REVIEW ARTICLE
THE EFFECTS OF EXERCISE TRAINING ON UPPER EXTREMITY FUNCTION FOR PERSONS WITH MULTIPLE SCLEROSIS: A SYSTEMATIC REVIEW
Valerie E. NEIRA, MS, Tamlynn D. NIEMIETZ, MS, and John W. Farrell III, PhD, CSCS
Clinical Biomechanics and Exercise Physiology Laboratory, Department of Health and Human Performance, Texas State University, San Marcos, TX 78666, USA
Objective: To evaluate the effects of exercise training on upper extremity physical function and physiological fitness outcomes in persons with multiple sclerosis (PwMS).
Methods: A search of 3 electronic databases (EMBASE, CINAHL, and ovidMEDLINE) was conducted following Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The included studies were randomized control trials that reported at least one outcome measure related to upper extremity function, contained a component of exercise training, and included PwMS.
Results: Of the 1381 articles retrieved from the electronic databases, 8 articles met the specific inclusion criteria. All the included articles incorporated strength training exercises into the rehabilitation intervention. Reported outcomes included the 9 Hole Peg Test (9HPT), Action Research Arm Test (ARAT), and Fugl-Meyer Assessment, with 3, 3, and 0 reporting significant improvements, respectively. Only grip strength was included as a physiological fitness outcome, with 2 articles reporting significant improvements.
Conclusion: The results of this review suggest that strength training may elicit improvements in functional and physiological upper extremity outcomes for PwMS. Several limitations of the current review must be noted, including a limited number of studies and the combination of strength training with other rehabilitative modalities.
LAY ABSTRACT
Persons with multiple sclerosis experience reduced strength and function in their arms. The purpose of this review was to examine the evidence available on the effects of using exercise to improve strength and function in the arms of persons with multiple sclerosis. Our research team searched through 1381 studies and found 8 that were relevant to what we were looking for. The 8 studies suggest that exercise, specifically strength training (e.g., weight lifting or resistance exercise), can improve strength and function of the arms in persons with multiple sclerosis. However, the strength training was part of a larger rehabilitation program, and we cannot definitively conclude that all of the improvements were solely because of strength training. Regardless, strength training appears to be an important part of rehabilitation programs aiming to improve strength and function in the arms of persons with multiple sclerosis.
Key words: multiple sclerosis; upper extremity; exercise; strength training; function.
Citation: JRM-CC 2022; 5: jrmcc00087. DOI: http://dx.doi.org/10.2340/jrmcc.v5.2306
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jul 18, 2022; Published: Sep 29, 2022
Correspondence address: John Farrell, Department of Health and Human Performance, Texas State University, 700 Aquarena Springs Drive, San Marcos, TX 78666, USA. Tel: 512-245-3417. Fax: 512-245-2561. E-mail: jwf77@txstate.edu
Competing interests and funding: The authors have no conflicts of interest to declare. No funding supported this work.
Multiple sclerosis (MS) is a chronic inflammatory autoimmune disease of the central nervous system that is often diagnosed in young adulthood (1). Demyelination of axons and the resulting impaired nerve signal conduction are common consequences of MS that result in alterations to the peripheral tissue and the accumulation of disability (1). Persons with MS (PwMS) experience a variety of symptoms, such as fatigue, cognitive impairment, muscle weakness, muscle spasticity, and increased sensitivity to pain (2). Decreases in muscular fitness parameters (e.g., muscular strength and endurance) have been reported in both the upper and lower extremities for PwMS (3). However, rehabilitation interventions have primarily focused on improving muscular fitness of the lower extremities to maintain or enhance mobility outcomes, as mobility is one of the primary determinants of disability status when using the Expanded Disability Status Scale (EDSS).
The rehabilitation paradigm for PwMS has begun to shift to include an emphasis on reducing upper extremity impairment, with significant inverse associations between upper extremity impairment and the ability to perform activities of daily living, independence, and quality of life being observed in PwMS (4, 5). Upper extremity impairment is defined as a combination of motor and sensory symptoms in the proximal or distal aspects of the upper limb that inhibits the ability to perform activities of daily life, resulting in a decrease in quality of life (5). Additionally, a high prevalence of upper extremity impairment in PwMS has been observed, with a previous investigation reporting that of 205 PwMS, 50% reported upper extremity impairment (6). The highest prevalence of impairment was in those with primary or secondary progressive MS (6). The high prevalence and negative consequences of upper extremity impairment in PwMS signifies the importance of identifying efficacious rehabilitative strategies to reduce upper extremity impairment to either maintain or improve upper extremity function.
A plethora of data supports the benefits of exercise training in PwMS, with exercise training guidelines having been established for PwMS with mild-to-moderate disability (7–10). Participation in exercise training for PwMS has been observed to improve muscular strength and endurance, both of which have been significantly associated with improvements in functional outcomes, such as the functional reach test, 4-step square test, and 6-min walk test (3). Exercise training represents a potentially cost-effective and efficacious rehabilitative modality for PwMS for maintaining or improving upper extremity function. However, it should be noted that the majority of MS research for limb impairment has predominantly been done on the lower extremities or does not focus on full upper extremity function (e.g., manual dexterity).
