CASE REPORT
SAFETY AND TOLERABILITY OF STRENGTH TRAINING IN SPINAL AND BULBAR MUSCULAR ATROPHY: A CASE REPORT
VINCENT SHIEH, MD1 CRIS ZAMPIERI, PT, PhD1, PAUL STOUT, DPT1, GALEN O. JOE, MD1, ANGELA KOKKINIS, RN2, KENNETH H. FISCHBECK, MD2, CHRISTOPHER GRUNSEICH, MD2 AND JOSEPH A. SHRADER, PT1
From the 1Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, 2Neurogenetics Branch, National Institute of Neurological Disorders and Stroke and National Institutes of Health, Bethesda, MD, USA
Objective: Spinal and bulbar muscular atrophy is characterized by slow-progressive muscle weakness, decreased functional performance and falls. Research into the use of exercise in spinal and bulbar muscular atrophy has shown equivocal to negative results, although authors suggest that patients with spinal and bulbar muscular atrophy may benefit from both increased exercise intensity and shorter bout duration. The aim of this case report is to explore the safety of a moderate intensity strength training programme coupled with dynamic balance and function-specific training in a patient with spinal and bulbar muscular atrophy.
Case report: A 56-year-old man with spinal and bulbar muscular atrophy presented with multiple falls and declining performance in physical, vocational, and recreational activities. Examination revealed several musculoskeletal impairments that were sub-clinical to mild compared with an SBMA natural history cohort.
Intervention and outcome: A 15-week moderate intensity exercise programme combining weight-lifting and functional exercises was performed under clinical supervision. Exercise volume, frequency and intensity were adjusted based on patient-reported outcomes and muscle damage blood markers. Performance-based and self-reported functional improvements occurred that exceeded the minimal clinically important difference. The intervention was well tolerated and the patient nearly doubled his baseline 10-repetition maximums for weight-lifting exercises.
Conclusion: Exercise therapy combining weight-lifting and upright functional training led to meaningful performance improvements in this case of a patient with spinal and bulbar muscular atrophy and relatively low disease burden.
LAY ABSTRACT
Spinal and bulbar muscular atrophy (SBMA) is a rare neuromuscular disease characterized by slow-progressive muscle weakness, decreased functional performance, and falling. With limited research for guidance, medical practitioners often advise patients with SBMA to avoid weight lifting or intensive exercise. The patient was a high-functioning 56-year-old man with SBMA who struggled with performing daily activities and intensive physical work demands. He participated in a closely monitored 15-week exercise program that combined weight lifting and functional exercises. The patient safely tolerated the program, self-reported physical improvements, and nearly doubled the weight for lifting exercises. This case report highlights one individual with SBMA who benefitted from moderate-intensity exercise, including weight lifting, under careful clinical supervision. More research is needed before this intervention can be recommended for people with SBMA.
Key words: motor neurone disease; spinal and bulbar muscular atrophy; weight lifting; neuromuscular disease.
Citation: JRM-CC 2022; 5: jrmcc00084. DOI: https://doi.org/10.2340/jrmcc.v5.2513
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jun 22, 2022; Published: Aug 18, 2022
Correspondence address: Cris Zampieri, Functional & Applied Biomechanics Section, National Institutes of Health - Clinical Center, Bldg 10 CRC Rm 1-1469, 10 Center Drive, Bethesda, MD 20892, zampierigallac@mail.nih.gov
Competing interests and funding: The authors have no conflicts of interest to declare.
This project was supported in part by intramural funds from the National Institute of Neurological Disorders and Stroke and the National Institutes of Health Clinical Center.
Spinal and bulbar muscle atrophy (SBMA), also known as Kennedy’s disease, is a rare, progressive neuromuscular disease caused by polyglutamine expansion of the androgen receptor gene on the X chromosome. Dysarthria, muscle weakness and cramping, sensory and balance disturbances may emerge in the 5th or 6th decade of life, presenting with decline in physical function, frequent falls, need for gait-assistive devices, and increased caregiver support (1).
