ORIGINAL REPORT
SWALLOWING ACTIVATION USING SENSORY STIMULATION IN PATIENTS WITH SEVERE DISORDERS OF CONSCIOUSNESS
Grégoire PRUM, MD1, Rémi MALLART, MD1, Margaux BEATRIX1 and Eric VERIN, MD-PHD1,2
From the 1Rouen University Hospital, Rouen, Normandy and 2EA 3830, GRHV, Rouen Normandy University, Rouen, France
Objective: Swallowing disorders are systematically present in patients with severe brain injury, disorders of consciousness, and subsequently poor quality of life. The study hypothesis was that taste and smell could improve swallowing function and quality of life in such patients, who are fed by gastrostomy tube.
Methods: Eight patients with unresponsive wakefulness syndrome were included in this study. All patients had been in a stable state for at least 2 years, and the delay between the neurological event and the study was always more than 2 years. Strong tastes and smells were selected using the Pfister olfactory classification. Taste and smell stimulations were performed every weekday, Monday to Friday, for 1 week (5 sessions) by a speech and language therapist. Evaluation of swallowing was performed before the first session and after the fifth session, and included the number of spontaneous swallows during 10 min, the presence of drooling, and spontaneous tongue and velum mobility.
Results: The number of spontaneous swallows at the initial evaluation was 6.8 ± 5.1 n/min. At the final evaluation there was a significant increase in the number of spontaneous swallows (9.1 ± 4.1 n/min, p < 0.01).
Conclusion: This clinical observation has shown that taste and smell stimulations are relevant in clinical practice to improve spontaneous swallowing.
LAY ABSTRACT
Swallowing disorders are systematically present in patients with severe brain injury, disorders of consciousness, and subsequent poor quality of life. The study hypothesis was that taste and smell could improve swallowing function in such patients who require exclusive feeding by gastrostomy tube. The study showed that, in patients with severe DoC, taste and smell stimulations increased the number of spontaneous swallows. Based on these findings, taste and smell stimulations could be used in clinical practice to improve swallowing function and overall quality of life.
Key words: consciousness disorders; traumatic brain injury; dysphagia; deglutition disorders; taste; smell.
Citation: JRM-CC 2022; 5: jrmcc00079. DOI: http://dx.doi.org/10.2340/jrmcc.v5.2448
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Mar 31, 2022; Published: June 13, 2022
Correspondence address: Eric Verin, Rouen Normandy University Hospital, 1 rue de Germont, 76031 Rouen cedex. E-mail: eric.verin@univ-rouen.fr
Competing interests and funding: The authors have no conflicts of interest to declare.
Swallowing disorders and oropharyngeal dysphagia are systematically present in patients who have severe brain injury and disorders of consciousness (DoC), who mostly require exclusive feeding by gastrostomy (1). Clinical observations indicate that swallowing and hypersalivation might be induced by taste and smell. Indeed, taste and smell have many links with central neuronal structures, such as the amygdala, the hippocampus, the insula and the orbital frontal cortex, which are also involved in the organization of swallowing (Fig. 1) (2). Olfactory signals reach the olfactory receptor neurones either directly, via the orthonasal route, or, for the majority of signals, indirectly, via the retronasal route from the oral cavity behind the soft palate. After stimulation of the neurones of the olfactory epithelium, the information is transmitted via an axon that crosses the cribriform plate of the ethmoid bone and synapses at the level of the olfactory bulbs, to the cortical olfactory areas (septal area, uncus and amygdala body) and the hypothalamus (3).
