ORIGINAL REPORT
SARCOPENIA IS A FREQUENT DISEASE IN SARS-COV-2 INFECTION
Sandro IANNACCONE, MD1, Luigia BRUGLIERA, MD, PhD1, Alfio SPINA, MD2, Gianluca NOCERA, MD2, Andrea TETTAMANTI, PT, MSc1, Alessandra GIORDANI, MSc1, Sara ANGELONE, MD1, Paola CASTELLAZZI, MD1, Paolo CIMINO, MD1, Jeffrey D. PADUL, MD1, Elise HOUDAYER, PhD1 and Federica ALEMANNO, PsyD, PhD1
From the 1Department of Rehabilitation and Functional Recovery and 2Department of Neurosurgery and Gamma Knife Radiosurgery, IRCCS San Raffaele Scientific Institute, Milan, Italy
Objective: We aimed to investigate the clinical symptoms and specific care requirements of SARS-CoV-2 patients who were admitted to a COVID-19 Rehabilitation Unit while still infectious for SARS-CoV-2 and in the subacute phase of the disease.
Methods: Patients admitted to our COVID-19 Rehabilitation Unit from March 2020 to December 2020 were evaluated for sarcopenia, and they also completed the following assessments: functional independence measure, short physical performance battery and Hamilton Rating Scale for Depression. Age and body mass index and symptoms of dysosmia or dysgeusia were also recorded.
Results: A total of 126 patients were enrolled (50 women, median age 72 years, 18.7 years), of whom 82% of patients presented with low grip strength. Sarcopenia was diagnosed in 52 patients. Sarcopenic patients were older than non-sarcopenic ones (median age 73.4 years, IQR 13.2 vs 63.9 years, IQR 14.5, respectively, p = 0.014). Sarcopenia was associated with the presence of depression (p = 0.008), was more common in women (p = 0.023) and was associated with greater functional deficits (functional independence measure and short physical performance battery analyses, p < 0.05). Sarcopenic patients also had a lower body mass index than other patients (p < 0.01).
Conclusion: More than 40% of our patients suffered from sarcopenia, which was associated with ageing, depression, low body mass index, reduction in functional autonomy and being a woman. Such data provide evidence for the need to assist hospitalized COVID-19 patients by means of a multidisciplinary specialist team.
LAY ABSTRACT
Many COVID-19 patients who require hospitalization in the first phase of the disease benefit from respiratory, motor or cognitive rehabilitation before being dismissed from the hospital. During this rehabilitative phase, these patients are still positive for SARS-CoV-2 and potentially infectious, although their symptoms might differ from the symptoms they encountered in the first days. The objective of this study was to examine the clinical condition of 126 COVID-19 patients in a COVID-19 rehabilitation ward. Our data demonstrated that 41% of these patients presented with sarcopenia, which represents a drastic loss of muscle mass. We noticed that the risk factors associated with sarcopenia were ageing, depression, being a woman and having more issues with being independent in daily life. These results reveal the importance of providing such COVID-19 patients with specific care by multidisciplinary teams of healthcare professionals.
Key words: SARS-CoV-2; sarcopenia; rehabilitation; COVID-19.
Citation: JRM-CC 2023; 6: jrmcc00089. DOI: https://doi.org/10.2340/jrmcc.v6.2222
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Oct 23, 2022; Published: Jan 31, 2023
Correspondence address: Elise Houdayer, Department of Rehabilitation and Functional Recovery, IRCCS San Raffaele Hospital, via Olgettina 60, 20132, MI, Milan, Italy. Phone: + 39 0226435739. E-mail: houdayer.elise@hsr.it
Competing interests and funding: The authors have no conflicts of interest to declare.
There is no funding to disclose.
