ORIGINAL REPORT
HIGH-VOLUME AND HIGH-INTENSITY FUNCTIONAL TRAINING IN PATIENTS WITH MULTIPLE SCLEROSIS: A PILOT STUDY ON FEASIBILITY AND FUNCTIONAL CAPACITY
Tom C. A. DERIKX, MSc.1, Ingrid M. H. BRANDS, MD, PhD1, Arne T. GOEDHART1, Wouter H. HOENS1, Majanka H. HEIJENBROK–KAL, PhD2,3 and Rita H. J. G. VAN DEN BERG–EMONS, PhD2
From the 1Libra Rehabilitation & Audiology, Eindhoven, The Netherlands, 2Erasmus University Medical Centre, Rotterdam, The Netherlands and 3Rijndam Rehabilitation, Rotterdam, The Netherlands
Objective: To evaluate the feasibility of a high-volume and high-intensity functional training programme in patients with multiple sclerosis (MS), and to explore whether functional capacity improves. A further objective was to explore changes in muscle strength and aerobic capacity.
Methods: This pilot study comprised a 12-week intervention, with an 8-week follow-up period. The intervention consisted of 3 weekly 3-h training sessions, comprising functional resistance-, endurance-, and skills training. Feasibility (questionnaire), functional capacity (Timed Up and Go Test, 10-Meter Walk Test, and 6-Minute Walk Test), aerobic capacity (cardiopulmonary exercise test) and muscle strength (1 repetition maximum (RM) leg press) were evaluated.
Results: Seven patients completed the study. Patients attended a mean of 93% of the training sessions. One adverse event was reported, which was not related to the training programme. Patients scored positive or very positive on 86% of the feasibility aspects and scored an overall grade of 8.9 on a scale of 1–10 regarding satisfaction with the training programme. Functional capacity, aerobic capacity, and muscle strength seemed to be improved after the training programme, but the improvements were not always sustained.
Conclusion: This new high-volume and high-intensity functional training programme appeared to be feasible in patients with MS, and may improve their functional capacity, aerobic capacity and muscle strength. A large-scale controlled trial over a longer period of time is required to evaluate the added value of the training programme.
LAY ABSTRACT
Multiple Sclerosis (MS) is an autoimmune disorder which affects 2.3 million people worldwide. People with MS often have impaired physical fitness, which may induce fatigue. In this pilot study we evaluated a new and high-intensive training program. Patients trained for 12 weeks, three days a week, three hours a day. We explored whether the training program is feasible in MS, and whether patients improve their physical fitness.
Seven patients completed the study, and attended on average 93% of the training sessions. Patients scored an overall grade of 8.9 on a scale of 1 to 10 regarding satisfaction with the training program. Physical fitness seemed to be improved, but improvements did not always preserve eight weeks after the training program. We concluded that the training program appears to be feasible in patients with MS, and may improve their physical fitness. However, a large controlled study is necessary to confirm these findings.
Key words: multiple sclerosis; exercise therapy; rehabilitation.
Citation: JRM-CC 2022; 5: jrmcc00078. DOI: http://dx.doi.org/10.2340/jrmcc.v5.2047
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Feb 11, 2022; Published: Apr 7, 2022
Correspondence address: Tom Derikx, Libra Revalidatie & Audiologie, Locatie Blixembosch, Toledolaan 2, 5629CC, Eindhoven, The Netherlands. E-mail: t.derikx@libranet.nl
Competing interests and funding: The authors have no conflicts of interest to declare.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by the CZ Fonds [201700286].
Multiple sclerosis (MS) is an autoimmune disorder of the central nervous system, which affects 2.3 million people worldwide (1). In the Netherlands, the prevalence of MS is 60–100 per 100,000 inhabitants (1). Patients with MS have 20% lower aerobic capacity (2) and 30–70% lower muscle strength (3) compared with healthy controls. These deficits may induce fatigue, which is highly prevalent in this population (4). Overall, this may result in hampering or loss of activities in daily life, which may initiate a cycle of deconditioning and worsening of symptoms (5).
Increasing evidence favours physical exercise therapy as a method for overall symptom management in MS (6). Low- and moderate-intensity endurance- and resistance-training in MS have been shown to be safe (i.e. they do not precipitate additional relapses or more rapid disease progression (2, 4, 7–10)) and effective in terms of maintaining aerobic capacity and muscle strength (7, 8, 11). Regarding high-intensity exercise training in MS, studies suggest larger improvements in aerobic capacity and muscle strength (increases of 25–60%) compared with moderate-intensity training (increases of 10–15%) (9, 12), which is in agreement with studies in healthy controls and several other patient groups (13, 14). These high-intensity regimes, whether or not combined with resistance training, have also been found to be safe and well-tolerated in patients with MS (9, 12, 15–17). Regarding fatigue, the effects of physical exercise therapy are not consistent in MS, but fatigue does not seem to be exacerbated (4, 7).