Reducing upper extremity impairment in PwMS through exercise training may positively affect the ability to perform activities of daily living (ADLs) and enhance quality of life. Thus, the purpose of the current review was to evaluate the effects of exercise training on upper extremity physical function and fitness outcomes in PwMS. Current research for exercise training for PwMS has focused on the lower extremity with scarce research on the effects of exercise training on upper extremity function. Because of a lack of research on upper extremity aerobic exercise, this review will contribute to literature by providing guidance for clinicians and researchers on appropriate upper extremity strength training interventions and functional outcome assessments for PwMS. The results of this systematic review will provide a summary of current evidence for upper extremity exercise training as a rehabilitative approach to increase upper extremity functional outcomes for PwMS and will provide recommendations for future investigations and interventions.
METHODS AND MATERIALS
Article inclusion criteria and search strategy
The purpose of the current review was to summarize the available studies that examined the efficacy of interventions incorporating exercise training modalities on upper extremity function for persons diagnosed with MS. Exercise training was defined as “planned structured and repetitive bodily movement performed to improve or maintain one or more components of physical fitness” (11). Engaging in exercise has the potential to improve and maintain functional ability, strength, and quality of life for PwMS (12). Physical function was defined as “the ability to perform both basic and instrumental activities of daily living” (13). Physical function is important to maintain in PwMS because it impacts quality of life, independence, and activities of daily living (14). MS disease status can determine both functional and physiological outcomes. As MS progresses the ability to perform ADLs or participate in exercise is reduced, leading to dependence (15).
This review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The search was conducted using electronic databases (EMBASE, CINAHL, and ovidMEDLINE) using the search terms “Upper extremit*” OR “upper limb*” AND rehab* OR therap* OR treatment* OR intervention*or exercise or physical activity AND “multiple sclerosis or ms”. The search strategy was developed with the assistance of a university librarian and was conducted in February 2021 by VN. Articles were screened by VN and TN with conflicts resolved through discussion.
Inclusion criteria involved full text articles in the English language that included (1) participants diagnosed with either primary progressive, secondary progressive, or relapse-remitting MS; (2) participants over the age of 18 years; (3) interventions that contained a component of exercise training; (4) at least one outcome measure related to upper extremity function; and (5) a randomized controlled trial (RCT) study design was utilized.
Article quality assessment
The quality of each article that met all inclusion criteria following full-text screening was assessed using the Tool for the Assessment of Study Quality and Reporting in Exercise (TESTEX). TESTEX is a tool designed for assessing the quality of articles reporting all exercise training parameters (e.g., intensity, duration, frequency, and mode). This tool uses a 15-point rating scale with 10 points for reporting exercise parameters and 5 points for overall study quality. Greater study quality is indicated by higher scores. Articles were evaluated independently by VN and JWF, with discrepancies resolved through discussion.
Descriptive approach
Data were extracted relative to participant characteristics (e.g., disability status or disease duration), exercise training characteristics (e.g., modality), and physical function and/or physiological outcomes. Data were first extracted by VN and then checked by JWF. Data were categorized by the exercise training intervention. The number of studies that reported statistically significant changes in upper extremity function or physiological outcomes were summarized using descriptive statistics. A meta-analytic approach was not attempted, given the limited number of studies retrieved.
RESULTS
Fig. 1 illustrates the literature search, article screening process, and specifies reasons for article exclusion. The electronic database search retrieved 1381 articles. A total of 336 duplicates were removed, leaving 1045 articles for title, abstract, and full text screening. A total of 1038 articles did not meet specific inclusion criteria, leaving 8 studies included in the systematic quantitative review.
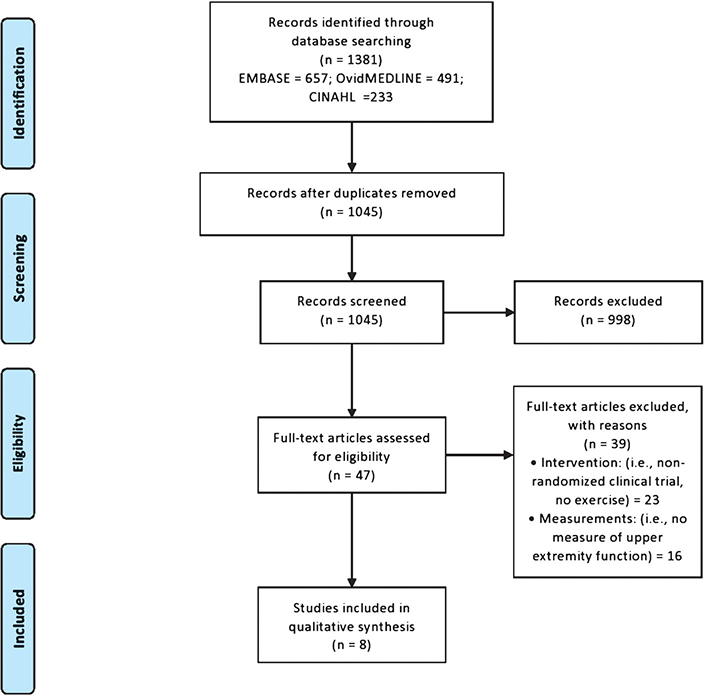
Fig. 1. PRISMA (the preferred reporting items for systematic review and meta-analyses) flow diagram for the literature review process.