Over the past year, many communications have reported clinical signs and symptoms of COVID-19. The most common symptoms during the acute phase of the disease are fever, dry cough and asthenia (1, 2). Other less common symptoms have also been reported, such as muscle pain, sore throat, diarrhea, conjunctivitis, headache and loss of taste and smell (3–10). Several communications reported the need for functional rehabilitation in about 15–20% of patients hospitalized for COVID-19 (11, 12). These patients were in the subacute phase of the disease (about 10–30 days after the onset of symptoms); were still positive for SARS-CoV-2 using RT-PCR, posing a risk of being infectious; did not require respiratory assistance (or no more than 2 L/min) and had areas of dependence on the functional independence measure (FIM) (13). Following the acute phase of the disease, patients were admitted to COVID-19 rehabilitation units to receive specialized rehabilitation for cardiorespiratory, motor and/or cognitive dysfunctions (11, 12, 14). Understanding the symptoms and clinical condition of those patients is of extreme importance for planning their multi-specialist assistance. In fact, several clinical complications of hospitalization for acute COVID-19 may persist during the subacute phase of COVID-19, such as nerve compression injuries (7), amputation (15) or dysphagia (16).
In the subacute phase of the disease, our study aimed to investigate the clinical condition of patients admitted to a COVID-19 Rehabilitation Unit. Particularly, we aimed to evaluate the presence of sarcopenia, patient age, body mass index (BMI), mood and functional autonomy in order to determine the optimal care for each patient.
MATERIALS AND METHODS
Patients
Patients admitted to the COVID-19 Rehabilitation Unit of the San Raffaele Scientific Institute (Milan, Italy) from 27 March 2020 to 29 December 2020 were included in this study.
Criteria to admit COVID-19 patients in this Unit were as follows: positive swab for SARS-CoV-2, stable SatO2 (≥ 92%) and respiratory rates (RR < 40 breaths/min), no need for respiratory assistance less than or equal to 2 L/min, absence of fever since at least 4 days and with areas of dependence on the FIM evaluation (the FIM is an 18-item measurement tool that explores an individual’s physical, psychological and social functioning) [11–13]. These patients had been previously admitted to the Emergency Room (ER), Intensive Care Units (ICU), Respiratory High Dependency Care Units (RHDCU) or Infectious Diseases Units. Fig. 1 depicts the patient’s comorbidities, symptoms and treatments. We excluded patients who were treated for cognitive dysfunctions, patients who were under anti-depressant drugs before their recovery and patients presenting with COVID-19 encephalitis.
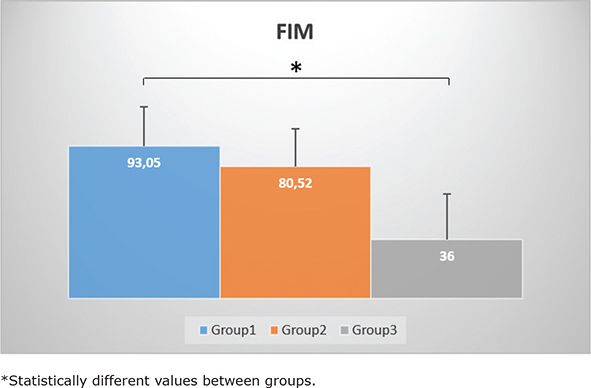
Fig. 1. The functional independence measure (FIM) scores among the different groups. Group 1: no sarcopenia with normal grip strength, Group 2: no sarcopenia with low grip strength, Group 3: sarcopenia. Data are presented in mean FIM scores+standard deviations.
Oral and written consents were obtained from all participants in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki), and the study was approved by the local Ethics committee of the San Raffaele Hospital.
Assessments
Every patient underwent the following assessments.
Sarcopenia. A sarcopenia evaluation was performed by a physiatrist according to the EWGSOP-2 guidelines (17).
Sarcopenia was considered as probable in patients with low muscle strength (measured using a handgrip). In order to confirm the presence of sarcopenia, measurements of muscle mass were performed using Bioelectric Impedance Analysis (BIA), a non-invasive method to obtain an estimate of the body composition. Patients with low grip strength and low muscle mass were diagnosed with confirmed sarcopenia.
The short physical performance battery (SPPB) (18) was used to define the severity of sarcopenia. The SPPB is a series of 3 tests to assess lower extremity physical function, including a 4-m walk at a natural pace, the time to complete 5 unassisted chair stands and 3 standing balance tests, each held for 10 s, with progressively more difficult stances. Each test is scored on a 0–4 scale using previously validated norms and summed for an overall score range of 0–12, where 0 indicates the lowest physical performance and 12 indicates the highest performance.