SBMA is considered a slowly progressive disease, and men can gradually lose approximately half of their peak strength (2) and are frequently advised to avoid strenuous physical activity and high-resistance exercises, such as weight-lifting. This advice stems from dated research limited primarily to muscular dystrophies (3, 4). However, recent research suggests that people with muscular dystrophies can tolerate and benefit from supervised, resistance exercise (5–7). Sheikh & Vissing describe a generally positive response to moderate intensity strength training in people with various muscle diseases, but a comparatively muted response for those with motor neurone diseases, such as SBMA (8). SBMA appears to share pathophysiological features of both motor neurone and myopathic diseases, as a toxic gain in function is seen in both motor neurones and muscle cells (9).
People with SBMA experience fatigue and reduced exercise tolerance to sustained cycling, but appear to better tolerate and benefit from infrequent, shorter duration high-intensity cycle training (10, 11). The authors surmise the shorter duration may reduce neuronal fatigue, and suggest that higher intensity strength-training programmes be explored as well for people with SBMA (9). To date, only 1 strengthening exercise randomized controlled trial (RCT) is available to help guide exercise prescription in SBMA. The study compared upright functional body-weight exercises with resistance bands with a control group who performed stretching only. Individuals with higher baseline function improved their activity level compared with the control group (measured via accelerometers over 10-day periods at baseline and 12-week follow-up), but functional capacity improved only in those with lower baseline function (12). The authors intended to promote moderate exercise intensity by initially prescribing up to 70% of baseline repetitions completed in 30 s and slowly increasing the intensity. However, the exercise group reported lower than expected ratings of perceived exertion, indicating moderate intensity may not have been achieved for some. The authors speculate the programme intensity may have been too low, especially for higher functioning individuals with SBMA.
This case report aims: (i) to explore the safety and tolerability of moderate intensity resistance exercise, including weight-lifting, coupled with upright functional and balance exercises for a high functioning man with SBMA; and (ii) to identify objective and self-reported measures that capture motor deficits and improvements.
CASE REPORT
Patient history and systems review
The patient was a 56-year-old man, working 50 h per week as a police officer. His medical history was significant for left-sided decompressive spine surgery at age 49 years to relieve L5 nerve root compression radiculopathy, which partially resolved lower extremity weakness and resulting foot drop. The patient was capable of independent community ambulation, self-care, and daily living activities, but reported progressive balance difficulties when walking on uneven terrain. He also reported difficulties ascending 2 flights of stairs consecutively and a considerable decline in his ability to lift weights, which was an occupational requirement. He was diagnosed with SBMA at age 54 years following a muscle biopsy and genetic testing. Genetic testing found 41 CAG repeats in the androgen receptor gene, where 36 is the minimum for diagnosis and 47 is mean for those with SBMA. The patient provided informed consent to participate in this study, which was approved by the National Institutes of Health Institutional Review Board (NIH IRB).
The patient attributed most of his physical difficulties to perceived upper and lower body weakness and was referred to rehabilitation services by his NIH neurologist. He had performed a self-directed functional exercise programme for several months that he reported was not sufficiently challenging. Concerned about passing his annual occupational physical examination, the patient requested guidance in safe weight-training to maintain his strength, functional performance and fitness. He planned to retire soon and his primary goal was to transition to an active outdoor lifestyle with his 2 teenage sons.
Examination
The patient was jointly evaluated by a physical therapist and a physiatrist with experience treating individuals with SBMA. Initial examination did not reveal considerable muscle weakness or functional limitations. He achieved the maximum score of 45 on the Adult Myopathy Assessment Tool (AMAT), indicating relatively high function. Manual muscle testing (MMT) revealed normal 10/10 strength for all major muscle groups, except bilateral intrinsic hand muscles, and left ankle dorsiflexion scoring a grade 9/10 (13). Full active range of motion was observed throughout all joints. He was able to maintain single limb and tandem stances for ≥10 s bilaterally. In addition, he completed 615 m, or 90% of his predicted distance on a timed six-minute walk test (T6MWT) while wearing a left posterior leaf spring ankle foot orthosis (AFO) (14, 15).