All the included studies utilized upper extremity strength training (i.e., exercises targeting improvements in muscular strength) as part of the intervention (n = 8). Table I summarizes the study, participants, and exercise training characteristics. The age of participants in each study ranged from 42.2 to 63.0 years (16–23). Two of the eight articles reported participants with primary and secondary progressive MS (16, 21). Five of the articles in the current review had participants with primary progressive, secondary progressive, and relapse remitting MS (18–20, 22). The included studies consisted of participants with EDSS scores ranging from 3.5 to ≤ 9.0. The following studies reported EDSS scores without standard deviations (16, 19, 20, 23) as summarized in Table I. Disease duration ranged from 11.7 ± 9.2 to 27.0 ± 10.0 years for all the included studies (16–23). Overall, exercise training prescriptions included 30–60 min per session, 2–3 sessions/week for 5–18 weeks. Carpinella et al. reported only total amount of sessions without duration, frequency, or session length (23). In addition to exercise training, other intervention components included task-oriented rehabilitation (16, 17), and robotic and virtual reality training (18 – 21, 23). Overall, the studies included in this review lacked a description of the specifics of strength training (i.e., movements, number of repetitions, number of sets and progression).
| Study characteristics | Participant characteristics | Exercise training characteristics | ||||||
| Reference (Quality) | n (Ex 1; Ex 2; Con) | Age (years) Mean ± SD | Type of MS | EDSS | Disease duration (years) Mean ± SD | Duration (weeks) | Frequency (x/week) | Session length (min) |
| Boffa et al. 2020 (11) | 13; 13; X | 52.0 ± 13.0; 57.0 ± 7.0 | PP, SP | ≤ 7.5 | 19.0 ± 10.0 | 18 | 2 | 60 |
| Bonzano et al. 2019 (11) | 15; 15; X | 49.7 ± 10.5 | RR, SP | 4.3 ± 1.4 | 11.7 ± 9.2 | 8 | 3 | NR |
| Carpinella et al. 2012 (13) | 11; 11; X | 50.8 ± 9.6 | PP, SP, RR | < 9.0 | 20.5 ± 0.0 | 8 sessions | NR | NR |
| Cuesta-Gomez et al. 2020 (14) | 16; X; 14 | 49.8 ± 2.5 | PP, SP, RR | 5.4 ± 0.3 | 15.2 ± 2.4 | 10 | 2 | 60 |
| Feys et al. 2015 (10) | 9; X; 8 | Median 58.0 | PP, SP, RR | 3.5 – 8.5 | Median 25.0 | 8 | 3 | 30 |
| Gandolfi et al. 2018 (11) | 23; X; 21 | 51.9 ± 10.9 | PP, SP, RR | 4.0 – 7.5 | 13.48 ± 7.82 | 5 | 2 | 50 |
| Gijbels et al. 2011 (12) | 9; X; X | 63.0 ± 10.0 | PP, SP | 7.9 ± 0.5 | 27.0 ± 10.0 | 8 | 3 | 30 |
| Ortiz-Rubio et al. 2016 (14) | 19; X; 18 | 42.2 ± 7.5 | PP, SP, RR | 5.7 ± 0.8 | NR | 8 | 2 | 60 |
| Ex 1: experimental group 1; Ex 2: experimental group 2; Con: control group; X: group not present; NR: not reported; MS: multiple sclerosis; EDSS: Expanded Disability Status Scale; PP: Primary Progressive; SP: Secondary Progressive; RR: Relapse Remitting. | ||||||||
Physical function and fitness outcomes are summarized in Table II. The following studies reported scores for both the 9 Hole Peg Test (9HPT) and the Action Research Arm Test (ARAT) for assessing function (16, 17, 20, 22, 23). Feys et al. is the only study included in this review to use the Fugl-Meyer assessment along with ARAT for physical function (19). Five studies reported scores for grip strength as a physiological fitness outcome (17 – 19, 22). Four of the previously listed studies apart from Cuesta-Gomez et al. also reported physical function outcomes with the ARAT.
