Background: Most patients with polio recover from the initial infection, but develop muscle weakness, pain and fatigue after 15–40 years, a condition called post-polio syndrome. Although poliovirus has been almost eliminated, 12–20 million people worldwide still have polio sequelae. The pain is described mainly as nociceptive, but some patients experience neuropathic pain. The aim of this study was to further characterize post-polio pain.
Patients and methods: A total of 20 patients with post-polio syndrome participated in the study. Physical examination was performed, and questionnaires containing pain drawing and visual analogue scales (VAS) for pain intensity during rest and motion and VAS for fatigue were completed. A walk test was performed to evaluate physical performance.
Results: Pain intensity was high (42/100 on the VAS at rest and 62/100 while moving). The pain was localized in both joints and muscles. Pain in the muscles was of “deep aching” character, included “muscle cramps” and was located mainly in polio-weakened limbs.
Conclusion: Muscle pain in patients with post-polio syndrome does not fulfil the criteria for either nociceptive or neuropathic pain; thus, it is suggested that the pain is termed “post-polio muscular pain”. The intensity of post-polio muscular pain is higher while moving, but does not influence physical function, and is separate from fatigue.
Key word: post-polio syndrome; muscle pain; visual analogue pain scale.
Accepted Nov 12, 2021; Published Jan 22, 2022
JRM-CC 2022; 5: jrmcc00077
Correspondence address: Eva Melin, Division of Rehabilitation Medicine, Department of Clinical Sciences, Danderyd Hospital, Karolinska Institutet, Stockholm, Sweden. E-mail: eva.melin@ki.se
Lay Abstract
INTRODUCTION
Poliomyelitis, often called polio, is caused by an enteroviral infection transmitted faeco-orally; for example, via contaminated food or water. Polio is considered a paediatric disease, as it mainly affects children under the age of 5 years. Appproximately 1 out of 100 infections leads to paralysis due to affection of the anterior horn cells in the spinal cord. Among paralysed patients, 5–10% will experience hypoventilation due to paralysed respiratory muscles, which can lead to death (1–3). In 1955 a poliovirus vaccine became available, and polio has since been eradicated from most countries worldwide, and is now endemic only in Afghanistan and Pakistan (4).
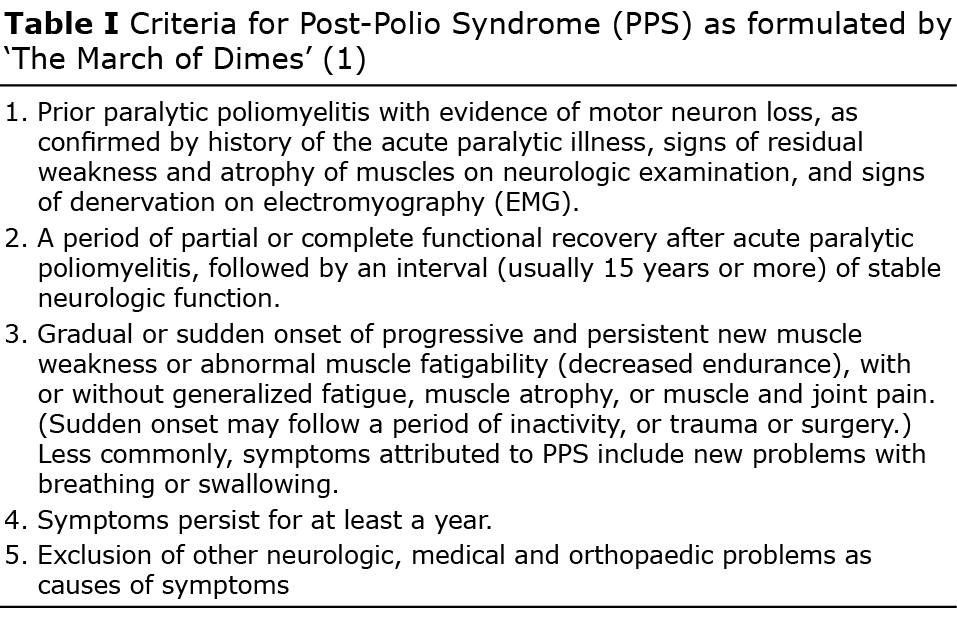
Most people survive the acute phase of poliomyelitis, but many have persistent neurological dysfunction. After a period of at least 15 years, increasing symptoms may occur, including increased muscle weakness and atrophy, joint and muscle pain and fatigue. This condition is known as post-polio syndrome (PPS). The March of Dimes has formulated the most commonly used criteria for PPS, which are listed in Table I (1).
