ORIGINAL ARTICLE
THE EFFECT OF IN-BED LEG CYCLING EXERCISES ON MUSCLE STRENGTH IN PATIENTS WITH INTENSIVE CARE UNIT-ACQUIRED WEAKNESS: A SINGLE-CENTER RETROSPECTIVE STUDY
Ayato SHINOHARA, MSc1, Hitoshi KAGAYA, MD, PhD2,3, Hidefumi KOMURA, MD, PhD4, Yusuke OZAKI, MSc1, Toshio TERANISHI, PhD5, Tomoyuki NAKAMURA, MD, PhD4, Osamu NISHIDA, MD, PhD4 and Yohei OTAKA, MD, PhD2
From the 1Department of Rehabilitation, Fujita Health University Hospital, 2Department of Rehabilitation Medicine I, School of Medicine, Fujita Health University, 3Department of Rehabilitation Medicine, National Center for Geriatrics and Gerontology, 4Department of Anesthesiology and Critical Care Medicine, School of Medicine, Fujita Health University, and 5Faculty of Rehabilitation, School of Health Science, Fujita Health University, Fujita, Japan
Objective: To examine the effect of in-bed leg cycling exercise on patients with intensive care unit-acquired weakness (ICU-AW).
Design: Single-center retrospective study.
Subjects/Patients: Patients admitted to the ICU between January 2019 and March 2023 were enrolled in the ergometer group, and those admitted to the ICU between August 2017 and December 2018 were enrolled in the control group.
Methods: The ergometer group performed in-bed leg cycling exercises 5 times per week for 20 min from the day of ICU-AW diagnosis. Furthermore, the ergometer group received 1 early mobilization session per day according to the early mobilization protocol, whereas the control group received 1 or 2 sessions per day. The number of patients with recovery from ICU-AW at ICU discharge and improvement in physical functions were compared.
Results: Significantly more patients in the ergometer group recovered from ICU-AW than in the control group (87.0% vs 60.6%, p = 0.039). Regarding physical function, the ergometer group showed significantly higher improvement efficiency in Medical Research Council sum score (1.0 [0.7–2.1] vs 0.1 [0.0–0.2], p < 0.001).
Conclusion: In-bed leg cycling exercise, in addition to the early mobilization protocol, reduced the number of patients with ICU-AW at ICU discharge.
LAY ABSTRACT
Recent developments in intensive care have dramatically improved the short-term prognosis of critically ill patients; however, intensive care unit-acquired weakness (ICU-AW) is a specific systemic muscle weakness that occurs in these patients. ICU-AW has been demonstrated to reduce health-related quality of life, as well as contribute to cognitive impairment and functional disability, in survivors of critical illnesses for months to years. Therefore, the development of effective interventions to prevent and improve ICU-AW is required. The study showed that in-bed leg cycling exercises 5 times per week for 20 min in addition to usual care improved muscle strength in patients with ICU-AW. In-bed leg cycling exercises may be a potentially useful exercise therapy for patients with ICU-AW.
Key words: early mobilization; exercise; intensive care unit; muscle strength; muscle weakness; rehabilitation exercise.
Citation: JRM-CC 2023; 6: jrmcc18434. DOI: https://doi.org/10.2340/jrmcc.v6.18434
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Nov 14, 2023; Published: Dec 28, 2023
Correspondence address: Yohei Otaka, Department of Rehabilitation Medicine I, School of Medicine, Fujita Health University, 1-98 Dengakugakubo, Kutsukake-cho,Toyoake, Aichi 470-1192, Japan. E-mail: otaka119@mac.com
Competing interests and funding: The authors have no conflicts of interest to declare.
Recent developments in intensive care have dramatically improved the short-term prognosis of critically ill patients (1); however, ICU-AW is a specific systemic muscle weakness that occurs in these patients (2, 3).