Studies have shown that MMT has lower sensitivity than maximal voluntary isometric contraction (MVIC) dynamometry to detect muscle weakness in people with neuromuscular diseases (16, 17). This is especially true for the large and strong hip, thigh, and calf muscles. Given that weakness was the patient’s primary complaint and is a prominent feature of SBMA, this study aimed to examine strength with quantitative muscle assessment (QMA). The MVIC for 7 bilateral muscle groups was measured with a system consisting of a fixed-frame dynamometer (AEVERL Medical, LLC, Gainesville, GA, USA) and load cells (Interface, Scottsdale, AZ, USA) with computer-assisted data acquisition. Strength values are presented in Table I as a comparison to normative data, which account for sex, age, weight, and height (18–20). Objective motor weakness was revealed at the bilateral ankles, shoulders and hips that were not detected by MMT (Table I).
The initial clinical impression was that the patient appeared to be in good physical health based on common rehabilitation screening tests. However, these results did not align with his complaints of loss of function and weakness. Objective MVIC strength testing revealed impaired strength in 6 out of 14 tested muscle groups, which could help explain his symptoms. This prompted further examination, including additional tests and measures, not yet used in SBMA research, but proven valid and reliable in other populations, to better detect any sub-clinical physical impairments.
Table II shows the patient completed a timed 30-s sit-to-stand (STS) test with a total of 10 repetitions, placing him in the 10th percentile of older, healthy adult men aged 60–64 years (21). He self-reported reduced vitality on the Fatigue Severity Scale (FSS) with a score of 28/63, where scores above 36 suggests further medical evaluation is needed (22, 23). He selected and ranked climbing 2 flights of stairs and raking the yard as difficult (5/10) and using a screwdriver above his head as less difficult (8/10), on the Patient-Specific Functional Scale (PSFS) (24). He scored 52/80 on the Lower Extremity Functional Scale (LEFS) and 19.2/100 on the Disability of Arm, Shoulder, and Hand index (DASH), revealing mild functional deficits (25, 26).
Objective forceplate-based balance testing was performed on a NeuroCom Smart EquiTest System (previously Natus Medical Inc., Seattle, WA, USA) and results were compared with an age-referenced normative dataset provided by the manufacturer. Quiet stance tests included the modified Clinical Test of Sensory Interaction on Balance (mCTSIB) and motor control test (MCT). Dynamic balance tests included the Limits of Stability (LOS), and Forward Lunge (FL) tests. Test descriptions can be found in Nashner 1997 (27). The mCTSIB revealed normal use of sensory input to control balance (Fig. 1), which appears to be atypical in individuals with SBMA and may relate to his relatively new onset of symptoms and high functional level (28). However, his preferred Center of Gravity alignment (COG) alignment was abnormal (43% forward of his theoretical limit of stability) during quiet standing. MCT, which tests reactive postural control, identified delayed automatic postural corrections forwards and backwards. LOS testing (intentional weight shifting towards 8 targets) revealed reduced maximal and endpoint excursions (Fig. 2). Other LOS parameters were within healthy reference ranges, including reaction times, movement velocities, and directional control and are not reported. FL testing did not reveal deficits.
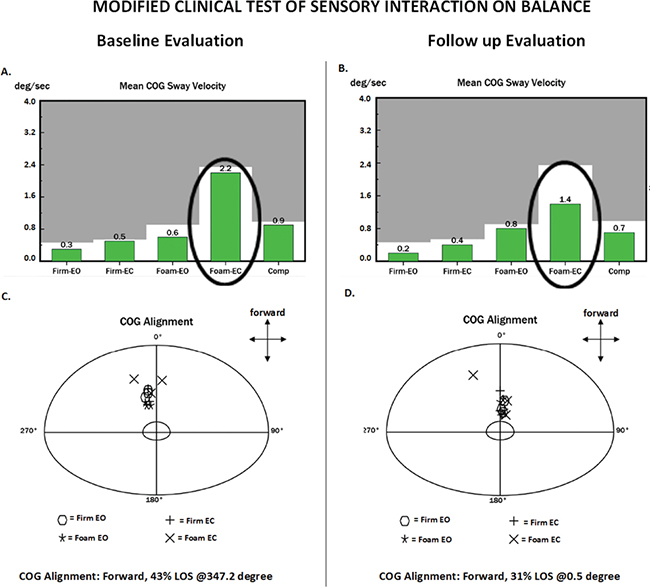
Fig. 1. NeuroCom report of the modified Clinical Test of Sensory Interaction in Balance (mCTSIB) test at baseline (A and C) and follow-up (B and D) evaluations. In this test the patient is required to stand still on firm surface with eyes open (Firm EO), firm surface with eyes closed (Firm EC), foam surface with eyes open (Foam EO) and foam surface with eyes closed (Foam EC). Three trials of 10 s are performed per condition. Green bars on plots A and B represent the mean centre of gravity (COG) sway velocity of the 3 trials per condition. Grey represents the abnormal area. Notice how this patient dramatically decreased his sway velocity on the Foam EC condition from baseline to follow-up (oval circle). The bottom plots (C and D) show a coordinate system denoting the preferred alignment of the patient’s COG during each trial for each condition. The centre circle delimits perfect COG alignment. During testing, the patient is instructed to look straight ahead at a fixation point and receive no feedback about their COG position, so they are unaware of their alignment. The patient achieved a more centred COG preferred alignment at follow-up.