Functional independence measure. The FIM is an 18-item scale that assesses function in six areas, including self-care, continence, mobility, transfers, communication and cognition. Each of the 18 items is graded on a scale of 1–7 based on level of independence (1 = total assistance required, 7 = complete independence). The score ranges between 18 and 126.
FIM and SPPB were performed by a physiotherapist.
Body mass index. BMI was measured by a nutritionist and expressed as the ratio: kg/m2 ([weight in kilograms]/[height in meters square]).
Hamilton Rating Scale for Depression. Hamilton Rating Scale for Depression (HDRS) is a 17-item semi-structured interview to assess depressive symptoms (19). The items are rated on 3- or 5-point scales, and the total score can range from 0 to 53, with higher scores indicative of higher levels of depression. A total score ranging from 0 to 7 indicates no or minimal symptoms of depression, from 8 to 17 mild depression, from 18 to 25 moderate depression and 26 and above severe depression. HDRS was performed by a psychologist (20).
Dysosmia and dysgeusia. Dysosmia and dysgeusia were self-reported by direct interview with the patients. The type of ventilation (orotracheal intubation vs non-intubated) that patients benefited from in the acute phase of the disease was reported.
Statistical analyses
Age, sex, HDRS, type of ventilation, taste, smell, FIM value and BMI were obtained at each patient’s COVID-19 rehabilitation admission and were statistically evaluated. Patients were divided into 3 groups for statistical analyses: Group 1 (no sarcopenia with normal grip strength), Group 2 (no sarcopenia with low grip strength) and Group 3 (sarcopenia). Normality of the continuous variable was estimated through visual inspection of density and QQ-plots and formally assessed through Shapiro–Wilk’s test. Relationships between continuous variables (Age, FIM and BMI) and groups were evaluated through one-way ANOVA or Kruskal–Wallis rank sum test, depending on the distribution of the continuous variable analysed. Post hoc analyses were conducted using Tukey Dunn’s or a similar procedure using Bonferroni p-value adjustment. The relationship between categorical variables was determined using Pearson’s Chi-squared test or Fisher’s exact test (if expected cell frequencies were lower than 5). Post hoc analyses were run with standardized residuals using Bonferroni adjusted p-values.
To identify any potential predictor of sarcopenia, a multinomial logistic regression was built in a stepwise fashion. All variables collected were analysed, then overall significance of variables was assessed with Wald test and only predictors with p-values lower than 0.2 were inserted in the final model. Continuous variables were treated like categorical ones. Patients were divided into groups according to their mean or median.
A p < 0.05 was considered statistically significant unless otherwise specified.
Statistical analysis and graphs were performed using R (R Core Team, 2020), package ggplot2 (H. Wickham, 2016).
RESULTS
Descriptive analyses
A total of 126 consecutive patients were enrolled: 76 were men (60%) and 50 were women (40%). Median age was 72 years (IQR 18.7). FIM median value was 80 (IQR 47).
In the acute phase of the disease, 25 patients received orotracheal intubation and ventilation (20%), while 101 patients (80%) received non-invasive ventilation, oxygen support or did not need any kind of respiratory support. Mean BMI was 25 (standard deviation [SD] ± 4.78). Eleven patients (8.7%) were underweight (BMI < 18.5), 53 (43%) overweight or obese (BMI > 25) and 61 (48.3%) had a normal BMI. Thirty-seven patients had dysosmia (29%) and 24 (19%) had dysgeusia.
Sarcopenia
As reported in Table I, 22 patients (18%) did not present clinical signs of sarcopenia (Group 1), while 104 patients (82%) met the EWGSOP-2 criteria for probable sarcopenia. Out of these 104 patients, 52 (41% of the total sample) exhibited low grip strength and no other signs of sarcopenia; thus, sarcopenia was excluded for these patients (Group 2). The remaining 52 patients (41% of the total sample) were diagnosed with confirmed sarcopenia (Group 3).