Worldwide, 12–20 million people have sequelae of poliomyelitis, according to Post-Polio Health International (5). In Sweden PPS is one of the most common neuromuscular disorders and it is one of the most prevalent motor neurone disorders in the USA. The underlying mechanism of PPS is not fully understood (6). However, it is known that ongoing denervation of motor units occurs, compensated by reinnervation. This mechanism reaches an endpoint at which reinnervation is not possible and thus uncompensated denervation results in a decrease in muscle tissue, followed by muscle weakness (5). Other causes, such as a persistent virus or immunological mechanisms, have also been suggested (7).
Since acute polio is decreasing (8), most physicians never encounter a case of polio and thus often lack the experience to diagnose it and its long-term pathology. PPS is a relatively newly recognized disorder with a wide range of possible presenting symptoms, and thus its diagnosis and management are challenging (1).
One of the most common symptoms in PPS is pain (9). In a study of 126 patients with PPS, muscle pain was found in two-thirds of patients (10). Pain is defined as: “An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (11). Pain can occur as a physiological response to tissue damage and inflammation, called nociceptive pain, as it is derived from nociceptors. Nociceptors are high-threshold sensory receptors of the peripheral somatosensory nervous system, which is capable of transducing and encoding noxious stimuli (12). Another type of pain is neuropathic pain, which is a direct consequence of a lesion or disease affecting the somatosensory system (13). In contrast to nociceptive pain, neuropathic pain can be described as non-beneficial (14). In PPS the pain has been described as of “deep aching” character, similar to that experienced during acute paralytic poliomyelitis, usually occurring later in the day (9), but other types of pain have also been found (15). The pain intensity is high and most often located in parts of the body that were affected by polio (5). However, the quality of life (QoL) is not affected by the pain intensity (16). Recent studies have described PPS pain as mainly of nociceptive character, which should indicate that there is tissue damage (6). On the other hand, polio is an infection affecting the motor part of the nervous system. The pain could thus be expected to originate from overuse of weakened muscles, or secondary conditions, such as arthritis or ruptured tendons. Since the polio infection affects the anterior horn cells, i.e. the motor part of the nervous system, no neuropathic pain is anticipated. Taking this into account the appellation of pain in PPS is uncertain. The aim of this study was to further characterize PPS pain.
PATIENTS AND METHODS
A cross-sectional study design was used. A total of 20 patients were recruited from the post-polio outpatient clinic at Danderyd University Hospital, Stockholm, Sweden. All patients fulfilled the criteria for PPS according to the March of Dimes (Table I). The diagnosis had also been confirmed previously by neurophysiology. The study recruited all patients who had been in contact with the post-polio outpatient clinic within the last 2 years, who had presented with pain, and who did not have any exclusion criteria. Exclusion criteria were: patients without pain, age over 74 years, wheelchair users, and patients with other diseases clearly causing the pain.
The patients were contacted by phone and asked if they would like to participate in the study. If they agreed, they were sent a letter with information about the study and questionnaires.