ICU-AW is characterized by acute and symmetrical limb weakness that occurs after ICU admission in critically ill patients (4). It is associated with worse short-term outcomes, including increased mortality, duration of mechanical ventilation, and lengths of ICU and hospital stay (5, 6). Furthermore, ICU-AW has been established to reduce health-related quality of life (HRQOL) (7), as well as contribute to cognitive impairment (8) and functional disability (9), in survivors of critical illnesses for months to years. Therefore, ICU-AW is important in critically ill patients. The prevalence of ICU-AW has been reported to be 24–47% of patients on mechanical ventilation for more than 7 days (3, 10). Another systematic review showed that ICU-AW occurred in 40% of critically ill adult patients (11). Therefore, the development of effective interventions to prevent and improve ICU-AW is required.
Currently, there is no effective intervention for ICU-AW, and the reduction of risk factors and avoidance of deep sedation (12) are the first choices of management in clinical settings. Early mobilization, such as cycling, is a well-established intervention for critically ill patients. Cycling exercises are expected to improve proprioceptive sensitivity, muscle coordination, lower limb circulation, and range of motion through continuous repetitive motion. In addition, they can be safely performed in bed by critically ill patients without hemodynamic changes (13), making them one of the most versatile tools at a therapist’s disposal (13–15). A study examining the effects of in-bed cycling in critically ill patients who stayed in the ICU for more than 7 days showed that 20 min of cycling per day improved quadriceps muscle strength, 6-min walk distance (6 MWD), and HRQOL (15). In contrast, Nickels et al. (16) showed that in-bed cycling exercise for 30 min per day had no effect on the prevention of muscle atrophy, improvement of physical function, or HRQOL. Thus, the effects of in-bed cycling exercises in critically ill patients remain controversial.
We hypothesized that in-bed leg cycling exercises are effective for improving ICU-AW. The aim of this study was to retrospectively examine the effect of in-bed leg cycling exercise on ICU-AW in critically ill patients.
METHODS
Study design and setting
This was a single-center retrospective study conducted in the ICU of Fujita Health University Hospital. This is a closed ICU with 18 beds and is used as a general ICU for all departments and all ages. It is staffed by 3 intensivists and has a nurse-to-patient ratio of 1:2. In addition, 2 physical therapists (PTs) are on staff, and early mobilization is performed based on a standard protocol. Furthermore, a protocol to perform in-bed cycle exercises for ICU-AW patients was introduced in January 2019. This study was approved by the Medical Research Ethics Review Committee of the Fujita Health University (No. HM23-073, approved on 07 June 2023) and has been reported in accordance with STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) guidelines. The requirement for informed consent was waived due to the retrospective study design, and individuals who did not opt out were included.
Participants
Consecutive patients admitted to the ICU between August 2017 and March 2023 and diagnosed with ICU-AW were retrospectively enrolled. Patients admitted to the ICU between January 2019 and March 2023 after the introduction of in-bed cycling exercises were included in the ergometer group, and those admitted to the ICU between August 2017 and December 2018 were included in the control group. The exclusion criteria for both groups were as follows: (i) motor paralysis due to stroke or neuromuscular disease, (ii) lower limb amputation, (iii) death in the ICU, (iv) age less than 18 years, (v) inability to exercise due to rest restriction, (vi) not independent in daily activities prior to ICU admission, (vii) ICU-AW was diagnosed 1 day prior to ICU discharge, and (viii) missing data. In addition, the ergometer group excluded patients who (i) performed in-bed cycling exercises prior to the diagnosis of ICU-AW, (ii) had a diagnosis of deep vein thrombosis, and (iii) were deemed inappropriate to use an ergometer by the therapists.
In-bed leg cycling exercise and ICU early mobilization protocol
The patients in the ergometer group performed one session of in-bed leg cycling exercises per day at least 5 times per week for a maximum of 20 min from the day of ICU-AW diagnosis. We used 2 types of equipment: Terasu Ergo III PLUS-20 (Showa Denki, Osaka, Japan) and Escargo electric cycle machine (Wellup, Yokohama, Japan). In-bed cycling exercises were performed according to an in-bed leg cycling exercise protocol described in a previous study by Kimawi et al. (17) (Fig. 1). The in-bed leg cycling exercise protocol was characterized by a step-by-step approach involving four 5-min cycling sessions. In the first session, the intensity level was started at 3 watt, and on the next day, the intensity level was started at 1 level lower than the intensity level at the end of the previous day. The intensity level was increased incrementally in 1 step if 5 min of active cycling was possible at each interval. The intensity level was lowered by 1 level for the next interval if 5 min of active cycling was difficult due to fatigue or other reasons. If active cycling was difficult even at 3 watt, passive cycling was performed using the electric cycle machine Escargo. The PT constantly encouraged the patient to actively cycle. The PT could stop the session before the 20-min protocol was completed at their discretion if further exercise was difficult owing to fatigue or other reasons. In-bed cycling exercises were performed during the stay in the ICU.
