapists shared the results of the evaluation and what they had undergone in the session, modifying the program goals and content as needed.
Patients were encouraged to implement compensatory strategies, resume hobbies, try to start new activities, and establish an exercise routine to achieve the patients’ or caregivers’ goals depending on the needs, situations, and conditions. The therapist assessed which activities were difficult for the patient and determined which hobbies to resume based on the needs of the patient and caregiver. The therapist then instructed the patient and caregiver on the target activities and the use of assistive devices to accomplish them, if required. The therapist also informed the caregivers about how to support the patient in the activity and how the difficulty with the activity was related to dementia symptoms. The therapists recommended walking and other exercises that can be performed at home for patients with no exercise habits, according to the patients’ functions. Moreover, they encouraged the patients to manage their exercises by using a diary to check the degree of implementation and devices such as pedometers so that they can continue after the program ends. Therapists also informed caregivers how to help patients continue their exercises.
Caregivers were trained to support patients and use compensatory strategies to achieve caregiver and patient goals and were informed about dementia symptoms and how to cope with them. Between each session, the caregivers practiced supporting the patient’s activities and interacting with the patient based on the therapist’s instructions and recorded their ability to implement them and the degree of anxiety and burden they experienced. The records were shared with the therapists and caregivers at each session and were referred to in the program when reflecting on the caregivers’ daily difficulties and the coping strategies they practiced. The therapist provided feedback on the caregivers’ efforts, including positive points and suggestions. As the program progressed, as new issues or targets in daily life were raised by the caregivers, the therapists and caregivers worked on them. The therapists observed the patients working on the program, with the caregivers as required, providing an opportunity for the caregivers to objectively evaluate what the patient can or cannot do and learn how the caregiver helps the patients perform activities. For participants requiring case management, occupational therapists worked with medical social workers to provide knowledge and coordinate service use. To provide comprehensive intervention to participants, case conferences were held at monthly intervals by a multidisciplinary team (physicians, physical therapists, and occupational therapists) to share program implementation and results, and to discuss subsequent plans.
Outcome measures
The patients and caregivers were assessed at the first (pre-program) and 8th sessions (post-program) of the program and 3 months after completion of the program (follow-up). Patients were assessed using the Timed Up and Go (TUG) test, Montreal Cognitive Assessment (MoCA), and QOL in Alzheimer’s disease (QOL-AD) scale. The TUG test is an assessment of standing balance and gait, measuring the time taken by a person to get up from a chair, move around a landmark 3 m away, and sit down in the chair again (27). This test has been verified for reliability and validity (27, 28). The MoCA is used to assess cognitive function by interview, with a score ranging from 0 to 30 with higher scores indicating better cognitive function; mild cognitive impairment is suspected in patients with a score of ≤ 25 (29). Its reliability and validity have been confirmed (29, 30). The QOL-AD scale is a QOL scale developed for patients with mild-to-moderate dementia, with total scores from 13 to 52, with higher scores indicating better QOL (31). Assessment can be performed by the patient themselves or by their caregivers as a proxy (32). In the present study, patients and their caregivers separately assessed patients’ QOL-AD scale. Its reliability and validity have been confirmed (32, 33).
Caregivers were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D) and Zarit Caregiver Burden Interview (ZBI). The CES-D is a self-administered depression questionnaire used as a screening tool for the early detection of depression (34). The total score ranges from 0 to 60 points, with ≥ 16 points indicating depression and higher scores indicating more marked depression. Its reliability and validity have been confirmed (35). The ZBI is a questionnaire that assesses the caregiver’s perception of care burden, including psychological, physical, social, and economic burdens, with scores ranging from 0 to 88 and higher scores indicating greater caregiving burden (36). Its reliability and validity have been confirmed (37).
Analysis
Differences in each outcome between the pre- and post-program periods and between the pre-program and follow-up periods were examined. The Wilcoxon rank-sum test was used for statistical analysis, and r was used to calculate the effect size (ES). Effect sizes were interpreted according to Cohen’s classifications as follows: 0.10, small; 0.30, medium; and 0.50, large (38). All statistical analyses were performed using SPSS version 28 (IBM Corp., Armonk, NY, USA). A p < 0.05 was considered statistically significant.
RESULTS
The results of patient assessments are shown in Fig. 1. The QOL-AD scale score given by caregivers significantly improved from the pre-program to post-program with a large ES (Z = 2.18, ES = 0.77, p = 0.029). There were no significant differences in other assessments from the pre- to post-program (p = 0.397–0.863). Furthermore, there were no significant differences in any assessment between the pre-program and follow-up periods (p = 0.526–0.999).