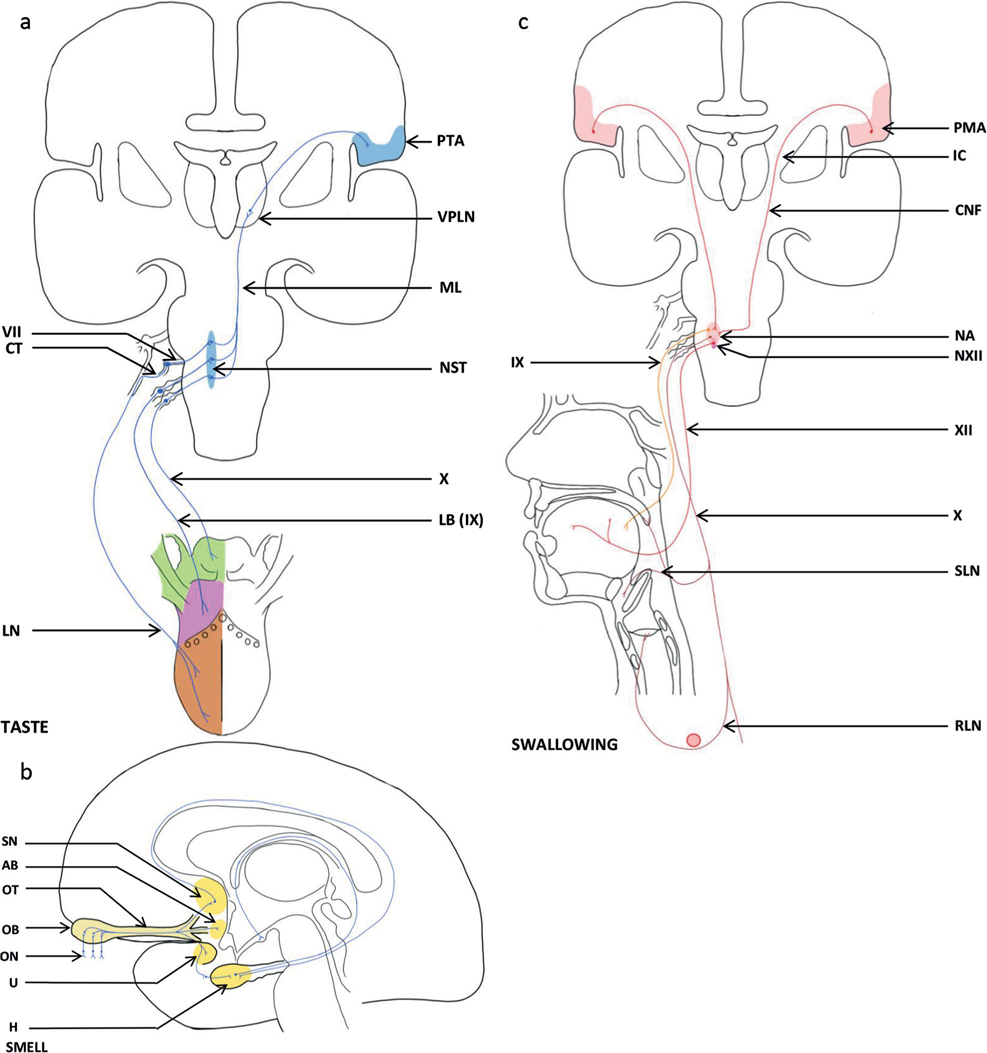
Fig. 1. Neuroanatomy of taste, smell and swallowing showing the different networks involved in: (a) Neuroanatomy of taste in sagittal plane of the brain, (b) Neuroanatomy of smell in median plane of the brain and (c) Neuroanatomy of swallowing in sagittal plane and projections in the oral cavity of swallowing. LN: lingual nerve (mandibular nerve Vc; trigeminal nerve (V)); CT: chorda tympani; VII: facial nerve ; LB (IX): lingual branches (glossopharyngeal nerve (IX)); X: vagus nerve; NST: nuclei of solitary tract (VII, IX, X); ML: medial lemniscus; VPLN: ventral posterolateral nucleus (thalamus); PTA: primitive taste area (post-central gyrus); ON: olfactory nerves; OB: olfactory bulb; OT: olfactory tract; SN: septal nuclei; U: uncus (parahippocampal gyrus; limbic lobe); AB: amygdaloid body; H: hippocampus; PMA: primitive motor area (pre-central gyrus); IC: internal capsule; CNF: corticonuclear fibres (pyramidal tract); NA: nucleus ambiguus (myelencephalon); XI: glossopharyngeal nerve; pharyngeal and stylopharyngeal branches; X: vagus nerve; pharyngeal branch; pharyngeal plexus; SLN: superior laryngeal nerve; RLN: recurrent laryngeal nerve (right: subclavian artery; left: aorta)); NXII: nucleus of hypoglossal nerve (myelencephalon); XII: hypoglossal nerve.
Taste comprises 4 basic gustatory modalities: sweet, sour, salty and bitter. Its transduction is initiated in the taste buds of the oropharynx, larynx and upper third of the oesophagus. The taste buds are innervated by the facial, glossopharyngeal and vagus cranial nerves (4). Peripheral gustatory fibres enter the brainstem and the nucleus tractus solitarius (NTS) before projecting to higher centres. It has been shown that interneurones in the NTS can be either excited or inhibited by taste stimuli (5). Oral sensory input, including taste, plays a critical role in the normal modulation of volitional swallowing (6). Therefore, by activating sensory receptors, taste and other sensory stimuli are likely to provide significant inputs to the NTS and higher centres by regulating swallowing activity. In our experience, patients with unresponsive wakefulness syndrome (UWS) (7) may have spontaneous saliva swallowing. Based on current knowledge of the network of taste and smell and their links with swallowing function, it was therefore hypothesized that taste and smell might induce swallowing in patients with UWS, thus contributing to the process of improvement in swallowing.