As a result of low aerobic capacity and decreased muscle strength, patients with MS experience limitations in functional capacity. Patients with MS are known to have reduced mobility, walking speed, and walking endurance (6). To additionally address this impaired functional capacity, we hypothesized that a functional approach in exercise programmes may be beneficial for this patient group. Therefore, we developed a comprehensive functional training programme including functional resistance training, endurance training, and skills training. This high-volume (high number of weekly training hours) training programme combines high-intensity training (functional resistance training) with low-intensity training (endurance- and skills training).
This pilot study evaluated the feasibility of this new training programme in patients with MS and explored changes in their functional capacity. Secondarily, the study explored changes in aerobic capacity and muscle strength. It was hypothesized that, although persons with MS are a vulnerable patient group, the high-intensity and high-volume functional training programme would be feasible, would not worsen fatigue, and would improve functional capacity.
METHODS
Experimental design
This was a pilot study in which patients completed 12 weeks of training, and an 8-week follow-up period. The numbers of patients who were eligible and included in the study, as well as demographic characteristics were collected at baseline. The feasibility of the training programme was measured directly after completion of the training programme. Pre- and post-training and at 8 weeks of follow-up, several physical measurements were performed, and fatigue was evaluated. The study was approved by the Medical Ethics Committee of Erasmus Medical Centre, Rotterdam, the Netherlands (NL64522.078.18).
Patients
Between April and December 2018, patients with MS who had a consultation with their Rehabilitation Specialist at Libra Rehabilitation & Audiology (Eindhoven, the Netherlands) and who were eligible, were asked to participate. Inclusion criteria were: age > 18 years, and able to walk at least 20 m, possibly with support (Expanded Disability Status Scale (EDSS) ≤ 6.5) (18). Participants with EDSS scores > 6.5 were not included, because of severe lower extremity functional limitations, which limit their ability to perform the training programme exercises. Exclusion criteria were: contra-indications to perform a progressive cardiopulmonary exercise test (CPET) (19), essential changes in disease-modifying drugs in the past 2 months, and an MS relapse in the past 3 months. All patients gave their written informed consent to participate in the study. In order to obtain good insight into feasibility and to explore the effects of the training programme, this pilot study aimed to include 15 patients.
Training programme
The training programme consisted of functional resistance training, endurance training, and skills training, 1 h each with sufficient rest in between, at a frequency of 3 times a week, for 12 weeks. In addition to these training sessions, patients had a 30-min individual physiotherapy session once a week to explain the training schemes, monitor possible (physical) inconveniencies related to the training programme, and prepare exercises for the skills training. All training sessions took place in small groups (n = 2–6) and were supervised by experienced physiotherapists and/or sports-therapists.
Functional resistance training. Functional resistance training consisted of 4 weeks of basic resistance training, 4 weeks of power training, and 4 weeks of capacity training. The aim of basic resistance training is to increase muscle strength. This phase is required for the subsequent training phases. The basic resistance training consisted of 4 lower-body exercises (squat, lunge, step-up, leg-press), 3 upper body exercises (bench press, fly, pull-over), and 1 core stability exercise (barbell rotation). Patients performed these exercises at 8 repetition maximum (RM), 4 sets, composed of 6–8 repetitions per set. Patients were instructed to have 90 s of rest between each exercise set. Load was increased by 10% (minimum of 1 kg) when 4 sets of 8 repetitions were completed (20).
The aim of power training is to increase speed during movements. The power training started with 5 cyclic exercises performed as rapidly as possible (squat, bench-press, pull-over, barbell-rotation, and explosive leg-press). After 2 weeks, these exercises were changed to acyclic and ballistic exercises (wall-ball, push-up, slam ball, Russian twist, and (box)jump). Patients performed exercises as rapidly as possible, at 12–16 RM, 4 sets, composed of 10 repetitions per set. Patients were instructed to take 3 min rest between each exercise set (20).
The aim of capacity training is to perform activities at high(er) speed and long(er) duration. Capacity training is an innovative and variable high-intensity training approach, based on principles of CrossFit, California, U.S. translated to rehabilitation purposes. CrossFit is a fitness programme that is constantly varied (exercises, duration, weights), at high-intensity, including only functional movements (21). Training sessions included combinations of power exercises, free weights, body-weight, weightlifting, gymnastics, walking/running, and rowing.