| Reference | Outcomes | |||
| Physical function | % Δ Physical function (Ex 1, Ex 2, Con) | Physical fitness | % Δ Physical fitness (Ex 1, Ex 2, Con) | |
| Boffa et al. 2020 | ARAT (pts) 9HPT (s) |
0.0, 0.0, NA − 5.2, 1.3, NA |
NA | NA |
| Bonzano et al. 2019 | ARAT R (pts)* ARAT L (pts)* 9HPT R (s)* 9HPT L (s)* |
6.7, 1.1, NA 7.0, 6.1, NA − 7.9, − 11.6, NA − 19.1, − 11.8, NA |
Grip strength R (kg)* Grip strength L (kg)* |
9.5, 5.4, NA 9.2, 7.1, NA |
| Carpinella et al. 2012 | ARAT (pts)* 9HPT (s)* |
7.8, 10.2, NA − 19.3, − 11.8, NA |
NA | NA |
| Cuesta-Gomez et al. 2020 | 9HPT MA (s) 9HPT LA (s) |
− 9.4, NA, 16.5 − 5.1, NA, − 15.2 |
Grip strength MA Grip strength LA*a |
23.5, NA, 0.0 − 6.2, NA, − 3.9 |
| Feys et al. 2015 | Fugl Meyer (pts) ARAT (pts) |
0.0, NA, 1.8 − 5.0, NA, − 2.8 |
Grip strength (kg) | − 1.4, NA, 4.3 |
| Gandolfi et al. 2018 | ARAT (pts) * 9HPT (s) * |
11.9, NA, 8.6 10.5, NA, 15.8 |
NA | NA |
| Gijbels et al. 2011 | ARAT (pts) 9HPT (s) |
8.9, NA, NA − 30.4, NA, NA |
Grip strength (kg) | 1.4, NA, NA |
| Ortiz-Rubio et al. 2016 | ARAT MA (pts) ARAT LA (pts) |
4.0, NA, − 0.3 1.5, NA, − 0.3 |
Grip strength MA (kg) Grip strength LA (kg) |
23.6, NA, 1.2 11.5, NA, –4.7 |
| Δ: change in outcome from pre to post. Ex 1: experimental group 1; Ex 2: experimental group 2; Con: control group; NA: not applicable; MA: more affected side; LA: less affected side; R: right; L: left; pts: points; kg: kilogram; s: seconds; ARAT: Action Research Arm Test; 9HPT: 9 Hole Peg Test. *Statistically significant within-group difference, p < 0.05. **Statistically significant between-group difference, p < 0.05. |
||||
Bonzano et al. saw significant within-group differences for both right and left arms for ARAT, 9HPT, and grip strength (17). Carpinella et al. had statistically significant within-group differences for both ARAT and 9HPT (23). Cuesta-Gomez et al. reported both statistically significant within- and between-group differences for only grip strength on the less affected side (18). Gandolfi et al.’s study indicated that both the ARAT and 9HPT assessments had statistically significant within-group differences (20). Boffa et al. reported only physical function outcome measurements and did not observe statistically significant differences between and within groups (16). The following studies reported both physical function and fitness outcomes but did not show any statistically significant differences for physical function or physical fitness (19, 21, 22).
Study quality
The median (interquartile range [IQR]) overall TESTEX score was 12.5 (2.25), with scores ranging from 10.0 to 14.0. The median (IQR) study quality and study reporting scores were 5.0 (0.25) and 8.0 (2.25), respectively. Overall, studies scored highly for specification of eligibility and randomization, allocation concealment of participants, and reporting similar groups at baseline. The studies in the current review scored poorly for not reporting adverse events, and not reporting exercise intensity, volume, or expenditure.
DISCUSSION
The purpose of the current review was to evaluate the effects of exercise training on upper extremity physical function and fitness outcomes in PwMS. Eight RCTs that included a component of exercise training as part of a rehabilitative intervention and met all inclusion criteria were reviewed (16, 18–23). Overall, significant within-group improvements were observed for the ARAT (17, 20, 23) and 9HPT in 3 studies each (17, 20, 23). Additionally, 2 studies reported significant within-group improvements in grip strength (17, 18). Although these results are promising, caution must be taken in the interpretation of these results because of a limited number of studies, discrepancies in results and reporting, and exercise training being used in combination with other modalities as part of a rehabilitation intervention.
All of the reviewed studies utilized strength training to potentially improve upper extremity function (16–23). Previous systematic reviews and meta-analysis have reported significant improvements in muscular strength and power in PwMS following strength training (9, 10). Researchers have identified enhanced motor unit recruitment, increases in muscle activation, and lean tissue mass as physiological adaptations for the observed improvements in muscular strength and endurance in PwMS (24, 25). These physiological adaptations may be a prerequisite for improvements in physical function. Improvements in muscular strength are significantly associated with improvements in walking performance, balance, hand grip strength, 9HPT scores, and ARAT scores (5). Overall, the reviewed studies prescribed strength training for 30–60 min a day, 2–3 days per week for 5–18 weeks (16–23). This is in line with recommendations from previous systematic reviews and meta-analysis, which recommend PwMS to engage in strength training 2–3 times per week on nonconsecutive days, for an hour and target major muscle groups (12, 26, 27).