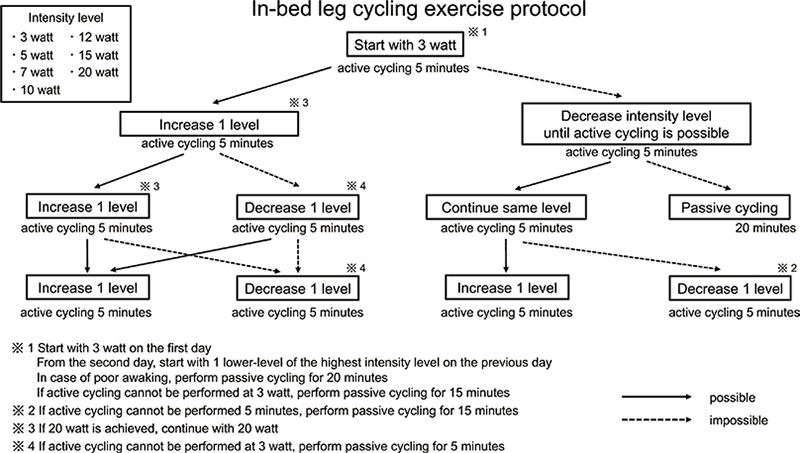
Fig. 1. In-bed leg cycling exercise protocol.
In addition to the cycling exercises, the ergometer group underwent 1 mobilization session per day according to the ICU early mobilization protocol (Fig. 2), whereas the control group underwent 1 or 2 sessions per day. The ICU early mobilization protocol was developed based on a previous report by Morris et al. (18–20). The protocol begins with chest physiotherapy and passive range of motion in step 0 and progresses step-by-step to ambulation in step 5. We proceeded with a step if the criteria for not proceeding with a step did not apply. In contrast, if the discontinuation criteria established during the implementation of rehabilitation were met, rehabilitation was discontinued.
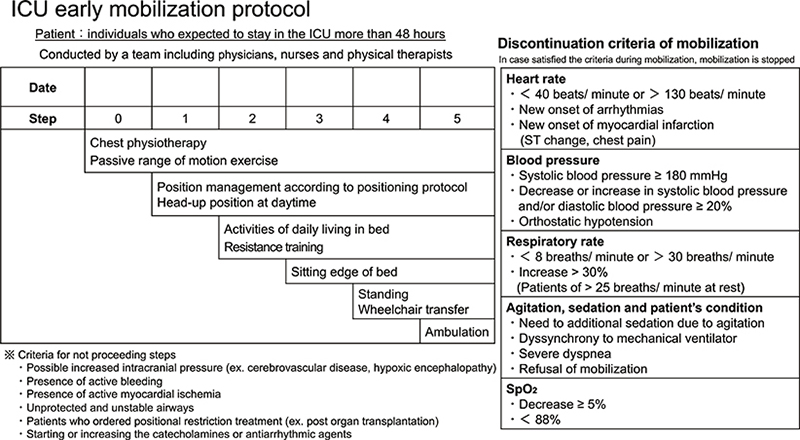
Fig. 2. ICU early mobilization protocol. ICU: intensive care unit; SpO2: saturation of percutaneous oxygen.
After discharge from the ICU, both groups underwent conventional physical therapy programs, including muscle strength exercises, sitting at the edge of the bed, standing, endurance training, and activities of daily living until hospital discharge.