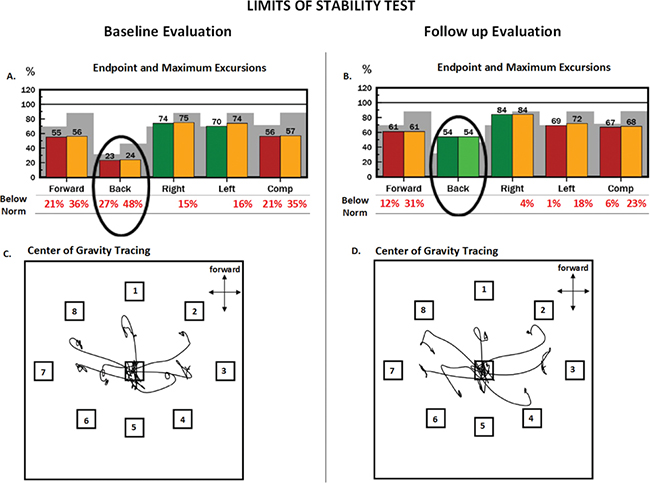
Fig. 2. NeuroCom report of the limits of stability (LOS) test at baseline (A and C) and follow-up (B and D) evaluations. In this test the patient is required to purposefully lean his body toward targets displayed in 8 directions. These targets represent 100% of his theoretical limits of stability. During the leaning task, the patient must maintain a straight posture, only moving at the ankle, like an inverted pendulum. He received visual feedback about the movement of his centre of gravity (COG). Red and yellow bars on plots A and B represent abnormal endpoint and maximum excursions, respectively. Endpoint excursion is how far the patient moves his COG at the initial motion, and maximum excursion is how far the patient moves his COG during the entire 8-s trial. Green bars represent normal excursions. Grey shading represents abnormal excursions that are 2 standard deviations (SD) from normative mean values. Notice how this patient dramatically increased excursions in the backward direction from baseline to follow-up (oval circle). The bottom plots (C and D) show this patient’s COG tracings during the leaning task. During testing, the patient starts in the centre box and leans toward each target in a clockwise manner, starting with target 1. The patient achieved further excursions toward targets 4, 5 and 6, which compose the backward direction, at follow-up.
While the patient’s strength was relatively preserved, he showed impairments in peak muscle strength, function and balance, which explained his subjective complaints. A rehabilitation plan was therefore devised with moderate-intensity exercises that incorporated weight-lifting, upright body-weight function training, and balance training. His relatively low disease burden coupled with the medical teams’ familiarity with SBMA and ability to closely monitor his response to care, in a research-hospital setting, provided a unique opportunity to explore this intervention.
Exercise programme
Physical therapists designed a 15-week moderate-intensity exercise programme that included elements of previous SBMA functional exercise research, the American College of Sports Medicine Guidelines for Strength Training and the Physical Activity Guidelines for Americans (12, 29, 30). The programme combined free-weights, a global gym multi-station apparatus, balance and functional exercises that could be performed supervised in the National Institute of Health Clinical Center’s gym once a week and unsupervised at an independent health club twice per week. The strengthening exercises targeted the upper and lower body muscle groups with overhead presses, chest presses, arm flies, latissimus pull-downs, bent-over rows, 30-s prone forearm planks, and double-limb knee extensions. Function-specific exercises included single-limb standing, repeated sit-to-stand, squats, step-ups, and lunges that targeted upright postural control and dynamic balance.