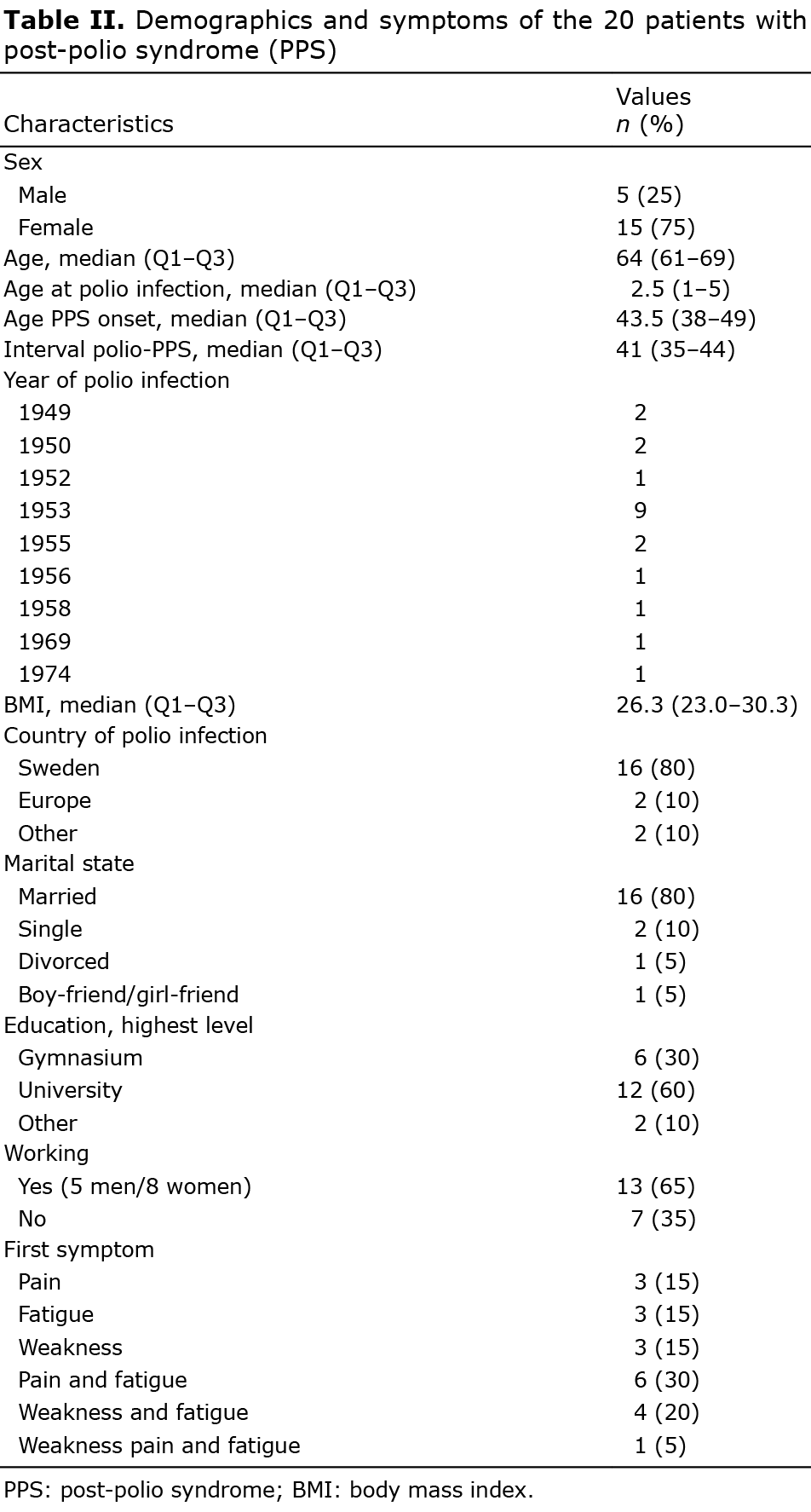
In order to obtain a clear description and characterization of the pain in PPS, the patients completed a pain drawing, evaluating 9 different types of pain, on which they marked both localization and quality of pain. The questionnaire also included information about the population’s demographic characteristics (Table II).
An experienced neurologist (KB) performed physical examination of the patients, including a neurological examination. Muscle weakness, atrophy, and tendon reflexes were evaluated. Sensory testing was performed to evaluate sensory function in the extremities, including pain, temperature, vibration and cold intolerance. The medical records were examined to determine the location of acute polio weakness.
Scales and inventories
Two visual analogue scales for pain (VAS-pain) were created as a 100-mm lines for assessing the current subjective pain intensity experienced at rest and while moving (where 0 mm represented “no pain” and 100 mm the “worst imaginable pain”) (17, 18).