Endurance training. Endurance training consisted of low-intensity interval training (LIT) on a cycle ergometer and treadmill. Cycle ergometer intensity was based on the load (Watt), heart rate (HR), and Borg Category Ratio Scale (6–20) (22) at the anaerobic threshold (AT) determined during CPET (Appendix I) (23). Walk intensity started at comfortable walking speed, based on HR and Borg Scale (22). The training protocol began with walking and cycling in 4 blocks of 5 min, alternated with passive rest periods of 2 min. This increased progressively to 1 block of 25 min each, and the intensity increased by 10%. Patients were free to choose to start with walking or cycling.
Skills training. The purpose of skills training is to automate techniques and skills to facilitate performing tasks during daily life. Exercises were selected based on individual goals during the individual physiotherapy. Intensity was based on each patient’s rating of exhaustion using the Borg Category Ratio Scale (6–20) (22). The intensity of each activity and rest periods were adjusted in order to maintain a maximum of 11 (“light”) on the Borg Scale. During the skills training, patients repeated different daily life activities, such as transfers, pick-up techniques, walking, and static and dynamic balance.
Primary outcomes
Feasibility. The feasibility of the programme was assessed with a questionnaire developed for the study (available on request) on the following aspects: time investment, time schedule, intensity, and injury risk. Patients’ perceptions of these aspects were assessed using a 5-point Likert scale: “strongly disagree”, “disagree”, “neutral”, “agree”, and “strongly agree”. Scores more favourable than neutral were considered as “satisfied”. In addition, an overall satisfaction grade was scored (0–10), with higher scores indicating higher satisfaction with the programme. The numbers of patients eligible, attendance, dropouts and adverse events were recorded. Finally, fatigue was measured using the Fatigue Severity Scale (FSS) (24).
Functional capacity. To evaluate functional capacity, tests on mobility (Timed Up and Go (TUG)) (25), walking speed (10-Meter Walk Test (10MWT)) (26), and walking endurance (6-Minute Walk Test (6MWT)) (25) were included.
Secondary outcomes
As secondary outcome parameters, aerobic capacity was assessed using CPET on a cycle ergometer (Appendix I) (27), and muscle strength using a 1 RM leg-press (28). Furthermore, patient and demographic characteristics, including age, sex, body mass index (BMI), EDSS score, disease course, disease duration and years since diagnosis, were recorded at baseline.
Data analysis
Descriptive statistics were used to analyse the feasibility of the training programme. Changes in fatigue, functional capacity, aerobic capacity, and muscle strength were analysed using Friedman’s analysis of variance (ANOVA) and a Wilcoxon signed-rank test as post hoc, using the statistical analysis software package SPSS (V23.0, SPSS Inc., Chicago, IL, USA), with alpha < 0.05. Because of the small sample size and pilot character of the study, this study focusses on trends rather than significance, describing and graphically presenting changes after the training programme.
RESULTS
Patients and feasibility
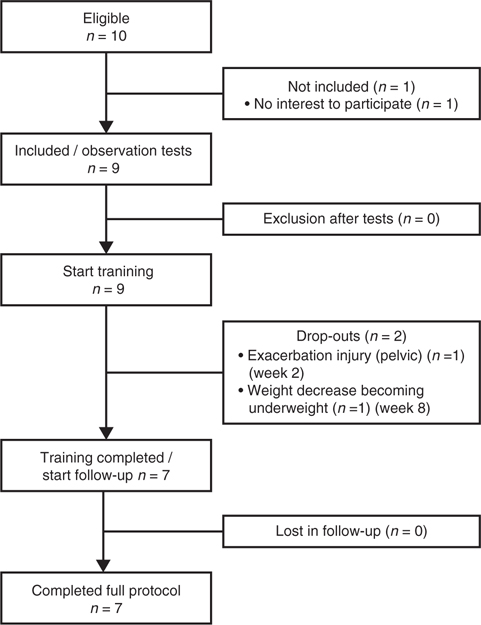
During the study period, 16 patients with MS were assessed by their rehabilitation specialist regarding functional capacity. Ten of these (63%) were eligible for the study. Nine patients were included in the study and began training (Fig. 1). Over the course of the training programme, 2 patients withdrew from the study. One patient withdrew in week 2 due to exacerbation of a previous pelvic injury (which had been considered to be recovered) as a result of the CPET. The second patient withdrew in the eighth training week due to weight loss and becoming underweight. Due to psychological factors and an unstable home environment, the patient was unable to counteract the weight loss. One further patient (MS_05) was excluded from the analyses on physical outcomes and fatigue, because of essential changes in disease-modifying drugs during the intervention period. Nevertheless, this patient was included in the analysis of feasibility. Table I shows the patient and demographic characteristics.
The results regarding feasibility are shown in Table II. Overall, patients were satisfied with the training programme (overall score 8.9 out of 10). In general, patients were satisfied with the feasibility aspects, except the time investment was considered to be high. The mean attendance was 94% (range 86–100%).