The 9HPT is an upper extremity function assessment and is a component of the Multiple Sclerosis Composite Score for disability assessment (5, 28). The 9HPT was utilized in 6 of the 8 articles in this review (16–18, 20, 21, 23). The 9HPT is often used for upper extremity functional outcome assessment because of easy administration, ability to detect disease and disability progression over time, and high reliability and validity (5). However, many researchers criticize the 9HPT as an upper extremity functional outcome, citing that it is a better assessment of fine motor skills and manual dexterity rather than gross motor function of the upper extremities because it requires fingers and hands to complete the tasks (5). Three of the 6 studies that recorded 9HPT reported significant within-group differences (17, 20, 23). A commonality between these 3 articles is that in addition to exercise training, there was also task-oriented training incorporated in the interventions (17, 20, 23). Task-oriented training can be effective for relearning motor movement patterns similar to activities of daily living. Bonzano et al. found that task-oriented training for PwMS reorganizes brain activity toward a pattern of brain activation similar to what can be observed in healthy individuals (17). This could be because of repetition and goal-oriented voluntary limb movement, which results in the inhibition of maladaptive brain plasticity processes that are induced by functional impairment and disuse (17). Boffa et al. observed an effect of task-oriented training in modulating several functional networks that are involved in sensory processing and motor control (16). Strength training in combination with task-oriented training is effective for inducing neuromuscular adaptations, such as increasing the magnitude of efferent neural output from the CNS to activate muscle fibers that enhance muscular power, motor skills, and functional recovery (29, 30). Because of the limitation of the 9HPT being a manual dexterity assessment, future investigations should pair the 9HPT with an upper extremity gross motor function assessment for PwMS.
If assessing upper extremity function, it has been suggested that the 9HPT be used in conjunction with a gross motor functional assessment, such as the ARAT (14). Seven of the studies included in this review used the ARAT to measure the physical function of PwMS (16, 17, 19–22, 31). The ARAT includes lifting and manipulating objects as well as gross movements (5). The ARAT is commonly used in stroke, brain injury, and PwMS as an upper limb functional outcome because of its relatively quick administration and its capability to evaluate both the arm and hand while executing tasks directly related to ADLs (31).The ARAT has notable drawbacks, such as the outcome is limited to a subjective score that describes quality of performance, and has a poor sensitivity to mild impairment (31). However, the ARAT is highly recommended for evaluating full upper extremity functional outcomes. Three of the 7 studies that included the ARAT showed significant within-group differences (17, 20, 32). These 3 studies incorporated task-oriented training in addition to strength training, and also used the 9HPT as an additional functional assessment (17, 20, 32). A possible explanation for the 3 studies that showed improvements for the ARAT is that those studies included task-oriented movement similar to the ones performed during an ARAT assessment (17, 20, 32). The task-oriented training included pinching, grasping, reaching, and manipulating objects (17, 20, 32). The objects would either get heavier or decrease in size over time, making the task more difficult to complete. The studies with significant differences for the ARAT are analogous with the studies that showed significant results for the 9HPT, in that task-oriented training was a component of the rehabilitation intervention. Therefore, future research is necessary to examine whether interventions with strength training alone would elicit improvement in the functional outcomes for PwMS.
Grip strength is a convenient and simple measure of upper extremity function and a good indicator of overall health and strength (20). It was the most common upper extremity physical fitness outcome utilized by the reviewed articles, with 5 of the 8 having included hand grip strength (17–19, 21, 22). Hand grip strength has been significantly associated with an increased odds ratio for experiencing disability and limiting ADLs in PwMS, and has been recommended to be included as a component of routine health assessments (33–35). A drawback of using a grip strength measurement is that participants who have severe swelling or pain in one or both of their hands may be reluctant to participate because of discomfort (34). Two studies (17, 18) that included grip strength reported significant within-group differences, with 1 study (18) reporting significant between-group differences. It should be noted that Bonzano et al. only showed within-group differences for the least affected side (17). Cuesta-Gomez et al. designed one of their virtual games to target grip muscle strength, which could be the reason why a significant result was observed (18). Further investigation is necessary on other possible physiological fitness measurements for the upper extremity for PwMS. A measurement closer in line with activities of daily living, such as assessing elbow flexors and extensors, should be looked into further.