| Total population | Group 1 (no sarcopenia, normal grip strength) | Group 2 (no sarcopenia, low grip strength) | Group 3 (sarcopenia) | |
| Age | 69.51 ± 13.96 | 63.86 ± 13.67* | 68.02 ± 15.4 | 79.5 ± 11.29* |
| Sex | 50 F; 76 M | 6 F; 16 M | 16 F; 36 M | 28 F; 24 M |
| HDRS | 6.45 ± 4.62 | 4.18 ± 3.25 | 6.31 ± 4.35 | 12 ± 5.06 |
| BMI | 24.69 ± 4.73 | 26.93 ± 4.85* | 26.26 ± 4.45* | 20.94 ± 3.75* |
| FIM | 79.53 ± 26.01 | 93.05 ± 23.96* | 80.52 ± 23.18 | 36 ± 27.54* |
| SPPB | 4.16 ± 3.77 | 6.32 ± 3.44* | 3.80 ± 3.89* | 3 ± 3.49* |
| Dysosmia | 29% (37) | 19% (7) | 57% (21) | 24% (9) |
| Dysgeusia | 19% (24) | 21% (5) | 58% (14) | 21% (5) |
| Data are presented in means±standard deviations. Age is expressed in years. | ||||
| F: Female; M: Male; HDRS: Hamilton Depression Rating Scale; BMI: body mass index; N: numbers of patients involved; FIM: functional independence measure; SPPB: short physical performance battery. | ||||
| *Statistically different values between groups. | ||||
Age and FIM distributions differed between patient groups. Pairwise comparison for each variable was performed using Dunn’s procedure with a Bonferroni correction for multiple comparisons. These post hoc analyses revealed statistically significant differences in age between Group 1 (median 63.9 years, IQR 14.5) and Group 3 (median 73.4 years, IQR 13.2, p = 0.014). Median age of Group 2 (68.1 years, IQR 22) was not significantly different from the other 2 groups. Moreover, patients with orotracheal intubation were significantly younger than the others (p < 0.001), with no significant correlation in the presence of sarcopenia (p = 0.15) or depression (p = 0.65).
FIM values were statistically higher in Group 1 (median 102.5, IQR 31.7) compared to that in Group 3 (median 65.5, IQR44.2, p = 0.003). SPPB score differed between groups (p = 0.008). Pairwise comparison was performed using Dunn’s procedure. The SPPB score was significantly different between Group 1 and Group 2 (p = 0.028), and between Group 1 and Group 3 (p = 0.009).
BMI differed between the 3 groups, as evaluated through a one-way ANOVA (p < 0.01). Tukey post hoc analysis revealed that BMI of Group 3 (mean 17.8; SD ± 3.8) was lower than Group 2 (mean 26.3; SD ± 4.5, p < 0.01) and Group 1 (mean 30; SD ± 4.8, p < 0.01).
There was a statistically significant association between sex and sarcopenia (χ2 7.5, p = 0.023). Indeed, 56% of women presented with confirmed sarcopenia compared to 31.5% of men. Sarcopenia was also related to the presence of depression (χ2 9.68, p = 0.008). According to the HDRS evaluation, 48 patients (38% of the total sample) presented with mild-to-moderate depression (2 patients in Group 1 [9% of Group 1], 22 patients in Group 2 [42.3% of Group 2] and 24 patients in Group 3 [46.2% of Group 3]).
Dysosmia, but not dysgeusia, was inversely associated with sarcopenia (χ2 6.75, p = 0.03). Indeed, 48% of patients with normal smell were sarcopenic compared to 24% of patients with dysosmia (see Table I).
Multinomial logistic regression was run to evaluate the multivariate effect of the collected variables on the likelihood of developing sarcopenia. Our reference category was Group 1 (non-sarcopenic patients with normal grip strength). Four variables were inserted in the final model (age, sex, depression and BMI). Continuous variables were treated like categorical ones. Patients were divided into 2 groups according to age (younger or older than 72 years) and into 2 groups based on BMI (less than 25 or greater than 25). The model significantly predicted the outcome variable better than the null model (p < 0.001) with a 61% accuracy. The model explained 32% (Nagelkerke R2) of the variance in sarcopenia. Three of the 5 predictors were statistically significant: age, depression state and BMI. Increasing age was associated with an increased likelihood of developing sarcopenia (OR = 5.67, p < 0.01). Depression was associated with an increased probability of receiving a diagnosis of probable or confirmed sarcopenia (OR = 8, p = 0.01, OR = 7.4, p = 0.02, respectively). Increasing BMI decreased the probabilities of developing sarcopenia (OR = 0.2, p < 0.01).