One adverse event occurred during the training programme. This patient (MS_04) had an epileptic seizure at home after the second training day in week 12. The neurologist concluded that this was a side-effect of medication and was probably not related to the training programme. The patient immediately stopped taking this medication.
At the group level, there was no change in fatigue (p = 0.513) (Table III). At the individual level (Fig. 2), fatigue decreased in 4 of 6 patients, whereas 2 patients experienced more fatigue after the training programme. One of these 2 patients was the patient who had the epileptic seizure. Four patients experienced less fatigue at follow-up compared with before the training programme.
| Parameter | Pre | Post | Follow-up | Friedman’s ANOVA | Wilcoxon post hoc | |||
| pre-post | pre-FU | post-FU | ||||||
| Fatigue (FSS) | Mean ± SD | 4.7 ± 1.1 | 4.5 ± 1.0 | 4.3 ± 0.6 | p = 0.513 | –0.2 | –0.4 | –0.2 |
| Median (min–max) | 5.5 (1.9–6.2) | 4.2 (3.4–6.1) | 4.5 (3.4–5.0) | |||||
| Mobility (TUG; s) | Mean ± SD | 17.1 ± 12.9 | 13.8 ± 10.7 | 13.9 ± 10.2 | p = 0.069 | –3.3 | –3.2** | 0.1 |
| Median (min–max) | 15.1 (6.3–41.5) | 10.3 (6.5–34.7) | 10.7 (6.2–33.0) | |||||
| Walking speed (10MWT; km/h) | Mean ± SD | 3.9 ± 2.5 | 4.4 ± 2.7 | 5.1 ± 2.3 | p = 0.022 | 0.5 | 1.2* | 0.7 |
| Median (min–max) | 3.6 (1.9–7.4) | 4.4 (2.5–7.5) | 6.3 (2.4–7.5) | |||||
| Walking endurance (6MWT; m) | Mean ± SD | 315.0 ± 218.7 | 386.8 ± 242.7 | 368.8 ± 242.5 | p = 0.115 | 71.8** | 53.8** | –18.0 |
| Median (min–max) | 279.5 (91.0–605.0) | 389.0 (81.0–665.0) | 377.5 (87.0–628.0) | |||||
| Aerobic capacity (CPET; ml/min/kg) | Mean ± SD | 23.5 ± 7.9 | 27.2 ± 9.6 | 25.3 ± 10.0 | p = 0.016 | 3.7* | 1.8 | –1.9 |
| Median (min–max) | 22.8 (12.3–34.3) | 26.6 (14.3–38.3) | 23.0 (13.6–42.2) | |||||
| Muscle strength (1 RM leg press; kg) | Mean ± SD | 88.8 ± 25.4 | 97.7 ± 28.7 | 103.8 ± 33.8 | p = 0.091 | 8.9 | 15.0 | 6.1 |
| Median (min–max) | 98.0 (46.0–111.0) | 109.0 (43.0–123.0) | 109.0 (46.0–132.0) | |||||
| FU: follow-Up; CPET: cardiopulmonary exercise test; SD: standard deviation; ANOVA: analysis of variance; FSS: Fatigue Severity Scale; TUG: Timed Up and Go Test; 10MWT: 10-Meter Walk Test; 6MWT: 6-Minute Walk Test; RM: repetition maximum. *Friedman’s ANOVA and Wilcoxon post hoc both revealed significant results: p < 0.05. **Wilcoxon post hoc revealed a significant result: p < 0.05. |
||||||||
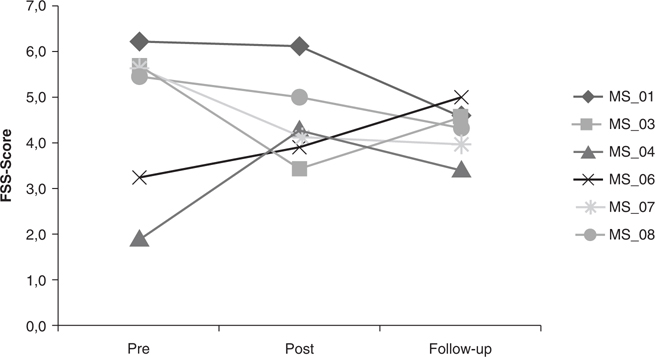
Fig. 2. Fatigue (Fatigue Severity Scale; FSS) in individual patients with multiple sclerosis (MS).
Functional capacity
At the individual level, each patient seemed to have improved his/her performance in the functional capacity tests after the training programme, with the exception of patient MS_04 (Fig. 3). Trends in mobility at the group level were found between pre-training and follow-up (19%; p = 0.028), in walking speed between pre-training and post-training (14%; p = 0.075) and between pre-training and follow-up (32%; p = 0.043) (Table III).