Only 4 of the 8 studies in this review showed significant improvements for physical function (i.e., 9HPT, ARAT), or physical fitness (i.e., grip strength) outcome measures (17, 18, 20, 32). The EDSS classifications for these studies were moderate to severe disability, with EDSS scores ranging from 4.0 to 7.5. A commonality among the studies with significant within- and between-group differences was high-intensity progressive resistance training that also incorporated task-oriented training into the rehabilitation intervention. The exercise prescription utilized in these 4 studies, described by the authors as high-intensity progressive strength training, included progressive incremental increases in duration, force application, number of repetitions, and load, with progressive incremental decreases in object size during task-oriented training (17, 18, 20, 32). Fimland et al. observed that maximal strength training in PwMS was effective in augmenting enhanced efferent motor output of spinal motor neurons, which alleviated some neuromuscular symptoms of MS (24). It has also been observed that progressive strength training in PwMS leads to muscular hypertrophy of type II muscle fibers (36). Another commonality between the studies that showed significant within-group differences was the use of an assistive device, such as robotics (e.g., Armeo spring).The Armeo spring is an adjustable exoskeleton apparatus that allows variable levels of gravity support during movement (21). Partial relief of upper limb weight enables severely affected PwMS to actively produce a larger range of motion (21). However, there is not enough research done on robotics and assistive devices for PwMS to indicate why there could be potential benefits to using assistive devices for upper extremity exercise training.
Previous investigations have observed significant differences between contralateral limbs in both physiological fitness and physical function in PwMS with mild-to-severe disability (35, 37). Additionally, no discernable pattern has been detected as to which side is predominately affected varying from participant to participant. This highlights significant issues with assessments of one side only or classifying limbs based on right and left or dominant or non-dominant, without classifying the limbs as more or less affected. Five of the included studies in the current review reported handgrip strength, with 3 of these studies reporting values for only one side or reporting values for the right and left hand. Either way, it appears no consideration was given as to the appropriate classification of the limbs. This creates a potential scenario where within a group of participants the limb being compared may be the most affected for some and the least affected for others. This has the potential to lead to misinterpretation or distortion of the analysis. Future investigations should consider assessing both limbs for the desired outcome measure and using these results to classify the limbs in a manner, such as “more affected” or “least affected,” to ensure appropriate comparisons for within and between groups.
An interesting finding of the current review was the lack of interventions using upper extremity aerobic exercise. It has been suggested that aerobic exercise can be used as a technique to prime the brain before rehabilitation in order to enhance cortical activation to promote brain plasticity (38). Improvements in finger movement rate with reductions in fatigue and motor fatigability of the upper extremities were observed in PwMS when combining arm cycling and task-oriented training (38). Although no significant improvements were reported for 9HPT and grip strength, the researchers speculated that this may be because of a reduced training intensity and frequency when compared to other interventions observing significant improvements in upper extremity function and manual dexterity (38). Future investigations are required to explore the effects of aerobic upper extremity exercise on upper extremity function for PwMS.
Research targeting upper extremities in MS and other neurological diseases is scarce compared to lower extremity research. A limitation of this current review is the discrepancy in the definition of upper extremity function within the included studies. Some studies refer to manual dexterity as upper limb function. Additionally, it should be noted that some studies used assisted strength training programs (e.g., robotics, Armeo spring, passive motion) instead of minimal assistance. To date, exercise studies for PwMS have not adequately controlled or tracked frequency, duration, and intensity of exercise (39). The studies included in this review also lacked reporting specific descriptions of strength training (i.e., movements, number of repetitions, number of sets and progression).