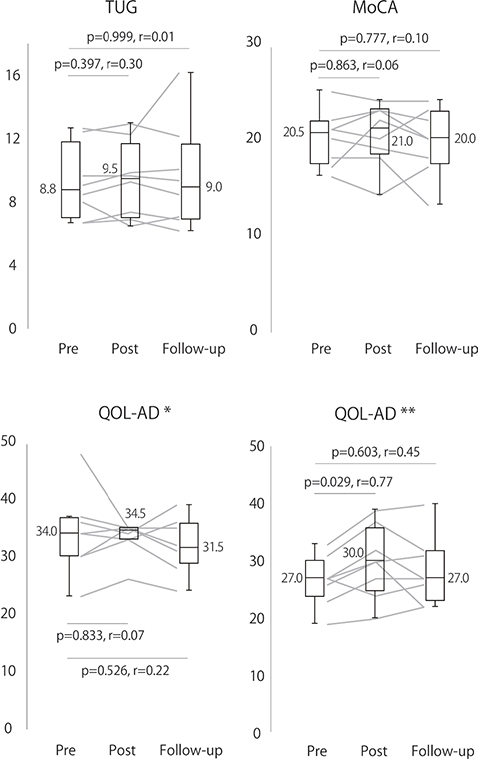
Fig. 1. Patient assessments. TUG: timed up-and-go test; MoCA: Montreal Cognitive Assessment; and QOL-AD: Quality of Life in Alzheimer’s Disease. QOL-AD* indicates the patient’s assessment of their own quality of life, and QOL-AD** indicates the caregiver’s assessment of the patient’s quality of life. Assessments were conducted during the first (pre-program) and last (post-program) sessions of the dementia rehabilitation program and 3 months after completion (follow-up). The horizontal lines inside the boxplots and the values beside the boxplot represent the median values; the edges of the boxplots represent the upper and lower quartiles, and the whiskers represent the maximum and minimum values. Line graphs show the results for individual patients.
The results of the caregiver assessment are shown in Fig. 2. There was a significant decrease in ZBI scores with a large ES between the pre- and post-program (Z = 2.04, ES = 0.72, p = 0.042) and a marginally significant decrease with a large ES in CES-D scores (Z = 1.94, ES = 0.69, p = 0.051). Neither assessment showed a statistically significant difference between the pre-program and follow-up periods; however, both scores tended to decrease with large ES (CES-D: Z = 1.47, ES = 0.52, p = 0.141; ZBI: Z = 1.42, ES = 0.50, p = 0.156).
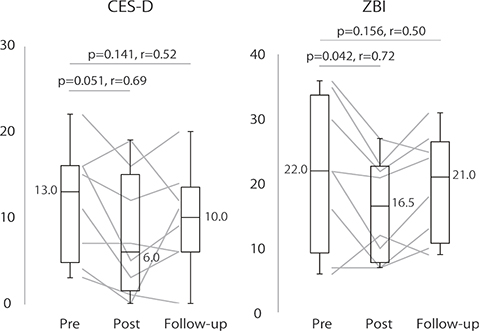
Fig. 2. Caregiver assessments. CES-D: Center for Epidemiologic Studies Depression Scale; and ZBI: Zarit Caregiver Burden Interview. Assessments were conducted during the first (pre-program) and last (post-program) sessions of the dementia rehabilitation program and 3 months after completion (follow-up). The horizontal lines inside the boxplots and the values beside the boxplot represent the median values; the edges of the boxplots represent the upper and lower quartiles, and the whiskers represent the maximum and minimum values. Line graphs show the results for individual patients.
DISCUSSION
This study examined the effects of a dementia rehabilitation program on patients with dementia and caregivers by analyzing the changes in related indicators before and after it. Post-program, the patients’ QOL improved, and the caregivers’ depression and care burden decreased.
In this study, interventions to improve real-life daily activities for patients with mild to moderate dementia did not change patients’ function but improved their QOL assessed by their caregivers. Improving activities of daily living in patients with mild to moderate dementia directly improves patients’ performance and satisfaction with activities, not their functions or symptoms (20). The patients in the present study may also have increased their performance and satisfaction with activities by working on their real-life daily activities, resulting in an improved QOL. A systematic review examining the effects of person-centered care on patients with dementia showed that improvements in QOL were more likely to occur in patients with less severe dementia (15). This study and previous findings indicate that individualized interventions are effective in improving QOL for patients with mild to moderate dementia.