METHODS
To examine this hypothesis, this study evaluated taste and smell stimulation in 8 patients with UWS (Table I). All patients had been in a stable state for at least 2 years. The patients’ families gave consent for the patients to participate in this non-invasive and observational study, which was approved by the local ethics committee (Rouen university hospital (E 2021-71)).
Table I. Characteristics of the 8 included patients
| Characteristics of the population |
mean SD |
| Age (y) |
49.6 ± 12.9 |
| Sex (female) |
2 |
| Duration since injury, in years |
8.3 ± 6.4 |
| WHIM (max 62) |
7 ± 6 |
| CRS-R (max 23) |
5 ± 3 |
| SD: standard deviation; WHIM: Wessex Head Injury Matrix; CRS-R: Coma Recovery Scale revised. |
Assessment of level of consciousness was performed using the Coma Recovery Scale revised (CRS-R) (8) and the Wessex Head Injury Matrix (WHIM) (9) at the beginning of the protocol. Strong tastes and smells were selected using the Pfister olfactory classification. Four different tastes were used: fruity (orange, banana), empyreumatic (chocolate, coffee), vegetal (tea, garlic) and spicy (vanilla, mint). To stimulate the sense of smell, these different substances were crushed and placed 5 cm in front of the patient’s nose for 10 min. To stimulate the sense of taste, orange juice, crushed banana, chocolate yogurt, vanilla yogurt, espresso coffee, tea, and mint syrup were used. The different substances were placed on a large cotton swab, which was positioned on the patient’s tongue for 10 min. The swab had a long tail, which was held by the operator. The taste and smell stimulations were performed every weekday, Monday to Friday, for 1 week (5 sessions) by a speech and language therapist, with a washout of 30 min. Before the first session and after the fifth session, the number of spontaneous swallows was counted with 2 fingers placed on the hyoid bone for 10 min. The presence of drooling, spontaneous tongue mobility and velum mobility during gag reflex induced by touching the velum with a tongue depressor (rated from 0 to 4, 0 being the absence of movement) were also assessed.
Data are presented as means and standard deviation (SD). The number of spontaneous swallows, tongue mobility and velum mobility were compared before the first evaluation and after the last evaluation using a Student’s t-test. Differences were considered statistically significant if p < 0.05.
RESULTS
Patients’ characteristics are shown in Table I. All 8 patients had a prolonged DoC, and none of them had an oral diet. All underwent the different tests without any sign of refusal or discomfort. The tests were performed in 6 patients in a sitting position in a wheelchair and in 2 patients in a supine position in bed. Three patients required initial labial stimulation to open their mouth, and no patient rejected the swab placed in their mouth. Head positioning was enabled by the adapted wheelchair. The number of spontaneous swallows at the first evaluation was 6.8 ± 5.1 n/min. Four patients had drooling suggestive of severe oropharyngea dysphagia. At the final evaluation, there was a significant increase in the number of spontaneous swallows (9.1 ± 4.1 n/min, p < 0.01). There was no change in tongue mobility or velum mobility. Drooling was either not clinically modified, or increased slightly, during the test.
DISCUSSION
This simple clinical observational study shows that taste and smell stimulations, performed once a day during 5 consecutive days in patients with UWS increased the number of spontaneous swallows. Two questions should be discussed: first, the mechanism involved in the increased number of swallows after taste and smell stimulations, and secondly, the role of sensory stimulation in the rehabilitation of patients with UWS in arousal unit.
Regarding the effects of taste and smell, it is possible that the heightened sensory input may have been enhanced in our patients who had received no sensory stimulation for at least 2 years. Perhaps all the tasting substances induced a heightened awareness of an unusual flavour in the mouth or nose. Indeed, attention and other emotive dimensions associated with swallowing function have been implicated in functional imaging studies, which have demonstrated activation of, for example, the anterior cingulate cortex during the task of swallowing. Neurological control of swallowing is achieved through the action of cranial nerves, brain stem control centres and the cortex. The mostly mixed pairs of cranial nerves convey the information of the afferent and efferent pathways. Brainstem control of swallowing involves cortical afferents and efferents, allowing the voluntary control of swallowing, which could argue for cortical activity in the study patients. At the cortical level, unlike other somatic functions, the swallowing muscle is bilateral, and asymmetrical with a minor and major hemisphere. The descending pathways are the cortico-nuclear fibres, the internal capsule, and the subthalamus. This influx is projected at the level of the dorsal swallowing group (DSG) and modulates swallowing. The ascending sensory pathways, via the brainstem and the thalamus, transmit information from the buccopharyngeal and laryngeal region to the cortical area (10). Although none of the current patients showed a complete oral phase of swallowing (lip grasping, lingual propulsion and no oral stasis after swallowing), it is important to investigate the existence of an effective oral phase considered as a cortex-mediated reaction.