DISCUSSION
Patients included in this study presented a reduction in functionality and autonomy (FIM evaluation), justifying the need to admit them to a rehabilitation ward (11). Fatigue, reduction of muscle mass, generalized asthenia, weight loss and sarcopenia were the main clinical conditions observed in this subacute phase, demonstrating the need for multidisciplinary clinical assistance (11, 12, 21).
Approximately 40% of our patients were diagnosed with sarcopenia. Sarcopenia has been defined as a progressive and generalized skeletal muscle disorder that is associated with an increased likelihood of adverse outcomes, including falls, fractures, physical disability and mortality (17). Sarcopenia has been associated with cardiac disease (22), respiratory disease (23) and cognitive impairments (24). It is mainly characterized by low muscle strength and is associated with low muscle quantity and quality (17). Sarcopenia has long been associated with ageing, especially following an acute or chronic disease (25). There is evidence showing that sarcopenia can begin earlier in life and can have many causes beyond ageing (26). Indeed, secondary sarcopenia can be caused by systemic diseases, especially in the case of inflammatory processes, such as tumours or organ failures (17). SARS-CoV-2 infection is particularly characterized by aggressive inflammatory responses that can be strongly implicated in the resulting damage to the airways (27). The overproduction of inflammatory cytokines or chemokines such as interleukin-6 and tumour necrosis factor together with low levels of type I interferons generate an unbalanced immune response that can cause severe systemic symptoms (28). Regarding our results, it is highly probable that sarcopenia might have been induced, at least in part, by the elevated inflammatory response in these patients who were in need of hospitalization. Other factors might have influenced the outcome of sarcopenia, such as physical inactivity because of prolonged bed rest, or an inadequate intake of energy or protein (17). SARS-CoV-2 infection has been associated with a risk of malnutrition that might be related to a reduction of food intake caused by nausea, diarrhea and loss of appetite (16).
Our data showed that sarcopenia is a clinical condition that should be taken into consideration in SARS-CoV-2 patients. Adequate clinical investigations should be undertaken upon admission of the patient in order to counteract underdiagnosed sarcopenia, and the patient’s clinical status should be carefully monitored during the hospitalization by a multidisciplinary team. Indeed, these results further confirm the need for a multidisciplinary team composed of neurologists, physiatrists, psychologists, nutritionists, cardiologists and physiotherapists in the rehabilitation of subacute COVID-19 patients (11).
Our results indicated that sarcopenic patients were the most compromised patients from a functional point of view, the most depressed, with BMI indicative of being underweight, the most aged patients, more often women and with no signs of dysosmia. Our data showed that approximately 40% of patients suffered from mild to moderate depression. It has been reported that psychiatric illnesses, cognitive disorders and depression are frequent in this subacute phase of the disease, as well as in the long term, and are often present concurrently (9, 14, 29–31). These clinical signs of early depression can worsen in the following weeks, leading to the appearance of post-traumatic stress disorder, especially in patients who underwent orotracheal intubation and who have been recovered in ICU in the acute phase (14, 32, 33). Sarcopenia and depression seem to share several common risk factors, such as physical inactivity, upregulation of inflammatory cytokines and dysregulation of hormones in the hypothalamic–pituitary–adrenal axis (36, 37). In their meta-analysis, Chang et al. (38) showed that age, sex and BMI were covariates of sarcopenia (24). They also showed that other confounders were cognitive function, physical performance, activities of daily living, smoking and drinking habits, diabetes mellitus and cardiovascular disease (24). Our data also showed that sarcopenia was highly correlated with decreased functional autonomy, as demonstrated by the FIM evaluation. Our analyses of patients’ clinical symptoms showed a reduced proportion of dysosmia (29%) and dysgeusia (19%) among our population, compared to what has been described of the acute phase of the disease (from about 35% up to 80–90% of patients) in the literature (3, 4, 8–10).
The main limitation of this study lies in the fact that none of these patients benefited from nutritional status evaluation before SARS-CoV-2 infection. Thus, although history of sarcopenia had been excluded from the anamnesis, we cannot rule out the possibility that some of these patients might have already had signs of sarcopenia before their admission to the hospital.