The intervention in this study also focused on the caregivers, which reduced caregivers’ depression and burden of care. “Focus on the person with dementia as well as the caregiver” is an essential element for successfully supporting caregivers (39). Consistent with the current study, educational programs for caregivers of patients with dementia have been shown to reduce depression and the burden of care (40–42). In contrast, a recent systematic review reported that providing caregivers with knowledge related to the disease and lectures on how to self-manage symptoms had no significant effect on depression and a significant effect on the burden of care with a small ES (43). The greater effectiveness of the current program could be attributed to the fact that the interventions for caregivers were combined with those for patients with dementia. Based on the therapist’s assessment of the patient’s symptoms and performance of activities, the caregiver could understand and learn appropriate coping skills. Furthermore, the caregiver sometimes participates in the patient’s program activities; the caregiver might have been able to understand the patient’s symptoms and learn coping strategies better, leading to improved actual coping skills and a reduced psychological burden.
The improvement in the QOL of the patients and caregivers’ depression and burden may have been enhanced due to interactions between patients and caregivers. The improved QOL of patients assessed by caregivers may have been influenced by the reduction of the psychological burden on caregivers, which may have improved their ability to cope with problems and interact appropriately with patients. In addition, the reduction of psychological burden on caregivers may have made their evaluation of patients positive. A qualitative study has shown that the quality of the relationship between the person with dementia and their caregiver was affected not only by the caregiver’s depression and anxiety but also by the behavioral symptoms and QOL of the person with dementia (44). Therefore, interventions targeting the dyadic may be more effective than interventions targeting only the patient or caregiver. In support of this, previous studies of comprehensive interventions for dyadic have shown their effectiveness (20, 22, 45–48). The outpatient rehabilitation program for both dementia patients and caregivers in this study may have resulted in positive effects for both due to the interactions that occurred between them.Some of the assessments in this study with improved outcomes after the program had large ES between the pre-program and follow-up periods, but none of them were significantly different. However, in previous studies, the effects remained at follow-up; for example, Graff et al. reported that 10 client-centered occupational therapy sessions over 5 weeks improved patient and caregiver QOL and that these effects persisted 6 weeks later (21). Although differences in the frequency of interventions, outcomes, and the duration between program completion and follow-up make general comparisons impossible, the lack of sustained effects at follow-up indicates that the interventions in this study may not have produced sufficient behavioral modifications in patients or caregivers. Teri et al. reported that a total of 12 home-based interventions over 3 months that combined an exercise program for patients with coping skills training for caregivers and 3 follow-up sessions over 3 months resulted in functional maintenance for 2 years (45). Since it has been shown that the effects of the program can be maintained depending on the approach, we need to improve the program to maintain the effects in the future.
This was only a preliminary study that did not fully verify the effectiveness of the dementia program. After modifying the program, future comparative studies to evaluate its effectiveness should be conducted with a sample size calculated based on the results obtained in this study.
CONCLUSIONS
The patients’ QOL significantly improved, and their care burden was significantly reduced after our activity-oriented dementia rehabilitation program for people with dementia and their caregivers in outpatient settings. Further studies with a control to confirm the effectiveness of this program are warranted.
Acknowledgements
The study protocol was approved by the Committee of Ethics Review of Fujita Health University (approval number: HM20-233). This study was conducted in accordance with the Declaration of Helsinki of 1964, revised in 2013.