Several measures to ensure patient safety were implemented. His response was monitored during and following each on-site exercise session with a symptom checklist recording the presence of muscle cramps, fatigue, perceived recovery from the previous session and adverse events (i.e. falls, chest pain, shortness of breath, joint pain, and dizziness). The patient completed the OMNI rating of perceived exertion (RPE) for each exercise, where 0–2 was very easy, 3–4 was somewhat easy, 5–6 was somewhat difficult, 7–8 was very difficult, and 9–10 was so difficult that I need to stop (31). He also rated muscle soreness in response to the intervention within 48 h after training, using a scale where 0 was no soreness, 1–3 was mild soreness, 4–6 moderate soreness, and 7–10 was severe soreness. In addition, a neurologist evaluated blood levels of creatine kinase (CK), lactate dehydrogenase (LDH), and insulin-like growth factor (IGF-1) at the start of the programme and as needed if the patient reported increased soreness or lacked exercise progression.
Training initiation
Training programme details are outlined in Table III. To ensure moderate intensity, a preliminary training session determined the initial training weight by 10 repetition maximum (10RM). If our patient was unable to perform 10 repetitions of any exercise, the load was decreased. Physical therapists adjusted the exercise volume (sets and repetitions), frequency (sessions per week), and intensity (load), with the intention that he would report a RPE of 6/10 (somewhat difficult) and soreness rating at or below 3/10 (mild soreness) throughout the programme. Squats, step-ups, lunges, and planks were initially completed at body weight only.
RESULTS
Training progression
The patient attended 14 of 15 supervised sessions and 20 of 24 unsupervised sessions, missing 5 sessions total for personal and work-related reasons. It was decided to increase exercise volume, frequency, and load separately to help identify his tolerance for each. Exercise volume was increased initially at week 4 from 1 to 2 sets per session, and frequency was then increased 3 weeks later from 2 to 3 sessions each week. Exercise load was increased carefully and gradually from weeks 8 to 15: global gym double-knee extension increased progressively from 18.1 to 31.8 kg; other global gym exercises increased progressively from 22.7 to 36.3 kg; free-weight exercises increased from 6.8 to 11.3 kg each; squats included two 9.1 kg free-weights; planks and single-leg balance were increased from 30 to 60 s. After checking CK levels and the symptom checklist as training progressed, single-limb balance exercises were added to the programme at week 7. At week 12, free-weights were added to the squats.
Safety monitoring
During the 15-week training programme, the patient’s CK levels ranged between 551 and 989 U/L, a range well within expected values in non-exercising men with SMBA (12). His LDH (168–192) and IGF-1 (91–115 U/L) levels were within a healthy range (2, 12). The patient reported appropriate recovery and no adverse events throughout the intervention programme. He further reported no fatigue immediately after the intervention concluded. From weeks 1 to 7, his mean perceived exertion was 3.5 and soreness was 1.6, while increasing exercise volume and frequency. From weeks 8 to 11, he reported mean exertion of 3.6 and soreness of 2.9, while beginning to increase exercise load. From weeks 12 to 15, his mean exertion was 5.1 and soreness was 1.6 as load was further increased.
Strength
MVICs for all muscle groups at follow-up are presented in Table I as a percentage of predicted. The post-training QMA assessment revealed a full-body muscle strength increase by 34 kg, from 84% to 88% of predicted strength. Strength increased in lower extremities by 41 kg, from 83% to 93% of predicted, most of which came from bilateral plantarflexion and right hip extension increases. The upper extremity decreased by 7 kg, from 81% to 77%.
Functional performance
At the end of training the patient continued to achieve the maximum AMAT score, and his forward lunge dynamic balance parameters remained within normal limits. He walked an additional 5 m on the T6MWT, but this was not clinically significant. The timed 30-s STS performance improved from below the 10th to nearly the 50th percentile for age-matched healthy men, exceeding the minimally clinically import difference (MCID) (Table II) (32). He improved beyond the MCID for LEFS and 2 of his 3 selected PSFS tasks, except for screwdriver use overhead. His follow-up DASH improved, but did not quite reach the MCID. Finally, the patient self-reported that fatigue no longer impacted his daily activity, with the lowest possible rating of 9 on the FSS.