A visual analogue scale fatigue (VAS-fatigue) was created to evaluate the fatigue experienced on a100-mm horizontal line (where 0 mm represented “not tired at all” and 100 mm “extremely tired”) (19).
A pain drawing was used to differentiate between the different qualities of pain. A picture of the body was constructed, on which the participant could indicate the pain experienced in a specific part of the body. This information was categorized according to location of acute polio weakness, determined from the patient’s reports, and the different qualities were assessed.
The six-minute walk test (6MWT) was used to measure exercise tolerance (20). During the 6MWT patients are asked to walk as far as possible during 6 min, according to the guidelines of the American Thoracic Society (ATS) (21). The 6MWT has been frequently used for clinical and research purposes, as well as for PPS research (22, 23).
Statistical analysis
The statistical programme SPSS (IBM SPSS Statistics 21) was used to calculate median values and 25th and 75th percentiles for VAS-pain intensity, VAS-fatigue and the 6MWT. Correlations were analysed using Spearman’s test, as fewer than 30 patients were included in the study (24).
Ethics
Ethics approval was obtained from the regional Ethical Review Board in Stockholm, Sweden (Dnr 2014/1509-31).
RESULTS
Pain
A total of 20 patients (15 women and 5 men) completed the study. Sixteen patients were born in Sweden. All of the men were working and 8 of the women were working. Almost half of the patients had had acute polio in 1953. A completeoverview of the demographic data is shown in Table II.
The median VAS for pain intensity at rest was 42 mm (range 25–63 mm) and for pain intensity while moving was 65 mm (range 55–88 mm). The median VAS for fatigue was 62 mm (range 35–72 mm). The median 6MWT was 358 m (range 246–414 m) (n = 19).
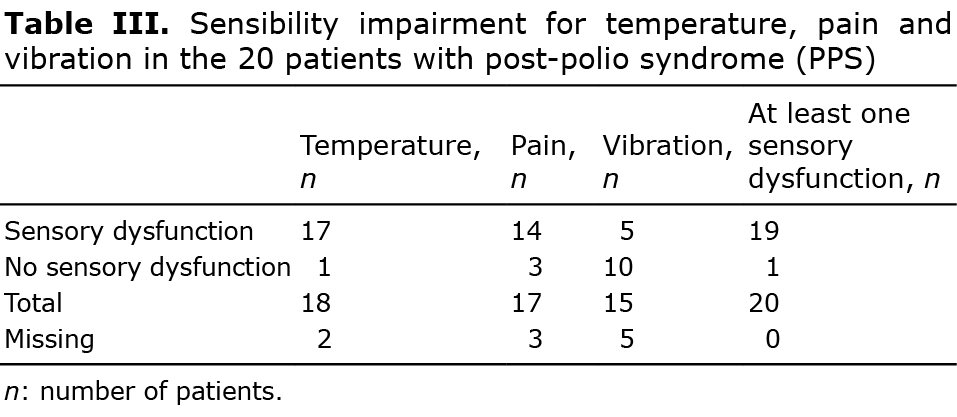
Nineteen patients had sensory dysfunction, 11 of whom reported cold intolerance. Data on sensibility impairment for temperature, pain and vibration are shown in Table III.