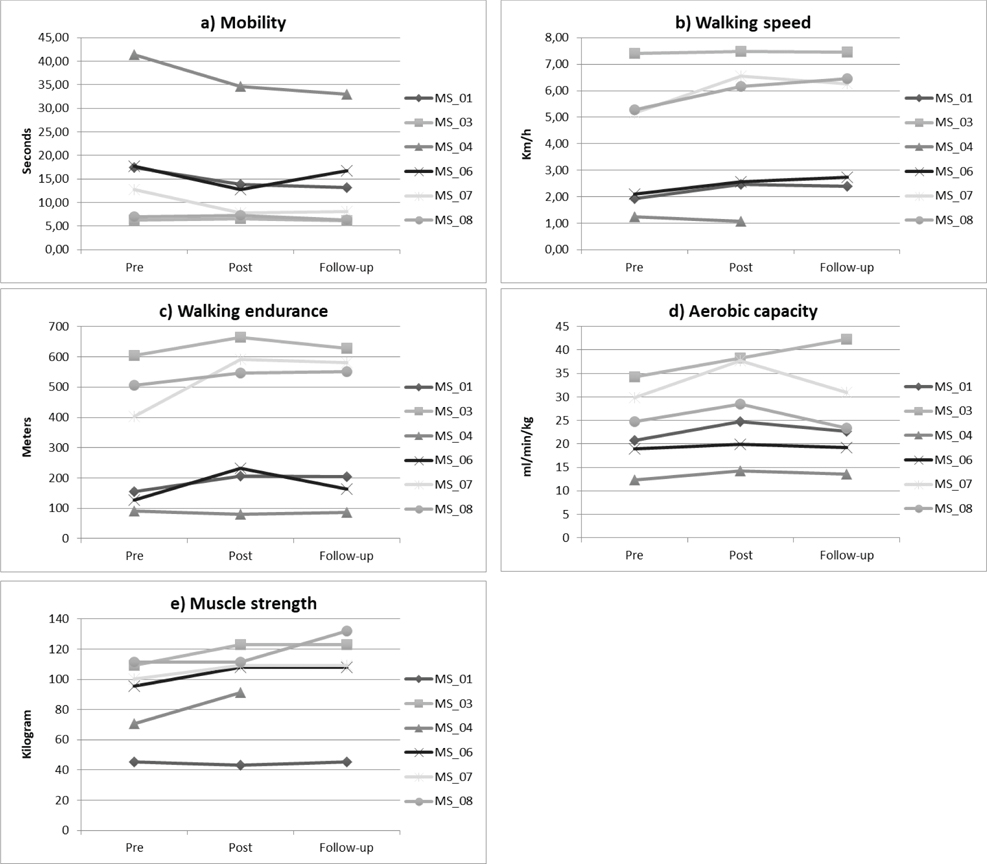
Fig. 3. Functional capacity: (a) mobility (Timed Up and Go Test; TUG), (b) walking speed (10-Meter Walk Test; 10MWT), (c) walking endurance (6-Minute Walk Test; 6MWT). Other: (d) aerobic capacity (cardiopulmonary exercise test; CPET), (e) muscle strength (1 repetition maximum (RM) leg press) in individual patients.
Aerobic capacity and muscle strength
At the individual level, all patients seemed to have improved their performance in aerobic capacity and muscle strength, with the exception of patient MS_01, who did not improve in muscle strength (Fig. 3). Trends at the group level were found in aerobic capacity between pre-training and post-training (16%; p = 0.027), and in muscle strength between pre-training and post-training (10%; p = 0.080) and between pre-training and follow-up (17%; p = 0.068) (Table III).
DISCUSSION
The results suggest that the high-intensity and high-volume functional training programme is feasible in patients with MS, does not appear to worsen fatigue, and may improve functional capacity, aerobic capacity and muscle strength. These results are in line with the study hypothesis. However, at follow-up, improvements were not always sustained.
The training programme appears to be feasible in several respects. Adherence was high (93%) and patients were very satisfied with the training programme. These favourable responses may, in part, reflect the small-group character of the training sessions, which makes exercising a more social activity (11). There were some dropouts and 1 adverse event, but these were not directly related to the training programme. These attrition rates are comparable to those of other studies involving participants with MS (10, 15–17). Finally, fatigue did not seem to worsen, which is an important finding given the high training volume and intensity.
Regarding time investment, the programme might benefit from optimization. Patients rated the commitment of 3 days per week in the rehabilitation centre, with 3 h training per day, as high. Performing the endurance training at home might be a solution, but such adaptation may decrease compliance. Shortening of the rest periods between the different training components may also be considered, but its appropriateness should be assessed on a case-by-case basis.