Data collection
Patient data were retrieved retrospectively from electronic medical records. Data characteristics of all enrolled patients were collected, including age, sex, height, body weight, body mass index (BMI), acute physiology and chronic health disease classification system II score (APACHE II score) (21), sequential organ failure assessment score (SOFA score) (22), ICU admission diagnosis, reason for ICU admission, length of ICU stay, duration from ICU admission to first rehabilitation, duration from ICU admission to diagnosis ICU-AW, duration from diagnosis of ICU-AW to ICU discharge, need for mechanical ventilation, duration of mechanical ventilation in the ICU, duration of hospitalization, destination of patient after hospital discharge, Medical Research Council sum score (MRC sum score) (23) at diagnosis of ICU-AW and ICU discharge, grip strength, functional status score for the ICU (FSS-ICU) (24), and functional independence measure (FIM) at hospital discharge. The APACHE II score was the worst value within 24 h of ICU admission.
The definition criteria for ICU-AW proposed by Stevens et al. (4) were used. These defined ICU-AW as an MRC sum score of less than 48 points on 2 occasions separated by 24 h.
Outcome measure
The primary outcome was recovery from ICU-AW at ICU discharge. Secondary outcomes were improvement in the MRC sum score, grip strength, FSS-ICU score, FIM score at discharge, and discharge destination. The improvement efficiency of the MRC sum score, grip strength, and FSS-ICU score was calculated by dividing the difference in these measures at the diagnosis of ICU-AW and at ICU discharge by the same period.
Statistical analyses
Continuous variables are presented as medians and interquartile ranges (IQRs), whereas categorical variables are presented as numbers and percentages. The clinical characteristics, physical function, and discharge destination in both groups were compared using the Wilcoxon sum rank test or Fisher’s exact test depending on the type of variable. The effect size was calculated using G*power. All analyses were performed using JMP (version 13.2; SAS Institute Inc., Cary, NC, USA). P-values less than 0.05 were considered to indicate statistical significance.
RESULTS
After applying the inclusion and exclusion criteria to 7,489 consecutive patients who were admitted to the ICU during the study period, 23 patients in the ergometer group and 33 patients in the control group were enrolled for analysis (Fig. 3).
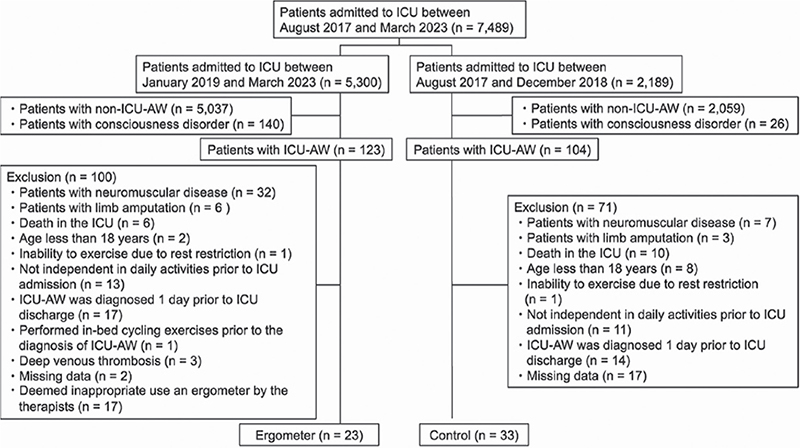
Fig. 3. Participants’ flow diagram. ICU: intensive care unit; ICU-AW: intensive care unit acquired weakness; ADL: activities of daily living.
The clinical characteristics of the enrolled patients are summarized in Table I. The BMI was significantly higher in the ergometer (23.6 [21.7–25.2] kg/m2) than in the control group (20.9 [18.4–23.1] kg/m2) (p = 0.009). The length of ICU stay (ergometer group: 33 [20.5–37.5] vs. control group: 13 [11–24] days, p < 0.001) and the duration from ICU admission to the diagnosis of ICU-AW (ergometer group: 13 [8.5–27.5] vs. control group: 6 [3–7] days, p < 0.001) were significantly longer in the ergometer group.
The in-bed cycling exercise data in the ergometer group are presented in Table II. A total of 284 in-bed cycling exercise sessions were conducted. The number of sessions performed by each patient was 8 [4–16] sessions. The total distance traveled during active cycling was 820 [490–1240] meters per session.