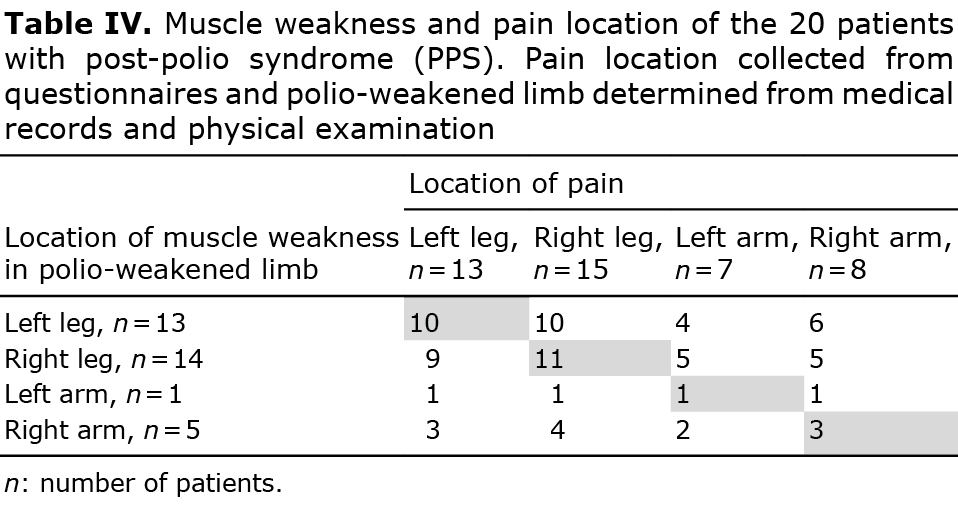
Ten patients had muscle weakness caused by polio infection in early life, in the left leg, and experienced pain in the same leg (Table IV). Eleven patients had weakness in the right leg and experienced pain in the same leg. One patient had weakness in the left arm and experienced pain in the same arm, and 3 patients had weakness in the right arm and experienced pain in the same arm (Table IV).
Fifteen patients also reported having pain in the torso, which was of “deep aching” character in 12 of the patients.
Quality of pain
Fifteen patients reported limb pain and pain in the joints (Table V). All except 1 patient reported muscle pain in the polio-weakened limb. The most commonly reported quality of limb pain by 9 of the 14 patients was “deep aching” pain and “’muscle cramps” (Table V). Nine patients had limb pain in the non-polio-weakened limb.
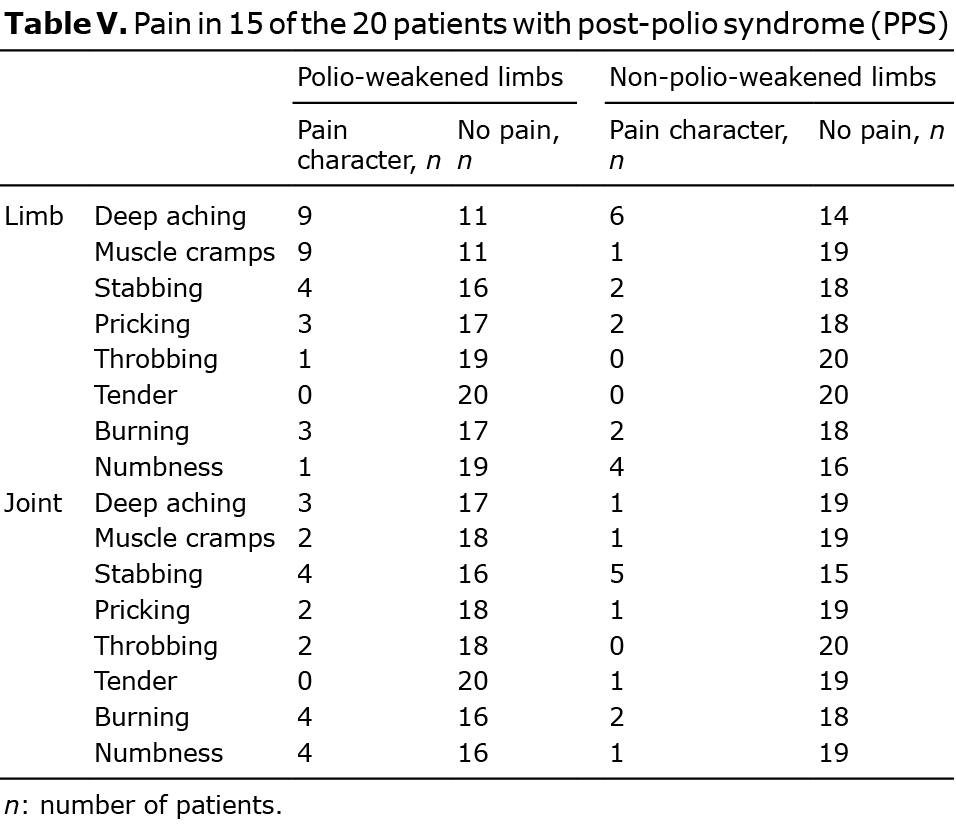
Joint pain was more often found in the polio-weakened limb (Table V). In the patients with joint pain in the polio-weakened limb, the quality of pain was evenly distributed regarding character. The diagnosis of polio in the weakened limbs was confirmed previously by neurophysiological examination.