REFERENCES
- World Health Organization. Fact sheets. Dementia 2022. [cited 2023 Feb 5]. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia
- World Health Organization, Resource Library. Countries with the oldest populations in the world. [cited 2023 Feb 5]. Available from: https://www.prb.org/resources/countries-with-the-oldest-populations-in-the-world/
- Ministry of Health Labour and Welfare, Comprehensive survey of living conditions 2016. 2017. [cited 2023 Feb 5]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa16/dl/05.pdf
- Ministry of Health Labour and Welfare, Comprehensive survey of living conditions 2019. 2020. [cited 2023 Feb 5]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa19/dl/05.pdf
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull 2003; 129: 946–972. https://doi.org/10.1037/0033-2909.129.6.946
- Jönsson L, Jönhagen ME, Kilander L, Soininen H, Hallikainen M, Waldemar G, et al. Determinants of costs of care for patients with Alzheimer’s disease. Int J Geriatr Psych 2006; 21: 449–459. https://doi.org/10.1002/gps.1489
- Chiao CY, Wu HS, Hsiao CY. Caregiver burden for informal caregivers of patients with dementia: a systematic review. Int Nurs Rev 2015; 62: 340–350. https://doi.org/10.1111/inr.12194
- Luijpen MW, Scherder EJ, Van Someren EJ, Swaab DF, Sergeant JA. Non-pharmacological interventions in cognitively impaired and demented patients – a comparison with cholinesterase inhibitors. Rev Neurosci 2003; 14: 343–368. https://doi.org/10.1515/revneuro.2003.14.4.343
- Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev 2013; 2013: CD003260. https://doi.org/10.1002/14651858.CD003260.pub2
- Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S. Exercise programs for people with dementia. Cochrane Database Syst Rev 2015; 2015: CD006489. https://doi.org/10.1002/14651858.CD006489.pub4
- Orgeta V, Qazi A, Spector AE, Orrell M. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database Syst Rev 2014; 2014: CD009125. https://doi.org/10.1002/14651858.CD009125.pub2
- Reilly S, Miranda-Castillo C, Malouf R, Hoe J, Toot S, Challis D, et al. Case management approaches to home support for people with dementia. Cochrane Database Syst Rev 2015; 1: CD008345. https://doi.org/10.1002/14651858.CD008345.pub2
- van der Steen JT, van Soest-Poortvliet MC, van der Wouden JC, Bruinsma MS, Scholten RJ, Vink AC. Music-based therapeutic interventions for people with dementia. Cochrane Database Syst Rev 2017; 5: CD003477. https://doi.org/10.1002/14651858.CD003477.pub3
- Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev 2012; 2: CD005562. https://doi.org/10.1002/14651858.CD005562.pub2
- Kim SK, Park M. Effectiveness of person-centered care on people with dementia: a systematic review and meta-analysis. Clin Interv Aging 2017; 12: 381–397. https://doi.org/10.2147/cia.s117637
- Dinapoli EA, Scogin F, Bryant AN, Sebastian S, Mundy MJ. Effect of individualized social activities on quality of life among older adults with mild to moderate cognitive impairment in a geriatric psychiatry facility. Aging Ment Health 2016; 20: 262–270. https://doi.org/10.1080/13607863.2015.1008990
- Cohen-Mansfield J, Thein K, Marx MS, Dakheel-Ali M, Freedman L. Efficacy of nonpharmacologic interventions for agitation in advanced dementia: a randomized, placebo-controlled trial. J Clin Psychiatry 2012; 73: 1255–1261. https://doi.org/10.4088/JCP.12m07918
- Cohen-Mansfield J, Libin A, Marx MS. Nonpharmacological treatment of agitation: a controlled trial of systematic individualized intervention. J Gerontol A Biol Sci Med Sci 2007; 62: 908–916. https://doi.org/10.1093/gerona/62.8.908
- Brown Wilson C, Arendt L, Nguyen M, Scott TL, Neville CC, Pachana NA. Nonpharmacological interventions for anxiety and dementia in nursing homes: a systematic review. Gerontologist 2019; 59: e731–e742. https://doi.org/10.1093/geront/gnz020
- Clare L, Linden DE, Woods RT, Whitaker R, Evans SJ, Parkinson CH, et al. Goal-oriented cognitive rehabilitation for people with early-stage Alzheimer disease: a single-blind randomized controlled trial of clinical efficacy. Am J Geriatr Psychiatry 2010; 18: 928–939. https://doi.org/10.1097/JGP.0b013e3181d5792a
- Graff MJ, Vernooij-Dassen MJ, Thijssen M, Dekker J, Hoefnagels WH, Olderikkert MG. Effects of community occupational therapy on quality of life, mood, and health status in dementia patients and their caregivers: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2007; 62: 1002–1009. https://doi.org/10.1093/gerona/62.9.1002
- Graff MJ, Vernooij-Dassen MJ, Thijssen M, Dekker J, Hoefnagels WH, Rikkert MG. Community based occupational therapy for patients with dementia and their care givers: randomised controlled trial. BMJ 2006; 333: 1196. https://doi.org/10.1136/bmj.39001.688843.BE
- Voigt-Radloff S, Graff M, Leonhart R, Schornstein K, Jessen F, Bohlken J, et al. A multicentre RCT on community occupational therapy in Alzheimer’s disease: 10 sessions are not better than one consultation. BMJ Open 2011; 1: e000096. https://doi.org/10.1136/bmjopen-2011-000096