The results of the physical function tests in both groups are shown in Table III. The primary outcome of recovery from ICU-AW at ICU discharge was significantly larger in the ergometer group than in the control group (ergometer group: 87.0% vs. control group: 60.6%, p = 0.039, effect size = 0.77). Regarding the secondary outcomes, the ergometer group showed significantly higher improvement efficiency in the MRC sum score (ergometer group: 1.0 [0.7–2.1] vs. control group: 0.1 [0.0–0.2], p < 0.001, effect size = 1.48). In addition, the improvement efficiency of the MRC lower limb sum score was significantly higher in the ergometer group (0.6 [0.3–0.9]) than in the control group (0.1 [0.0–0.2], p < 0.001, effect size = 1.57), while there was no significant difference in the improvement efficiency of the MRC upper limb sum score (p = 0.382), FIM at discharge (p = 0.257), and discharge destination (p = 0.498) were not significantly different between the 2 groups (Table IV).
DISCUSSION
The main finding of this study was that in-bed leg cycling exercises reduced the number of patients with ICU-AW at ICU discharge and increased lower limb muscle strength in patients with ICU-AW, which supports our hypothesis. To the best of our knowledge, this is the first study to demonstrate the effectiveness of in-bed leg cycling exercises for improving muscle strength in patients with ICU-AW.
The improvement in the MRC score was found only in the lower limbs (not in the upper limbs), indicating that the difference in muscle strength between the 2 groups was attributed to the effect of in-bed cycling exercise which targeted the lower limbs. Previous studies have shown that in-bed cycling exercises improve acute muscle atrophy, quadriceps strength, 6 MWD, and HRQOL (15, 25). A meta-analysis showed that in-bed cycling exercises in critically ill patients can be safely performed but do not show significant effects on physical function, duration of mechanical ventilation, duration of ICU stay, duration of hospitalization, quality of life, and hospital mortality (26). Although the participants included in the meta-analysis were critically ill, patients with ICU-AW were not specifically included, and there have been no studies of only patients with ICU-AW.
Although the mechanism of ICU-AW has been reported, it is not fully understood. Previous studies identified several potential risk factors of ICU-AW, including multiple organ failure, immobility, hyperglycemia, use of corticosteroids and neuromuscular blockers, and malnutrition (2, 27–29). Although using analgesics and sedatives induces circulatory depression and tissue perfusion defects (30, 31), appropriate analgesic and sedative management is recommended for critically ill patients to relieve pain and other treatment stress (32). In addition, the mechanisms of rapid muscle weakness have been shown to include decreased membrane excitability associated with sodium channel abnormalities (33), damage to the blood-nerve barrier, damage to axonal fibers caused by inflammatory mediators, and damage to muscles due to mitochondrial oxidative stress (34). ICU-AW has been classified into 3 categories: critical illness myopathy (CIM), critical illness polyneuropathy (CIP), and critical illness neuromyopathy (CINM) (4). However, it has been proposed that ICU-AW can also induce transitory reductions in muscle strength due to muscle deconditioning with normal nerve conduction velocity and compound motor action potential (35). Under the current concept of ICU-AW, such a variety of pathologies would be categorized as ICU-AW. The short-term improvement in muscle strength in this study suggests that many of the patients in the present study had pathologies that resulted in transitory reductions in muscle strength due to muscle deconditioning. In-bed cycling exercises are expected to improve proprioceptive sensitivity muscle coordination, and lower limb circulation through continuous repetitive motion. Factors that alter muscle output during muscle strength exercise therapy during the first 2 weeks include changes in the type and total number of motor units mobilized for muscle contraction and the frequency of alpha motor nerve firing, which are central nervous system factors (36). Rhythmic in-bed cycling exercises and the use of an in-bed leg cycling exercise protocol, as in this study, may improve cell perfusion defects, membrane hyperexcitability associated with sodium channelopathies, and damage to the blood-nerve barrier, resulting in an increase in the number of motor units mobilized for muscle contraction and in the frequency of alpha motor nerve firing, thereby improving muscle strength.