Correlations
The VAS score for pain at rest correlated moderately with age at onset of PPS (r=0.548, n = 20, p = 0.012). None of the results of the VAS scales for pain intensity correlated with the outcome of VAS fatigue, 6MWT or body mass index (BMI).
DISCUSSION
The aim of this study was to further characterize PPS pain. The results show that pain is common in PPS, which corroborates the results of earlier studies (6, 9, 16, 25–27). The mean pain intensity was high and increased while moving, in accordance with the results of an earlier study (16). Pain was most often described as “deep aching”, as also described in an earlier study by Widar & Ahlstrom (15). This pain and “muscle cramps” were mostly localized in the limbs with muscle weakness caused by polio. Thus, this “’deep aching” pain is suggested to originate from polio-weakened muscles, which is further supported by the common perception of “muscle cramps”. However, joint pain was as equally present as muscle pain. This contradicts earlier data from the study by Widar & Ahlstrom (25), in which the pain was described as located mainly in the joints. In the current study, patients had pain in multiple locations, which is in accordance with earlier studies (15, 25).
The pain in PPS was more often present in the polio-weakened limb. The “deep aching” pain combined with “muscle cramps” are not usual characteristics of a nociceptive pain. This might be due to overuse or inactivity, or secondarily to deformities due to weak muscles. Since nociceptive pain is caused by damage to body tissue (28), this could mean that the pain starts as nociceptive, even if its characteristics are unusual. Neuropathic pain is defined as pain caused by a lesion or disease of the somatosensory nervous system (29). Even though the patients often had sensory dysfunction, this did not clearly correlate with innervation of a peripheral nerve. Furthermore, the quality of the pain is not typical for neuropathic pain. The pain appears in a part of the body with a recovered motor-nerve injury, not a sensory-nerve injury. Thus, it should not be neuropathic pain, in the classic sense or definition. Other possibilities are that the close proximity of the anterior and posterior horn cells in the spinal cord has a secondary effect on the sensory system, or that the atrophy and structural changes in the polio limbs result in secondary damage to sensory nerves. Hence, one cannot fully exclude that the pain of the patients with PPS might have a neuropathic component. Werhagen & Borg (16) described neuropathic pain in 10% of patients with PPS. This pain was of another type than the one described in the current patients. Thus, the pain is neither convincingly nociceptive nor neuropathic, but may be due to a combination and/or a disturbed pain modulation. The background for this is unclear and further research is necessary to evaluate the character and origin of this pain.
It is suggested that the deep aching pain in combination with muscle cramps, as described in this study and other studies (15, 25) and located in the polio-weakened limb is specific to PPS. We therefore suggest naming the pain “post-polio muscular pain” (PPMP). Melin and co-workers (30) showed a high level of prostaglandin enzymes in blood vessels in muscular tissue, which could indicate a systemic infection and explain the deep aching pain found in the muscles. Future studies for achieving analgesia by treatment with prostaglandin blockers (for example non-steroidal anti-inflammatory drugs; NSAIDs) is of great interest. If achieved, it indicates a peripheral cause of the pain. Treatment with intravenous Ig (IvIg) has been shown to have an effect on pain in PPS (31). IvIg was given in doses high enough to cross the blood–brain barrier on the assumption that the inflammatory process in PPS was localized in the central nervous system (CNS), due to findings of increased cytokine level in the cerebrospinal fluid (22). However, if the pain is peripheral, one would not need high doses of IvIg in order to achieve analgesia.