Due to the pilot character of the study, the sample size was small, there was no control group, and no corrections were made for multiple comparisons; however, the results show some promising changes in patients following the training programme. Overall, the functional capacity (mobility, walking speed, walking endurance) seemed to be improved after the training programme, with the exception of 1 patient, who had an epileptic seizure.
Regarding mobility (TUG), 3 patients improved > 20%, which is considered a clinically relevant effect (29). The 2 patients who showed no improvements from pre- to post-treatment had low EDSS scores, and most likely did not improve because they were not limited in this task. The mean improvement of 19% in the current study is comparable or larger than improvements described in other studies (range 7–17%) (15, 30–33). Those studies included a wide variety of training methods, including high-intensity interval training (15), resistance training (31), or a combination of endurance- and resistance training (30, 32, 33). The larger improvements in the current study are most likely due to the high-intensity component and the high volume of training hours, whereas patients in other studies trained for only approximately 2 h per week (30–32).
All but 1 of the study participants walked faster after the training programme, as measured with the 10MWT, and 3 of them showed clinically relevant improvements (> 20%) (26). The degree of change found in the current study (14%) is within the range of changes found in other studies (3–24%) (28, 32–35). Other studies mainly conducted resistance training (28), and in some this was combined with endurance exercises (32, 33).
Regarding walking endurance (6MWT), the training programme seemed favourable in most patients. Mean improvement was 23%, which is comparable to or larger than improvements found in other training studies (3–21%) (15, 28, 30, 33, 36, 37), with the exception of the study of Learmonth et al. (31), who reported an overall increase of 37% after 12 weeks of circuit training, including mobility, balance and resistance training components. Other studies conducted mainly resistance training (28, 33, 36, 37), some combined with endurance training (33, 36). Walking was included in the training programme during the endurance training and capacity training, which may explain why walking endurance showed large improvements.
In addition to changes in functional outcomes, aerobic capacity seemed to improve (16%), and in 4 patients changes were clinically relevant (> 10%) (27). The findings of the current study are comparable to, or exceed, the improvements reported in other studies (range 4–15%) (15, 17, 38, 39), whereas 2 studies reported larger gains compared with ours (17–22%) (11, 40). Compared with Wens et al. (40), who performed 8 weeks of High-intensity Interval Training (HIT) , a HIT component was performed only in the last 4 weeks of the training programme. In addition, there may be a learning effect for the test used by Petajan et al. (11), whose study participants performed the test 4 times in 15 weeks.
Muscle strength seemed to improve in the current patients (10%), but most other studies reported larger gains in patients with MS (19–44%) (9, 28, 34). This could be explained by different measurement techniques (1 RM leg press vs maximal voluntary isometric contraction (MVIC)), but is most likely due to differences in training programmes. The current training programme aimed to improve muscle strength only during the first 4 weeks, as a prerequisite to the power- and capacity training, whereas other studies aimed to improve muscle strength for 8 weeks or longer.
Study limitations
This pilot study has some limitations. Only 9 patients were eligible in the study period, and only 7 completed the intervention. Furthermore, the study population was a mixed population of patients with varying levels of impairment, various types of MS, and varying times since diagnosis. The small and heterogeneous study population confirms the challenge to recruit and include patients in a high-volume intervention study. Finally, the study did not contain a control group with patients who did not receive the high-intensity and high-volume functional training programme. A large-scale controlled trial over a longer period of time is required to evaluate the added value of the training programme.
Clinical messages
- This high-volume and high-intensity functional training programme appears to be feasible in patients with MS (EDSS ≤ 6.5).
- Functional capacity, aerobic capacity and muscle strength all seem to improve with this training programme. A large-scale controlled trial over a longer period of time is required to evaluate the added value of the training programme.
ACKNOWLEDGEMENTS
All patients are acknowledged for their time and effort participating in this study.
REFERENCES
- Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, et al. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014; 83: 1022–1024.
- Langeskov-Christensen M, Heine M, Kwakkel G, Dalgas U. Aerobic capacity in persons with multiple sclerosis: a systematic review and meta-analysis. Sports Med 2015; 45: 905–923.
- Wens I, Dalgas U, Vandenabeele F, Krekels M, Grevendonk L, Eijnde BO. Multiple sclerosis affects skeletal muscle characteristics. PLoS One 2014; 9: 108–158.
- Heine M, van de Port I, Rietberg MB, van Wegen EE, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev 2015: CD009956.
- Motl RW. Physical activity and irreversible disability in multiple sclerosis. Exerc Sport Sci Rev 2010; 38: 186–191.