In our experience, the use of taste and smell stimulation is of interest in patients with severe DoC who are unable to initiate swallowing or perform safe swallowing. After a period of coma, some patients with severe brain injury remain in an altered state of consciousness. Patients with DoC classically receive hydration and nutrition through an enteral-feeding tube. A recent study (11) demonstrated that almost all patients with DoC had severe dysphagia. No patient with UWS had an effective oral phase, which suggests that its presence may be a sign of consciousness. The fact that stimulation of taste and smell could induce spontaneous swallowing could be used as a marker of the beginning of swallowing rehabilitation and a modification of arousal status. This point is of particular interest, since taste and smell involve cortical and hypocampical structures, but are not evaluated with different scales used in UWS, such as the WHIM or the CRS-R, and in our opinion they should be evaluated.
This study has several limitations. It is an uncontrolled study, conducted in a population of patients with prolonged DoC in UWS state, providing only clinical data. The number of swallows, muscle tone, and drooling were not evaluated by nasoendoscopy Fonctional endoscopic evaluation of swallowing (FEES) or videofluoroscopy, Electromyogram (EMG) was not used, and drooling was not quantified. In addition, the period of sensory stimulation studied was very short; hence the effect should be interpreted with caution. This study presents preliminary results, which could be used as the basis for future studies, with the aim of improving swallowing function in these patients with extremely poor quality of life.
It should be noted that, due to unsafe or absent swallowing in these patients with DoC, in clinical practice all nutrition is given via gastrostomy tube and the patient is therefore often not exposed to taste stimulation. Whilst taste stimulation, adapted to minimize the risk of aspiration, may be performed as part of sensory stimulation programmes, and could contribute to improving the quality of life of these patients, there are no established guidelines on this, and many patients do not receive such stimulation.
In conclusion, this simple clinical observational study highlights the possibility of oral activity and swallowing response in patients with UWS. Further studies into swallowing evaluation and rehabilitation in patients with UWS are needed.
ACKNOWLEDGEMENTS
The authors thank Nikki Sabourin-Gibbs, CHU Rouen, for her help in editing the manuscript.
REFERENCES
- Bremare A, Rapin A, Veber B, Beuret-Blanquart F, Verin E. Swallowing disorders in severe brain injury in the arousal phase. Dysphagia 2016; 31: 511–520.
- Marciani L, Pfeiffer JC, Hort J, Head K, Bush D, Taylor AJ, et al. Improved methods for fMRI studies of combined taste and aroma stimuli. J Neurosci Methods 2006; 158: 186–194.
- Cury KM, Uchida N. Robust odor coding via inhalation-coupled transient activity in the mammalian olfactory bulb. Neuron 2010; 68: 570–585.
- Rangachari PK, Rangachari U. Matters of taste: bridging molecular physiology and the humanities. Adv Physiol Educ 2015; 39: 288–294.
- Smith DV, Travers JB, Van Buskirk RL. Brainstem correlates of gustatory similarity in the hamster. Brain Res Bull 1979; 4: 359–372.
- Chee C, Arshad S, Singh S, Mistry S, Hamdy S. The influence of chemical gustatory stimuli and oral anaesthesia on healthy human pharyngeal swallowing. Chemical Senses 2005; 30: 393–400.
- Martino G, Pluchino S, Bonfanti L, Schwartz M. Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol Rev 2011; 91: 1281–1304.
- Kalmar K, Giacino JT. The JFK Coma Recovery Scale – Revised. Neuropsychol Rehabil 2005; 15: 454–460.
- Shiel A, Horn SA, Wilson BA, Watson MJ, Campbell MJ, McLellan DL. The Wessex Head Injury Matrix (WHIM) main scale: a preliminary report on a scale to assess and monitor patient recovery after severe head injury. Clin Rehabil 2000; 14: 408–416.
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 2001; 81: 929–969.
- Melotte E, Maudoux A, Delhalle S, Martial C, Antonopoulos G, Larroque SK, et al. Is oral feeding compatible with an unresponsive wakefulness syndrome? J Neurol 2018; 265: 954–961.