No statistically significant correlations between VAS pain intensity and 6MWT scores were found, suggesting that, although the patients experienced high pain intensity, it did not affect their physical performance. Measurement of the intensity of pain while moving and at rest showed that patients experienced more pain while moving. This is in accordance with a previous study in which patients with PPS experienced high pain intensity, but did not have decreased activity or QoL (16). In addition, no correlation was found between VAS-fatigue and pain intensity scores. This corresponds to the results of a study by Östlund et al. (27), which found that pain and fatigue were not strongly correlated, but is in conflict with data from a study by Sheth et al. (32) indicating a weak correlation between fatigue and pain. Further research is needed on this topic.
In the study by Werhagen & Borg (6), the age at which primary polio infection occurred was important for developing pain. However, the current study found that the age of developing PPS, not the age of polio infection, correlated mildly with the pain (r=0.548). The reason for this is unclear; it is possible that older patients may have developed effective coping strategies for the pain.
It was possible to assess the quality of pain by using the pain drawings. This method of describing the pain is different and more qualitative than data from earlier studies (15, 25, 26). From the interview and physical examination, the pain was not considered neuropathic. On the other hand, the pain drawings showed that some of the patients reported pain characteristics that might be of a neuropathic type. In addition, the intensity of pain was measured as a total perceived pain, and the intensity of pain may differ in different locations, as shown by data from a study by Stoelb (26). Thus, the methods used in the study may not be sensitive enough to determine the full character of the pain. In the study by Werhagen & Borg (6) 10% of patients reported neuropathic pain due to other neurological conditions; for example, compression neuropathies or disc hernias. The patients in the current study did not report this. However, the patients in the current study may not be representative of the entire group of patients with PPS, since they were all able to walk; hence this is a limitation of the current study.
This study focussed on the quality of pain; however, a great number of patients had sensory dysfunction, mainly decreased sensibility to temperature. It is not known why these patients have sensory dysfunction, as the region affected should be limited to the motor part of the nervous system. Other conditions, such as polyneuropathy, might be considered. In order to analyse sensibility impairment more thoroughly, one should perform sensory examination by means of semi-quantitative methods (17, 33).
Further limitations of the current study are the small number of patients. The results should therefore be confirmed in larger studies. In addition, the patients were recruited from the outpatient clinic, which might result in the study patients having more severe pain than the average post-polio patient.
In conclusion, pain in PPS is mostly of deep aching character and muscle cramps, localized in the polio-weakened limb. This type of pain seems to be specific to PPS. It is suggested that this pain is termed post-polio muscular pain (PPMP). Future studies should aim to characterize PPMP and to analyse the influence of motor and sensory dysfunction on the pain.
ACKNOWLEDGEMENTS
The authors thank Biomedical scientist Lisbet Broman for skilful technical assistance, and registered nurse Gunilla Forssberg and Fay Visser for their skilful assistance in collecting the data.
The authors have no conflicts of interest to declare.
REFERENCES
- March of Dimes. Identifying best practices in diagnosis and care. In: Dimes Mo, editor. International conference on post-poliosyndrome. White Plains: March of Dimes; 2000, p. 5–11.
- Melnick JL. Current status of poliovirus infections. Clin Microbiol Rev 1996; 9: 293–300.
- Howard RS, Wiles CM, Spencer GT. The late sequelae of poliomyelitis. Q J Med 1988; 251 : 219–232.
- Global polio eradication initiative applauds WHO African region for wild polio-free certification 2020. [updated 2020 Aug 25; cited 2021 Nov 28] Available from: https: //www.who.int/news/item/25-08-2020-global-polio-eradication-initiative-applauds-who-african-region-for-wild-polio-free-certification.
- Gonzalez H, Olsson T, Borg K. Management of postpolio syndrome. The Lancet Neurol 2010; 9: 634–642.
- Werhagen L, Borg K. Analysis of long-standing nociceptive and neuropathic pain in patients with post-polio syndrome. J Neurol 2010; 257: 1027–1031.
- Melin E. Post-polio syndrome: analysis of inflammation and immune modulation. [Dissertation] Inst för kliniska vetenskaper, Danderyds sjukhus/Department of Clinical Sciences, Danderyd Hospital; 2014.
- Poliomyelitis fact-sheets [Internet] 2021 [updated 2019 July 22; cited 2021 Nov 28]. https://www.who.int/news-room/fact-sheets/detail/poliomyelitis.