To conclude, our study revealed evidence that hospitalized COVID-19 patients who suffer from functional impairments that prohibit them from being autonomous should be provided with multidisciplinary rehabilitative care that addresses the specific clinical conditions encountered in the subacute phase of COVID-19, including sarcopenia. The complexity of these conditions justifies the need to provide patients with regular follow-ups after the end of the rehabilitative period.
REFERENCES
- Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol 2020; 92(6): 568–576. https://doi.org/10.1002/jmv.25748
- Gautret P, Million M, Jarrot PA, Camoin-Jau L, Colson P, Fenollar F, et al. Natural history of COVID-19 and therapeutic options. Expert Rev Clin Immunol 2020; 16(12): 1159–1184. https://doi.org/10.1080/1744666X.2021.1847640
- Zhou Y, Li W, Wang D, Mao L, Jin H, Li Y, et al. Clinical time course of COVID-19, its neurological manifestation and some thoughts on its management. Stroke Vasc Neurol 2020; 5(2): 177–179. https://doi.org/10.1136/svn-2020-000398
- Vacchiano V, Riguzzi P, Volpi L, Tappatà M, Avoni P, Rizzo G, et al. Early neurological manifestations of hospitalized COVID-19 patients. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 2020; 41(8): 2029–2031. https://doi.org/10.1007/s10072-020-04525-z
- Chen L, Liu M, Zhang Z, Qiao K, Huang T, Chen M, et al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol 2020; 104(6): 748–751. https://doi.org/10.1136/bjophthalmol-2020-316304
- Paliwal VK, Garg RK, Gupta A, Tejan N. Neuromuscular presentations in patients with COVID-19. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 2020; 41(11): 3039–3056. https://doi.org/10.1007/s10072-020-04708-8
- Brugliera L, Filippi M, Del Carro U, Butera C, Bianchi F, Castellazzi P, et al. Nerve compression injuries after prolonged prone position ventilation in patients with SARS-CoV-2: a case series. Arch Phys Med Rehabil 2021; 102(3): 359–362. https://doi.org/10.1016/j.apmr.2020.10.131
- Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis [serial online]. [cited 2020 Jun 30]. Available from: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa330/5811989
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77(6):683–690. https://doi.org/10.1001/jamaneurol.2020.1127
- Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020; 227: 2251–2261. https://doi.org/10.1007/s00405-020-05965-1
- Iannaccone S, Castellazzi P, Tettamanti A, Houdayer E, Brugliera L, de Blasio F, et al. Role of rehabilitation department for adult Covid-19 patients: the experience of the San Raffaele Hospital of Milan. Arch Phys Med Rehabil 2020; 101(9): 1656–1661. https://doi.org/10.1016/j.apmr.2020.05.015
- Brugliera L, Spina A, Castellazzi P, Cimino P, Tettamanti A, Houdayer E, et al. Rehabilitation of COVID-19 patients. J Rehabil Med 2020; 52(4): jrm00046. https://doi.org/10.2340/16501977-2678
- Pasqua F, Biscione GL, Crigna G, Gargano R, Cardaci V, Ferri L, et al. Use of functional independence measure in rehabilitation of inpatients with respiratory failure. Respir Med 2009; 103(3): 471–476. https://doi.org/10.1016/j.rmed.2008.09.007
- Alemanno F, Houdayer E, Parma A, Spina A, Del Forno A, Scatolini A, et al. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: a COVID-rehabilitation unit experience. PLoS One 2021; 16(2): e0246590. https://doi.org/10.1371/journal.pone.0246590
- Brugliera L, Spina A, Castellazzi P, Cimino P, Arcuri P, Deriu MG, et al. Rehabilitative of COVID-19 patients with acute lower extremity Ischemia and amputation. J Rehabil Med 2020; 52(9): jrm00094. https://doi.org/10.2340/16501977-2678
- Brugliera L, Spina A, Castellazzi P, Cimino P, Arcuri P, Negro A, et al. Nutritional management of COVID-19 patients in a rehabilitation unit. Eur J Clin Nutr 2020; 52(4): 1–4. https://doi.org/10.2340/16501977-2678
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48(1): 16–31. https://doi.org/10.1093/ageing/afy169