Balance
At follow-up, the patient’s sway velocity had reduced by 36% in the most difficult condition of the mCTSIB (standing on foam with eyes closed), which indicates improvement using sensory information for postural control (Fig. 1). His preferred COG alignment remained anterior, but improved from 43% to 31% of his theoretical LOS (Fig. 1). His automatic postural responses on the MCT remained abnormally delayed. He improved the LOS endpoint and maximal excursions in almost all directions, but most dramatically backwards (Fig. 2). Changes in FL were not observed.
DISCUSSION
Moderate intensity exercise, including weight lifting, was well tolerated
To our knowledge, this is the first report detailing weight-lifting techniques as an intervention for a high-functioning patient with SBMA. He safely performed a 15-week exercise programme, combining weight-lifting, dynamic balance and function-specific exercises, while avoiding muscle injury. No adverse symptoms were reported, and the OMNI rating scales of perceived exertion and soreness ensured that training eventually reached moderate intensity with minimal muscle soreness. Physiological markers, such as CK, LDH, and IGF-1 values, were not elevated beyond the reference ranges observed in patients with SBMA. Importantly, the patient did not report post-exertional fatigue with this hybrid resistance and functional training programme, as was seen in a study of aerobic training in people with SBMA (10).
Safely dosing weight-lifting for a person with SBMA
Very few published works were available to help guide us in dosing the exercise intervention. The initial training loads were determined with a 10RM technique because similar variations have been safely used in previous strength-training studies for groups with heterogeneous neuromuscular disease (4, 33). A 1-repetition maximum technique was considered, but not used due to risk of muscle injury. Despite acquiring quantitative MVIC data for 7 muscle groups, these laboratory-derived single-joint isometric muscle actions were not representative of the compound isotonic weight-lifting techniques and body-weight functional exercises. Therefore, it was decided not to dose the intervention on MVIC data.
The patient reported moderate isolated quadriceps soreness during the first 2 weeks of training. As a result, the global gym knee extension volume and frequency were not increased at the same rate as other exercises. In addition, lunge depth was reduced during this time, while acceptable CK values were ascertained. Our experience treating individuals with SBMA informed us that quadriceps muscles are often the most affected lower extremity muscle group and can be initially challenging to train due to soreness (2). With close monitoring, the patient eventually increased the global gym double-limb knee extensions to 31.8 kg, 75% more than his initial 10RM load, and performed full-depth lunges each at the same volume and frequency of other exercises. Improvements in quad strength and quad dominant functional exercises appeared to lag behind those seen in other muscle groups throughout the intervention requiring patience from the patient and clinicians. Many of the patient’s gains in submaximal strength were seen during the final 4 weeks, which could have been missed with a 12-week programme.
In a previous 12-week home-based functional exercise study, subjects with relatively high baseline function reported ratings of perceived exertion (RPE) in the 3/10 “somewhat easy” scale range (12). This participant reported the highest intensity “somewhat difficult” (mean RPE 5.1/10) during the last 4 weeks of the programme, when only the load was being increased. This training programme was performed in a “circuit” fashion at an intended higher intensity than was used in the previous study (12). Recent pilot work suggests that people with SBMA may respond better to exercise (cycling) containing higher intensity and shorter duration (11). While cause-effect relationships are beyond the scope of case reports, we postulate that the higher intensity employed by our patient, coupled with shorter bursts of exercise (each set of repetitions lasting 30–45 s) may have facilitated improvements in functional performance, while also keeping muscle soreness and post-exertional fatigue low.
Careful selection of clinical outcome measures
An important consideration for this case was selecting appropriate outcome measures to track the patient’s response to the intervention. Tools commonly used in SBMA research, such as AMAT, MMT, and 6MWT, were unable to identify impairments initially due to his high functional level. Continuous scale measures, such as muscle dynamometry, 30-s STS, and instrumented balance, testing along with patient-selected and patient-reported outcomes, were essential to detect the patient’s initial impairments and training response.