- Trojan DA, Cashman NR. Post-poliomyelitis syndrome. Muscle Nerve 2005; 31: 6–19.
- Vasiliadis H-M, Collet J-P, Shapiro S, Venturini A, Trojan DA. Predictive factors and correlates for pain in postpoliomyelitis syndrome patients. Arch Phys Med Rehabil 2002; 83: 1109–1015.
- Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 2020; 161: 1976–1982.
- IASP Terminology [Internet] 2021 [updated 2017 Dec 14; cited 2021 Nov 28]. Available from: iasp-pain.org/resources/terminology/#nociceptor.
- Merskey H, Bogduk N. Classification of chronic pain : descriptions of chronic pain syndromes and definitions of pain terms. 2nd edn. Seattle: IASP Press; 1994.
- Taverner T. Neuropathic pain: an overview. Br J Neurosci Nurs 2014; 10: 116–123.
- Widar M, Ahlstrom G. Experiences and consequences of pain in persons with post-polio syndrome. J Adv Nurs 1998; 28: 606–613.
- Werhagen L, Borg K. Impact of pain on quality of life in patients with post-polio syndrome. J Rehabil Med 2013; 45: 161.
- Huskisson EC. Measurement of pain. Lancet 1974; 304: 1127–1131.
- Joyce CRB, Zutshi DW, Hrubes V, Mason RM. Comparison of fixed interval and visual analogue scales for rating chronic pain. Eur J Clin Pharmacol 1975; 8: 415–420.
- Vasconcelos Jr OM, Prokhorenko OA, Kelley KF, Vo AH, Olsen CH, Dalakas MC, et al. A comparison of fatigue scales in postpoliomyelitis syndrome. Arch Phys Med Rehabil 2006; 87: 1213–1237.
- Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12–minute walking tests in respiratory disease. Br Med J (Clinl Res edn) 1982; 284: 1607–1608.
- Enright PL. The Six-Minute Walk Test. Respiratory care 2003; 48: 783–785.
- Gonzalez H, Sunnerhagen KS, Sjöberg I, Kaponides G, Olsson T, Borg K. Intravenous immunoglobulin for post-polio syndrome: a randomised controlled trial. Lancet Neurol 2006; 5: 493–500.
- Vreede KS, Henriksson J, Borg K, Henriksson M. Gait characteristics and influence of fatigue during the 6-minute walk test in patients with post-polio syndrome. J Rehabil Med 2013; 45: 924.
- Chan YH. Biostatistics 101: data presentation. Singapore Med J 2003; 44: 280.
- Widar M, Ahlstrom G. Pain in persons with post-polio: the Swedish version of the Multidimensional Pain Inventory (MPI). Scand J Caring Sci 1999; 13: 33–40.
- Stoelb BL, Carter GT, Abresch RT, Purekal S, McDonald CM, Jensen MP. Pain in persons with postpolio syndrome: frequency, intensity, and impact. Arch Phys Med Rehabil 2008; 89: 1933–1940.
- Östlund G, Wahlin Å, Sunnhagen KS, Borg K. Vitality among Swedish post-polio patients: a physiological phenomenon. 2008. J Rehabil Med 2008; 40: 709–714.
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain (Amsterdam) 2015; 156: 1003–1007.
- Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain (Amsterdam) 2019; 160: 53–59.
- Melin E, Lindroos E, Lundberg IE, Borg K, Korotkova M. Elevated expression of prostaglandin E2 synthetic pathway in skeletal muscle of prior polio patients. J Rehabil Med 2014; 46: 67–72.
- Gonzalez H, Sunnerhagen KS, Sjoberg I, Kaponides G, Olsson T, Borg K. Intravenous immunoglobulin for post-polio syndrome: a randomised controlled trial. Lancet Neurol 2006; 5: 493–500.
- Sheth MS, Sharma SS, Jadav R, Ghoghari B, Vyas NJ. Prevalence of post polio syndrome in Gujarat and the correlation of pain and fatigue with functioning in subjects with post polio syndrome. Ind J Physiother Occupat Ther 2014; 8: 230–235.
- Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain 2013; 154: 1807.