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 263–269. https://doi.org/10.1016/j.jalz.2011.03.005
- Albert MS, Dekosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 270–279. https://doi.org/10.1016/j.jalz.2011.03.008
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51: 1546–1554. https://doi.org/10.1212/wnl.51.6.1546
- Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991; 39: 142–148. https://doi.org/10.1111/j.1532-5415.1991.tb01616.x
- Ries JD, Echternach JL, Nof L, Gagnon Blodgett M. Test-retest reliability and minimal detectable change scores for the timed “up & go” test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys Ther 2009; 89: 569–579. https://doi.org/10.2522/ptj.20080258
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
- Roalf DR, Moberg PJ, Xie SX, Wolk DA, Moelter ST, Arnold SE. Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimers Dement 2013; 9: 529–537. https://doi.org/10.1016/j.jalz.2012.10.001
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med 2002; 64: 510–519. https://doi.org/10.1097/00006842-200205000-00016
- Torisson G, Stavenow L, Minthon L, Londos E. Reliability, validity and clinical correlates of the Quality of Life in Alzheimer’s disease (QoL-AD) scale in medical inpatients. Health Qual Life Outcomes 2016; 14: 90. https://doi.org/10.1186/s12955-016-0493-8
- Matsui T, Nakaaki S, Murata Y, Sato J, Shinagawa Y, Tatsumi H, et al. Determinants of the quality of life in Alzheimer’s disease patients as assessed by the Japanese version of the Quality of Life-Alzheimer’s disease scale. Dement Geriatr Cogn Disord. 2006; 21: 182–191. https://doi.org/10.1159/000090744
- Radloff LS. The CES-D Scale. Appl Psychol Meas 1977; 1: 385–401. https://doi.org/10.1177/014662167700100306
- Clark CH, Mahoney JS, Clark DJ, Eriksen LR. Screening for depression in a hepatitis C population: the reliability and validity of the Center for Epidemiologic Studies Depression Scale (CES-D). J Adv Nurs 2002; 40: 361–369. https://doi.org/10.1046/j.1365-2648.2002.02378.x
- Whitlatch CJ, Zarit SH, von Eye A. Efficacy of interventions with caregivers: a reanalysis. Gerontologist 1991; 31: 9–14. https://doi.org/10.1093/geront/31.1.9
- Wang G, Cheng Q, Wang Y, Deng YL, Ren RJ, Xu W, et al. The metric properties of Zarit caregiver burden scale: validation study of a Chinese version. Alzheimer Dis Assoc Disord 2008; 22: 321–326. https://doi.org/10.1097/WAD.0b013e3181902334
- Cohen J. Statistical power analysis for the behavioral science. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
- International Psychogeriatric Association. IPA Complete Guides to Behavioral and Psychological Symptoms of Dementia-Specialists Guide; 2015.
- Pinquart M, Sörensen S. Helping caregivers of persons with dementia: which interventions work and how large are their effects? Int Psychogeriatr 2006; 18: 577–595. https://doi.org/10.1017/s1041610206003462
- Parker D, Mills S, Abbey J. Effectiveness of interventions that assist caregivers to support people with dementia living in the community: a systematic review. Int J Evid Based Healthc 2008; 6: 137–172. https://doi.org/10.1111/j.1744-1609.2008.00090.x
- Marim CM, Silva V, Taminato M, Barbosa DA. Effectiveness of educational programs on reducing the burden of caregivers of elderly individuals with dementia: a systematic review. Rev Lat Am Enfermagem 2013; 21: 267–275. https://doi.org/10.1590/s0104-11692013000700033
- Kishita N, Hammond L, Dietrich CM, Mioshi E. Which interventions work for dementia family carers?: an updated systematic review of randomized controlled trials of carer interventions. Int Psychogeriatr 2018; 30: 1679–1696. https://doi.org/10.1017/s1041610218000947
- Spector A, Orrell M, Charlesworth G, Marston L. Factors influencing the person-carer relationship in people with anxiety and dementia. Aging Ment Health 2016; 20: 1055–1062. https://doi.org/10.1080/13607863.2015.1063104
- Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, et al. Exercise plus behavioral management in patients with Alzheimer disease. JAMA 2003; 290: 2015–2022. https://doi.org/10.1001/jama.290.15.2015
- McCurry SM, Gibbons LE, Logsdon RG, Vitiello MV, Teri L. Nighttime insomnia treatment and education for Alzheimer’s disease: a randomized, controlled trial. J Am Geriatr Soc 2005; 53: 793–802. https://doi.org/10.1111/j.1532-5415.2005.53252.x
- Gitlin LN, Winter L, Burke J, Chernett N, Dennis MP, Hauck WW. Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: a randomized pilot study. Am J Geriatr Psychiatry 2008; 16: 229–239. https://doi.org/10.1097/JGP.0b013e318160da72
- Chien WT, Lee IYM. Randomized controlled trial of a dementia care programme for families of home-resided older people with dementia. J Adv Nurs 2011; 67: 774–787. https://doi.org/10.1111/j.1365-2648.2010.05537.x