Clinical relevance of plantar flexor strengthening and weight-bearing functional exercises on dynamic balance
The patient initially reported loss of balance and falls, a common complaint in people with SBMA, but reported no falls during the intervention and follow-up periods (2). Objective balance measurements revealed impairments of dynamic and static postural control. Strength of bilateral ankle plantarflexion (APF) improved following the intervention from 60% to 100% of predicted. We speculate this strength gain may partially explain the improvements in LOS backward excursions and his improved mCTSIB COG alignment. There is evidence that calf strength plays a key role in anterior/posterior LOS, and APF strengthening improves dynamic balance in older adults (34, 35).
The improvement in preferred standing alignment in the foam with eyes closed mCTSIB condition could be clinically significant, since individuals with SBMA have poor performance while standing on foam with eyes closed, reflecting impaired proprioception (28). We speculate that the relatively high dose of upright balance and functional exercises (estimated 3,720 repetitions in total) may have augmented his APF strength and proprioception. There is evidence of improved proprioception in subjects with osteoarthritis who performed weight-bearing knee-strengthening exercises compared with those who performed similar non-weight-bearing strengthening exercises (36). Right hip extensor strength improvement also may have led to improved postural control during LOS testing; however, we did not make other objective core strength measurements. Finally, we suggest that the coupled effects of bringing his preferred COG from a more forward position towards centre and normalizing his backward LOS excursions may help reduce fall risk in the backward direction.
Functional improvements and increased exercise intensity despite few MVIC improvements
Isometric peak force does not always improve as functional performance does. While our patient’s MVIC generally did not improve, except for APF strength mentioned above, he demonstrated improved submaximal muscle performance capacity, advancing the 10RM load and volume for all exercises and increasing STS, LEFS, and PSFS outcomes. Reports have shown non-significant changes in isometric peak force after a 12-week progressive resistance strength training programme, while dynamic muscle strength improved, in patients with sporadic inclusion body myositis and children with Spinal muscular atrophy (SMA) (6, 37). Clearly, aspects of muscle performance other than peak force can lead to improved functional performance and balance but were beyond the scope of this report. Future research designs emphasizing muscle endurance, motor control, and dynamic balance may help clinicians to optimize function and quality of life for people with SBMA.
In conclusion, the patient was able to safely perform and tolerate 15-weeks of progressive resistance training 3 times per week with a combination of free and global gym weights and body-weight functional and dynamic balance exercises. Importantly, he received a high degree of supervision and consistent monitoring for adverse effects in an outpatient hospital setting. He reported high satisfaction with the intervention and functional improvements on several self-report questionnaires. Twelve months after the intervention, the patient reported that he had retired and was enjoying an outdoor recreational lifestyle, managing his 10-acre property. Research with appropriate design, adequate sample size, and objective continuous scale outcome measures is needed to determine if people with SBMA might benefit from similar training programmes.
REFERENCES
- Fratta P, Nirmalananthan N, Masset L, Skorupinska I, Collins T, Cortese A, et al. Correlation of clinical and molecular features in spinal bulbar muscular atrophy. Neurology 2014; 82 (23): 2077–2084. doi: 10.1212/WNL.0000000000000507. Epub 2014 May 9. PMID: 24814851; PMCID: PMC4075620.
- Rhodes LE, Freeman BK, Auh S, Kokkinis AD, La Pean A, Chen C, et al. Clinical features of spinal and bulbar muscular atrophy. Brain 2009; 132 (Pt 12): 3242–32451. doi: 10.1093/brain/awp258. PMID: 19846582; PMCID: PMC2792370.
- Johnson EW, Braddom R. Over-work weakness in facioscapulohuumeral muscular dystrophy. Arch Phys Med Rehabil 1971; 52 (7): 333-336. PMID: 5565885.
- Kilmer DD, McCrory MA, Wright NC, Aitkens SG, Bernauer EM. The effect of a high resistance exercise program in slowly progressive neuromuscular disease. Arch Phys Med Rehabil 1994; 75 (5): 560–563. PMID: 8185450.
- Sveen ML, Andersen SP, Ingelsrud LH, Blichter S, Olsen NE, Jønck S, et al. Resistance training in patients with limb-girdle and becker muscular dystrophies. Muscle Nerve 2013; 47 (2): 163–169. doi: 10.1002/mus.23491. Epub 2012 Nov 21. PMID: 23169433.