- Pearson M, Dieberg G, Smart N. Exercise as a therapy for improvement of walking ability in adults with multiple sclerosis: a meta-analysis. Arch Phys Med Rehabil 2015; 96: 1339–1348.
- Latimer-Cheung AE, Pilutti LA, Hicks AL, Martin Ginis KA, Fenuta AM, MacKibbon KA, et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil 2013; 94: 1800–1828.
- Kjolhede T, Vissing K, Dalgas U. Multiple sclerosis and progressive resistance training: a systematic review. Mult Scler 2012; 18: 1215–1228.
- Wens I, Dalgas U, Vandenabeele F, Grevendonk L, Verboven K, Hansen D, et al. High Intensity Exercise in Multiple Sclerosis: Effects on Muscle Contractile Characteristics and Exercise Capacity, a Randomised Controlled Trial. PLoS One 2015; 10: e0133697.
- Kerling A, Keweloh K, Tegtbur U, Kuck M, Grams L, Horstmann H, et al. Effects of a Short Physical Exercise Intervention on Patients with Multiple Sclerosis (MS). Int J Mol Sci 2015; 16: 15761–15775.
- Petajan JH, Gappmaier E, White AT, Spencer MK, Mino L, Hicks RW. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol 1996; 39: 432–441.
- Collett J, Dawes H, Meaney A, Sackley C, Barker K, Wade D, et al. Exercise for multiple sclerosis: a single-blind randomized trial comparing three exercise intensities. Mult Scler 2011; 17: 594–603.
- Sloth M, Sloth D, Overgaard K, Dalgas U. Effects of sprint interval training on VO2max and aerobic exercise performance: A systematic review and meta-analysis. Scand J Med Sci Sports 2013; 23: 341–352.
- Raymond MJ, Bramley-Tzerefos RE, Jeffs KJ, Winter A, Holland AE. Systematic review of high-intensity progressive resistance strength training of the lower limb compared with other intensities of strength training in older adults. Arch Phys Med Rehabil 2013; 94: 1458–1472.
- Orban A, Garg B, Sammi MK, Bourdette DN, Rooney WD, Kuehl K, et al. Effect of High-Intensity Exercise on Multiple Sclerosis Function and 31P MRS Outcomes. Med Sci Sports Exerc 2019.
- Campbell E, Coulter EH, Paul L. High intensity interval training for people with multiple sclerosis: A systematic review. Mult Scler Relat Disord 2018; 24: 55–63.
- Skjerbaek AG, Naesby M, Lutzen K, Moller AB, Jensen E, Lamers I, et al. Endurance training is feasible in severely disabled patients with progressive multiple sclerosis. Mult Scler 2014; 20: 627–630.
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452.
- Keytsman C, Eijnde BO, Hansen D, Verboven K, Wens I. Elevated cardiovascular risk factors in multiple sclerosis. Mult Scler Relat Disord 2017; 17: 220–223.
- American College of Sports M. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 2009; 41: 687–708.
- Glassman G. Understanding CrossFit. CrossFit Journal Articles. 2007; 56; 1–2.
- Borg G. Borg’s rating of perceived exertion and pain scales. Champaign, IL: Human kinetics 1998.
- Koch B, Schaper C, Ittermann T, Spielhagen T, Dorr M, Volzke H, et al. Reference values for cardiopulmonary exercise testing in healthy volunteers: the SHIP study. Eur Respir J 2009; 33: 389–397.
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46: 1121–1123.
- Bennett SE, Bromley LE, Fisher NM, Tomita MR, Niewczyk P. Validity and Reliability of Four Clinical Gait Measures in Patients with Multiple Sclerosis. Int J MS Care 2017; 19: 247–252.
- Bethoux F, Bennett S. Evaluating walking in patients with multiple sclerosis: which assessment tools are useful in clinical practice? Int J MS Care 2011; 13: 4–14.
- Langeskov-Christensen M, Langeskov-Christensen D, Overgaard K, Moller AB, Dalgas U. Validity and reliability of VO(2)-max measurements in persons with multiple sclerosis. J Neurol Sci 2014; 342: 79–87.
- Dalgas U, Stenager E, Jakobsen J, Petersen T, Hansen HJ, Knudsen C, et al. Resistance training improves muscle strength and functional capacity in multiple sclerosis. Neurology 2009; 73: 1478–1484.
- Learmonth YC, Dlugonski DD, Pilutti LA, Sandroff BM, Motl RW. The reliability, precision and clinically meaningful change of walking assessments in multiple sclerosis. Mult Scler 2013; 19: 1784–1791.
- Sabapathy NM, Minahan CL, Turner GT, Broadley SA. Comparing endurance- and resistance-exercise training in people with multiple sclerosis: a randomized pilot study. Clin Rehabil 2011; 25: 14–24.