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49(2): M85–M94. https://doi.org/10.1093/geronj/49.2.M85
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23(1): 56–62. https://doi.org/10.1136/jnnp.23.1.56
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6(4): 278–296. https://doi.org/10.1111/j.2044-8260.1967.tb00530.x
- Iannaccone S, Alemanno F, Houdayer E, Brugliera L, Castellazzi P, Cianflone D, et al. COVID-19 rehabilitation units are twice as expensive as regular rehabilitation units. J Rehabil Med 2020; 52(6): jrm00073. https://doi.org/10.2340/16501977-2704
- Bahat G, İlhan B. Sarcopenia and the cardiometabolic syndrome: a narrative review. Eur Geriatr Med 2016; 7(3): 220. https://doi.org/10.1016/j.eurger.2015.12.012
- Bone AE, Hepgul N, Kon S, Maddocks M. Sarcopenia and frailty in chronic respiratory disease. Chron Respir Dis 2017; 14(1): 85–99. https://doi.org/10.1177/1479972316679664
- Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Association between sarcopenia and cognitive impairment: a systematic review and meta-analysis. J Am Med Dir Assoc 2016; 17(12): 1164.e7–1164.e15. https://doi.org/10.1016/j.jamda.2016.09.013
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997; 127 5 Suppl: 990S–991S. https://doi.org/10.1093/jn/127.5.990S
- Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging 2008; 12(7): 427–432. https://doi.org/10.1007/BF02982703
- Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020; 20(6): 363–374. https://doi.org/10.1038/s41577-020-0311-8
- Neufeldt CJ, Cerikan B, Cortese M, Frankish J, Lee JY, Plociennikowska A, et al. SARS-CoV-2 infection induces a pro-inflammatory cytokine response through cGAS-STING and NF-κB. Commun Biol 2022; 5(1): 1–15. https://doi.org/10.1038/s42003-021-02983-5
- Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun 2020; 89: 594–600. https://doi.org/10.1016/j.bbi.2020.07.037
- Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry [serial online]. 2020 [cited 2020 Jun 30]. Available from: https://www.thelancet.com/journals/lanpsy/article/PIIS2215-0366(20)30287-X/abstract
- Holmes EA, O’Connor RC, Perry VH, Tracey I, Wessely S, Arseneault L, et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry 2020; 7(6): 547–560. https://doi.org/10.1016/S2215-0366(20)30168-1
- Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med 2015; 43(5): 1121–1129.
- Murray H, Grey N, Wild J, Warnock-Parkes E, Kerr A, Clark DM, et al. Cognitive therapy for post-traumatic stress disorder following critical illness and intensive care unit admission. Cogn Behav Ther [serial online]. [cited 2020 Oct 27];13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7251252/
- Kim NH, Kim HS, Eun CR, Seo JA, Cho HJ, Kim SG, et al. Depression is associated with sarcopenia, not central obesity, in elderly korean men. J Am Geriatr Soc 2011; 59(11): 2062–2068. https://doi.org/10.1111/j.1532-5415.2011.03664.x
- Hsu YH, Liang CK, Chou MY, Liao MC, Lin YT, Chen LK, et al. Association of cognitive impairment, depressive symptoms and sarcopenia among healthy older men in the veterans retirement community in southern Taiwan: a cross-sectional study. Geriatr Gerontol Int 2014; 14 Suppl 1: 102–108. https://doi.org/10.1111/ggi.12221
- Budui SL, Rossi AP, Zamboni M. The pathogenetic bases of sarcopenia. Clin Cases Miner Bone Metab Off J Ital Soc Osteoporos Miner Metab Skelet Dis 2015; 12(1): 22–26. https://doi.org/10.11138/ccmbm/2015.12.1.022
- Hallgren M, Herring MP, Owen N, Dunstan D, Ekblom Ö, Helgadottir B, et al. Exercise, physical activity, and sedentary behavior in the treatment of depression: broadening the scientific perspectives and clinical opportunities. Front Psychiatry [serial online]. 2016 [cited 2021 Mar 12];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4786540/
- Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing 2017; 46(5): 738–746. https://doi.org/10.1093/ageing/afx094