The results of this systematic review indicate that exercise training, specifically strength training, may elicit improvements of functional and physiological upper extremity outcomes for PwMS. Because of a limited number of studies incorporating upper extremity exercise and functional outcomes for PwMS, further research is necessary. Future studies should report in more detail the exercise intensity, frequency, and duration of strength training for this population. This systematic review provides guidance for clinicians and researchers on appropriate upper extremity strength training interventions and functional outcome assessments for PwMS.
REFERENCES
- Friese MA, Schattling B, Fugger L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat Rev Neurol 2014; 10(4): 225–238. https://doi.org/10.1038/nrneurol.2014.37
- Crayton HJ, Rossman HS. Managing the symptoms of multiple sclerosis: a multimodal approach. Clin Ther 2006; 28(4): 445–460. https://doi.org/10.1016/j.clinthera.2006.04.005
- Sabapathy NM, Minahan CL, Turner GT, Broadley SA. Comparing endurance- and resistance-exercise training in people with multiple sclerosis: a randomized pilot study. Clin Rehabil 2011; 25(1): 14–24. https://doi.org/10.1177/0269215510375908
- Lamers I, Feys P. Assessing upper limb function in multiple sclerosis. Mult Scler J 2014; 20(7): 775–784. https://doi.org/10.1177/1352458514525677
- Feys P, Lamers I, Francis G, Benedict R, Phillips G, LaRocca N, et al. The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult Scler J 2017; 23(5): 711–720. https://doi.org/10.1177/1352458517690824
- Holper L, Coenen M, Weise A, Stucki G, Cieza A, Kesselring J. Characterization of functioning in multiple sclerosis using the ICF. J Neurol 2010; 257(1): 103–113. https://doi.org/10.1007/s00415-009-5282-4
- Latimer-Cheung AE, Martin Ginis KA, Hicks AL, Motl RW, Pilutti LA, Duggan M, et al. Development of evidence-informed physical activity guidelines for adults with multiple sclerosis. Arch Phys Med Rehabil 2013; 94(9): 1829–1836.e7. https://doi.org/10.1016/j.apmr.2013.05.015
- Motl RW, Sandroff BM. Benefits of exercise training in multiple sclerosis. Curr Neurol Neurosci Rep 2015; 15(9): 62. https://doi.org/10.1007/s11910-015-0585-6
- Kjølhede T, Vissing K, Dalgas U. Multiple sclerosis and progressive resistance training: a systematic review. Mult Scler J 2012; 18(9): 1215–1228. https://doi.org/10.1177/1352458512437418
- Jørgensen M, Dalgas U, Wens I, Hvid L. Muscle strength and power in persons with multiple sclerosis – a systematic review and meta-analysis. J Neurol Sci 2017; 376: 225–241. https://doi.org/10.1016/j.jns.2017.03.022
- Bouchard C, Shephard RJ, Brubaker PH. Physical activity, fitness, and health: consensus statement. Med Sci Sports Exerc 1994; 26(1): 119. https://doi.org/10.1249/00005768-199401000-00024
- Latimer-Cheung AE, Pilutti LA, Hicks AL, Martin Ginis KA, Fenuta AM, MacKibbon KA, et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil 2013; 94(9): 1800–1828.e3. https://doi.org/10.1016/j.apmr.2013.04.020
- Garber CE, Greaney ML, Riebe D, Nigg CR, Burbank PA, Clark PG. Physical and mental health-related correlates of physical function in community dwelling older adults: a cross sectional study. BMC Geriatr 2010; 10(1): 6. https://doi.org/10.1186/1471-2318-10-6
- Lamers I, Kelchtermans S, Baert I, Feys P. Upper limb assessment in multiple sclerosis: a systematic review of outcome measures and their psychometric properties. Arch Phys Med Rehabil 2014; 95(6): 1184–200. https://doi.org/10.1016/j.apmr.2014.02.023
- Paltamaa J, Sarasoja T, Leskinen E, Wikström J, Mälkiä E. Measures of physical functioning predict self-reported performance in self-care, mobility, and domestic life in ambulatory persons with multiple sclerosis. Arch Phys Med Rehabil 2007; 88(12): 1649–1657. https://doi.org/10.1016/j.apmr.2007.07.032
- Boffa G, Tacchino A, Sbragia E, Schiavi S, Droby A, Piaggio N, et al. Preserved brain functional plasticity after upper limb task-oriented rehabilitation in progressive multiple sclerosis. Eur J Neurol 2020; 27(1): 77–84. https://doi.org/10.1111/ene.14059
- Bonzano L, Pedullà L, Tacchino A, Brichetto G, Battaglia MA, Mancardi GL, et al. Upper limb motor training based on task-oriented exercises induces functional brain reorganization in patients with multiple sclerosis. Neuroscience 2019; 410: 150–159. https://doi.org/10.1016/j.neuroscience.2019.05.004
- Cuesta-Gómez A, Sánchez-Herrera-Baeza P, Oña-Simbaña ED, Martínez-Medina A, Ortiz-Comino C, Balaguer-Bernaldo-de-Quirós C, et al. Effects of virtual reality associated with serious games for upper limb rehabilitation in patients with multiple sclerosis: randomized controlled trial. J Neuroeng Rehabil 2020; 17(1): 90. https://doi.org/10.1186/s12984-020-00718-x
- Feys P, Coninx K, Kerkhofs L, De Weyer T, Truyens V, Maris A, et al. Robot-supported upper limb training in a virtual learning environment : a pilot randomized controlled trial in persons with MS. J Neuroeng Rehabil 2015; 12(1): 60. https://doi.org/10.1186/s12984-015-0043-3
- Gandolfi M, Valè N, Dimitrova EK, Mazzoleni S, Battini E, Benedetti MD, et al. Effects of high-intensity robot-assisted hand training on upper limb recovery and muscle activity in individuals with multiple sclerosis: a randomized, controlled, single-blinded trial. Front Neurol 2018; 9: 905. https://doi.org/10.3389/fneur.2018.00905