This study has some potential limitations. First, it was a single-center retrospective study of patients with critical illnesses, and the results may not necessarily be generalizable to groups with dissimilar demographic characteristics. Second, the BMI was significantly higher in the ergometer group, and the patient backgrounds were not the same. Thus, the effects of the in-bed cycling exercises could have been influenced by the patients’ characteristics. Third, although approaches to CIM, CIP, CINM, and muscle deconditioning may differ according to their respective characteristics, electrophysiological testing was not performed in this study, and it was not possible to explore the relationship between the pathophysiological conditions for muscle weakness and the exercise effect. However, this study provides valuable information on the improvement of muscle strength in patients with ICU-AW by combining in-bed leg cycling exercises with early mobilization programs. Further randomized controlled trials of patients with specific ICU-AW types will confirm the effects of in-bed leg cycling exercise for patients with ICU-AW.
In conclusion, in-bed leg cycling exercises, in addition to the early mobilization protocol, significantly improved the efficiency of the MRC sum scores and MRC lower limb sum scores and reduced the number of patients with ICU-AW at ICU discharge. In-bed leg cycling exercises may be a potentially useful exercise therapy for improving muscle strength in critically ill patients with ICU-AW.
ACKNOWLEDGMENTS
This study was approved by the Medical Research Ethics Review Committee of the Fujita Health University (No. HM23-073, approved on 07 June 2023).
REFERENCES
- Cuschieri J, Johnson JL, Sperry J, West MA, Moore EE, Minei JP, et al. Benchmarking outcomes in the critically injured trauma patient and the effect of implementing standard operating procedures. Ann Surg 2012; 255: 993–999. https://doi.org/10.1097/SLA.0b013e31824f1ebc
- Schefold JC, Bierbrauer J, Weber-Carstens S. Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle 2010; 1: 147–157. https://doi.org/10.1007/s13539-010-0010-6
- Schweickert WD, Hall J. ICU-acquired weakness. Chest 2007; 131: 1541–1549. https://doi.org/10.1378/chest.06-2065
- Stevens RD, Marshall SA, Cornblath DR, Hoke A, Needham DM, de Jonghe B, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med 2009; 37: S299–S308. https://doi.org/10.1097/CCM.0b013e3181b6ef67
- Hermans G, Van Mechelen H, Clerckx B, Vanhullebusch T, Mesotten D, Wilmer A, et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2014; 190: 410–420. https://doi.org/10.1164/rccm.201312-2257OC
- Ali NA, O’Brien JM, Jr, Hoffmann SP, Phillips G, Garland A, Finley JC, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med 2008; 178: 261–268. https://doi.org/10.1164/rccm.200712-1829OC
- Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med 2014; 42: 849–859. https://doi.org/10.1097/CCM.0000000000000040
- Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010; 304: 1787–1794. https://doi.org/10.1001/jama.2010.1553
- Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364: 1293–1304. https://doi.org/10.1056/NEJMoa1011802
- Truong AD, Fan E, Brower RG, Needham DM. Bench-to-bedside review: mobilizing patients in the intensive care unit – from pathophysiology to clinical trials. Crit Care 2009; 13: 216. https://doi.org/10.1186/cc7885
- Appleton RT, Kinsella J, Quasim T. The incidence of intensive care unit-acquired weakness syndromes: a systematic review. J Intensive Care Soc 2015; 16: 126–136. https://doi.org/10.1177/1751143714563016
- Needham DM, Korupolu R, Zanni JM, Pradhan P, Colantuoni E, Palmer JB, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil 2010; 91:536–542. https://doi.org/10.1016/j.apmr.2010.01.002
- Camargo Pires-Neto R, Fogaca Kawaguchi YM, Sayuri Hirota A, Fu C, Tanaka C, Caruso P, et al. Very early passive cycling exercise in mechanically ventilated critically ill patients: physiological and safety aspects – a case series. PLoS One 2013; 8: e74182. https://doi.org/10.1371/journal.pone.0074182