- Learmonth YC, Paul L, Miller L, Mattison P, McFadyen AK. The effects of a 12-week leisure centre-based, group exercise intervention for people moderately affected with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil 2012; 26: 579–593.
- Cakt BD, Nacir B, Genc H, Saracoglu M, Karagoz A, Erdem HR, et al. Cycling progressive resistance training for people with multiple sclerosis: a randomized controlled study. Am J Phys Med Rehabil 2010; 89: 446–457.
- Sangelaji B, Kordi M, Banihashemi F, Nabavi SM, Khodadadeh S, Dastoorpoor M. A combined exercise model for improving muscle strength, balance, walking distance, and motor agility in multiple sclerosis patients: A randomized clinical trial. Iran J Neurol 2016; 15: 111–120.
- Dodd KJ, Taylor NF, Shields N, Prasad D, McDonald E, Gillon A. Progressive resistance training did not improve walking but can improve muscle performance, quality of life and fatigue in adults with multiple sclerosis: a randomized controlled trial. Mult Scler 2011; 17: 1362–1374.
- Kjolhede T, Vissing K, de Place L, Pedersen BG, Ringgaard S, Stenager E, et al. Neuromuscular adaptations to long-term progressive resistance training translates to improved functional capacity for people with multiple sclerosis and is maintained at follow-up. Mult Scler 2015; 21: 599–611.
- Ahmadi A, Arastoo AA, Nikbakht M, Zahednejad S, Rajabpour M. Comparison of the Effect of 8 weeks Aerobic and Yoga Training on Ambulatory Function, Fatigue and Mood Status in MS Patients. Iran Red Crescent Med J 2013; 15: 449–454.
- Garrett M, Hogan N, Larkin A, Saunders J, Jakeman P, Coote S. Exercise in the community for people with minimal gait impairment due to MS: an assessor-blind randomized controlled trial. Mult Scler 2013; 19: 782–789.
- Zimmer P, Bloch W, Schenk A, Oberste M, Riedel S, Kool J, et al. High-intensity interval exercise improves cognitive performance and reduces matrix metalloproteinases-2 serum levels in persons with multiple sclerosis: A randomized controlled trial. Mult Scler 2018; 24: 1635–1644.
- Heine M, Verschuren O, Hoogervorst EL, van Munster E, Hacking HG, Visser-Meily A, et al. Does aerobic training alleviate fatigue and improve societal participation in patients with multiple sclerosis? A randomized controlled trial. Mult Scler 2017; 23: 1517–1526.
- Wens I, Dalgas U, Vandenabeele F, Verboven K, Hansen D, Deckx N, et al. High Intensity Aerobic and Resistance Exercise Can Improve Glucose Tolerance in Persons With Multiple Sclerosis: A Randomized Controlled Trial. Am J Phys Med Rehabil 2017; 96: 161–166.
Appendix I.
Details of the cardiopulmonary exercise test (CPET)
Prior to the cardiopulmonary exercise test (CPET), patients were screened by the Lausanne Protocol (cardiovascular screening questionnaire), by electrocardiography (ECG) at rest, and by blood pressure measurement at rest, and their results were reviewed by their rehabilitation specialist. The CPET was administered on an electronically braked cycle ergometer (Corival V2, Lode, Groningen, The Netherlands) using a progressive ramp protocol. The test started with a 3-min rest period followed by a 3-min warm-up without resistance. The target pedal rate during the test was 60–70 revolutions/min, but not less than 50 revolutions/min. The magnitude of the increase in load during the CPET was calculated with the SHIP equation using data from healthy adults (age, sex, body weight), selecting a protocol with a 5–25 Watt increase per min (23). Based on the impact of the disease and professional experience, the predicted peak load was adjusted if necessary, to ensure that the total exercise time ranged between 8 and 12 min. The load of the selected protocol was equally divided over 60 s. Strong verbal encouragement was given throughout the test. During testing, pulmonary gas exchange (Master Screan CPX, Jaeger, CareFusion, San Diego, California, U.S. ) and heart rate (12-lead ECG, Jaeger Vyntus ECG, CareFusion, San Diego, California, U.S. ) were measured continuously. Blood pressure was measured once every two min (Bosotron 2, Bosch & Sohn, Jungingen, Germany). The test was ended when the patient voluntarily stopped due to exhaustion, the participant was unable to maintain the minimum pedal rate (> 50 revolutions/min), or ECG or blood pressure abnormalities were detected. Oxygen uptake (VO2) values were collected breath-by-breath and averaged every 10 s. VO2peak was reported as the highest value averaged over the 10-s blocks, or at the end of the test of a block of at least 5 s. The oxygen system was calibrated before each test using reference gases.