- Gijbels D, Lamers I, Kerkhofs L, Alders G, Knippenberg E, Feys P. The Armeo Spring as training tool to improve upper limb functionality in multiple sclerosis: a pilot study. J Neuroeng Rehabil 2011; 8(1): 5. https://doi.org/10.1186/1743-0003-8-5
- Ortiz-Rubio A, Cabrera-Martos I, Rodríguez-Torres J, Fajardo-Contreras W, Díaz-Pelegrina A, Valenza MC. Effects of a home-based upper limb training program in patients with multiple sclerosis: a randomized controlled trial. Arch Phys Med Rehabil 2016; 97(12): 2027–2033. https://doi.org/10.1016/j.apmr.2016.05.018
- Carpinella I, Cattaneo D, Bertoni R, Ferrarin M. Robot training of upper limb in multiple sclerosis: comparing protocols with or without manipulative task components. IEEE Trans Neural Syst Rehabil Eng 2012; 20(3): 351–360. https://doi.org/10.1109/TNSRE.2012.2187462
- Fimland MS, Helgerud J, Gruber M, Leivseth G, Hoff J. Enhanced neural drive after maximal strength training in multiple sclerosis patients. Eur J Appl Physiol 2010; 110(2): 435–443. https://doi.org/10.1007/s00421-010-1519-2
- Wens I, Dalgas U, Vandenabeele F, Grevendonk L, Verboven K, Hansen D, et al. High intensity exercise in multiple sclerosis: Effects on muscle contractile characteristics and exercise capacity, a randomised controlled trial. Earnest CP, editor. PLoS One 2015; 10(9): e0133697. https://doi.org/10.1371/journal.pone.0133697
- Dalgas U, Stenager E, Ingemann-Hansen T. Review: multiple sclerosis and physical exercise: recommendations for the application of resistance-, endurance- and combined training. Mult Scler J 2008; 14(1): 35–53. https://doi.org/10.1177/1352458507079445
- Cruickshank TM, Reyes AR, Ziman MR. A systematic review and meta-analysis of strength training in individuals with multiple sclerosis or Parkinson disease. Medicine (Baltimore) 2015; 94(4): e411. https://doi.org/10.1097/MD.0000000000000411
- Fischer JS, Rudick RA, Cutter GR, Reingold SC, National MS Society Clinical Outcomes Assessment Task Force. The multiple sclerosis functional composite measure (MSFC): an integrated approach to MS clinical outcome assessment. Mult Scler J 1999; 5(4): 244–250. https://doi.org/10.1177/135245859900500409
- Chowan NC, Singh P. The comparison of the effect of task-oriented training and progressive resistance training in stroke subjects on upper limb function and quality of life in stroke subjects – a randomized clinical trial. Natl J Integr Res Med 2019; 10(5): 19–27.
- da Silva PB, Antunes FN, Graef P, Cechetti F, Pagnussat AS. Strength training associated with task-oriented training to enhance upper-limb motor function in elderly patients with mild impairment after stroke: a randomized controlled trial. Am J Phys Med Rehabil 2015; 94(1): 11–19. https://doi.org/10.1097/PHM.0000000000000135
- Carpinella I, Cattaneo D, Ferrarin M. Quantitative assessment of upper limb motor function in multiple sclerosis using an instrumented Action Research Arm Test. J Neuroeng Rehabil 2014; 11(1): 67. https://doi.org/10.1186/1743-0003-11-67
- Carpinella I, Cattaneo D, Bertoni R, Rovaris M, Caputo D, Ferrarin M. Robot therapy for upper limb rehabilitation in multiple sclerosis: a virtual approach and a functional approach. Gait Posture 2009; 30: S28–S29. https://doi.org/10.1016/j.gaitpost.2009.07.012
- Koda H, Kai Y, Murata S, Osugi H, Anami K, Fukumoto T, et al. Relationship between muscle strength asymmetry and body sway in older adults. J Aging Phys Act 2018; 26(3): 457–461. https://doi.org/10.1123/japa.2017-0096
- McGrath R, Vincent BM, Jurivich DA, Hackney KJ, Tomkinson GR, Dahl LJ, et al. Handgrip strength asymmetry and weakness together are associated with functional disability in aging Americans. J Gerontol Ser A 2021; 76(2): 291–296. https://doi.org/10.1093/gerona/glaa100
- Farrell JW, Motl RW, Learmonth YC, Pilutti LA. Persons with multiple sclerosis exhibit strength asymmetries in both upper and lower extremities. Physiotherapy 2021; 111: 83–91. https://doi.org/10.1016/j.physio.2020.07.006
- Dalgas U, Stenager E, Jakobsen J, Petersen T, Overgaard K, Ingemann-Hansen T. Muscle fiber size increases following resistance training in multiple sclerosis. Mult Scler J 2010; 16(11): 1367–1376. https://doi.org/10.1177/1352458510377222
- Farrell JW, Bemben DA, Black CD, Larson DJ, Pardo G, Fjeldstad-Pardo C, et al. Evaluation of power production asymmetry during cycling in persons with multiple sclerosis. Int J Environ Res Public Health 2019; 16(18): 3445. https://doi.org/10.3390/ijerph16183445
- Gervasoni E, Cattaneo D, Bertoni R, Grosso C, Bisio A, Rovaris M, et al. Effect of arm cycling and task-oriented exercises on fatigue and upper limb performance in multiple sclerosis: a randomized crossover study. Int J Rehabil Res 2019; 42(4): 300–308. https://doi.org/10.1097/MRR.0000000000000362
- Collett J, Dawes H, Meaney A, Sackley C, Barker K, Wade D, et al. Exercise for multiple sclerosis: a single-blind randomized trial comparing three exercise intensities. Mult Scler J 2011; 17(5): 594–603. https://doi.org/10.1177/1352458510391836