- Kho ME, Martin RA, Toonstra AL, Zanni JM, Mantheiy EC, Nelliot A, et al. Feasibility and safety of in-bed cycling for physical rehabilitation in the intensive care unit. J Crit Care 2015; 30: e1411–e1415. https://doi.org/10.1016/j.jcrc.2015.07.025
- Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, Troosters T, et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med 2009; 37: 2499–2505. https://doi.org/10.1097/CCM.0b013e3181a38937
- Nickels MR, Aitken LM, Barnett AG, Walsham J, King S, Gale NE, et al. Effect of in-bed cycling on acute muscle wasting in critically ill adults: a randomised clinical trial. J Crit Care 2020; 59: 86–93. https://doi.org/10.1016/j.jcrc.2020.05.008
- Kimawi I, Lamberjack B, Nelliot A, Toonstra AL, Zanni J, Huang M, et al. Safety and feasibility of a protocolized approach to in-bed cycling exercise in the intensive care unit: quality improvement project. Phys Ther 2017; 97: 593–602. https://doi.org/10.1093/ptj/pzx034
- Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med 2008; 36: 2238–2243. https://doi.org/10.1097/CCM.0b013e318180b90e
- Adler J, Malone D. Early mobilization in the intensive care unit – A systematic review. Cardiopulm Phys Ther J 2012; 23: 1. https://doi.org/10.1097/01823246-201223010-00002
- Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373: 1874–1882. https://doi.org/10.1016/S0140-6736(09)60658-9
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–829. https://doi.org/10.1097/00003465-198603000-00013
- Vincent J-L, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707–710. https://doi.org/10.1007/BF01709751
- Kleyweg RP, van der Meché FGA, Meulstee J. Treatment of guillain-Barré syndrome with high-dose gammaglobulin. Ann Neurol 1988; 38: 1639–1641. https://doi.org/10.1212/WNL.38.10.1639
- Zanni JM, Korupolu R, Fan E, Pradhan P, Janjua K, Palmer JB, et al. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care 2010; 25: 254–262. https://doi.org/10.1016/j.jcrc.2009.10.010
- Hickmann CE, Castanares-Zapatero D, Deldicque L, Van den Bergh P, Caty G, Robert A, et al. Impact of very early physical therapy during septic shock on skeletal muscle: a randomized controlled trial. Crit Care Med 2018; 46: 1436–1443. https://doi.org/10.1097/CCM.0000000000003263
- Takaoka A, Utgikar R, Rochwerg B, Cook DJ, Kho ME. The efficacy and safety of in-intensive care unit leg-cycle ergometry in critically ill adults. A systematic review and meta-analysis. Ann Am Thorac Soc 2020; 17: 1289–1307. https://doi.org/10.1513/AnnalsATS.202001-059OC
- de Jonghe B, Lacherade JC, Sharshar T, Outin H. Intensive care unit-acquired weakness: risk factors and prevention. Crit Care Med 2009; 37: S309–S315. https://doi.org/10.1097/CCM.0b013e3181b6e64c
- Lee CM, Fan E. ICU-acquired weakness: what is preventing its rehabilitation in critically ill patients? BMC Med 2012; 10: 115. https://doi.org/10.1186/1741-7015-10-115
- Zink W, Kollmar R, Schwab S. Critical illness polyneuropathy and myopathy in the intensive care unit. Nat Rev Neurol 2009; 5: 372–379. https://doi.org/10.1038/nrneurol.2009.75
- Kress JP, Hall JB. Sedation in the mechanically ventilated patient. Crit Care Med 2006; 34: 2541–2546. https://doi.org/10.1097/01.CCM.0000239117.39890.E3
- Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013; 41: 263–306. https://doi.org/10.1097/CCM.0b013e3182783b72
- Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018; 46: e825–e873. https://doi.org/10.1097/CCM.0000000000003299
- Cannon SC, Bean BP. Sodium channels gone wild: resurgent current from neuronal and muscle channelopathies. J Clin Invest 2010; 120: 80–83. https://doi.org/10.1172/JCI41340
- Batt J, Mathur S, Katzberg HD. Mechanism of ICU-acquired weakness: muscle contractility in critical illness. Intensive Care Med 2017; 43: 584–586. https://doi.org/10.1007/s00134-017-4730-3
- Farhan H, Moreno-Duarte I, Latronico N, Zafonte R, Eikermann M. Acquired muscle weakness in the surgical intensive care unit: nosology, epidemiology, diagnosis, and prevention. Anesthesiology 2016; 124: 207–234. https://doi.org/10.1097/ALN.0000000000000874
- Basmajian JV. Muscle alive. 5th ed. Baltimore, MD: Williams & Wlkins; 1985, p. 19–251.