- Lewelt A, Krosschell KJ, Stoddard GJ, Weng C, Xue M, Marcus RL, et al. Resistance strength training exercise in children with spinal muscular atrophy. Muscle Nerve 2015; 52 (4): 559–567. doi: 10.1002/mus.24568. PMID: 25597614; PMCID: PMC4506899.
- van den Berg LE, Favejee MM, Wens SC, Kruijshaar ME, Praet SF, Reuser AJ, et al. Safety and efficacy of exercise training in adults with Pompe disease: evalution of endurance, muscle strength and core stability before and after a 12 week training program. Orphanet J Rare Dis 2015; 10: 87. doi: 10.1186/s13023-015-0303-0. PMID: 26187632; PMCID: PMC4506616.
- Sheikh AM, Vissing J. Exercise therapy for muscle and lower motor neuron diseases. Acta Myol 2019; 38 (4): 215–232. PMID: 31970320; PMCID: PMC6955630.
- Dahlqvist JR, Vissing J. Exercise therapy in spinobulbar muscular atrophy and other neuromuscular disorders. J Mol Neurosci 2016; 58 (3): 388–393. doi: 10.1007/s12031-015-0686-3. Epub 2015 Nov 19. PMID: 26585990.
- Preisler N, Andersen G, Thøgersen F, Crone C, Jeppesen TD, Wibrand F, et al. Effect of aerobic training in patients with spinal and bulbar muscular atrophy (Kennedy disease). Neurology 2009; 72 (4): 317–323. doi: 10.1212/01.wnl.0000341274.61236.02. PMID: 19171827.
- Heje K, Andersen G, Buch A, Andersen H, Vissing J. High-intensity training in patients with spinal and bulbar muscular atrophy. J Neurol 2019; 266 (7): 1693–1697. doi: 10.1007/s00415-019-09316-x. Epub 2019 Apr 19. PMID: 31004213.
- Shrader JA, Kats I, Kokkinis A, Zampieri C, Levy E, Joe GO, et al. A randomized controlled trial of exercise in spinal and bulbar muscular atrophy. Ann Clin Transl Neurol 2015; 2 (7): 739–747. doi: 10.1002/acn3.208. Epub 2015 May 7. PMID: 26273686; PMCID: PMC4531056.
- Harris-Love MO, Fernandez-Rhodes L, Joe G, Shrader JA, Kokkinis A, La Pean Kirschner A, et al. Assessing function and endurance in adults with spinal and bulbar muscular atrophy: validity of the adult myopathy assessment tool. Rehabil Res Pract 2014; 2014: 873872. doi: 10.1155/2014/873872. Epub 2014 May 5. PMID: 24876969; PMCID: PMC4026974.
- Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 1998; 158 (5 Pt 1): 1384–1387. doi: 10.1164/ajrccm.158.5.9710086. Erratum in: Am J Respir Crit Care Med 2020; 201 (3): 393. PMID: 9817683.
- Takeuchi Y, Katsuno M, Banno H, Suzuki K, Kawashima M, Atsuta N, et al. Walking capacity evaluated by the 6-minute walk test in spinal and bulbar muscular atrophy. Muscle Nerve 2008; 38 (2): 964–971. doi: 10.1002/mus.21077. PMID: 18642379.
- Andres PL, Skerry LM, Thornell B, Portney LG, Finison LJ, Munsat TL. A comparison of three measures of disease progression in ALS. J Neurol Sci 1996;139 Suppl:64–70. doi: 10.1016/0022-510x(96)00108-6. PMID: 8899661.
- Harris-Love MO, Shrader JA, Davenport TE, Joe G, Rakocevic G, McElroy B, et al. Are repeated single-limb heel raises and manual muscle testing associated with peak plantar-flexor force in people with inclusion body myositis? Phys Ther 2014; 94 (4): 543–552. doi: 10.2522/ptj.20130100. Epub 2013 Dec 5. PMID: 24309617; PMCID: PMC3973819.
- Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther 1996;76 (3): 248–259. doi: 10.1093/ptj/76.3.248. PMID: 8602410.
- Muscular weakness assessment: use of normal isometric strength data. The National Isometric Muscle Strength (NIMS) Database Consortium. Arch Phys Med Rehabil 1996; 77 (12): 1251–1255. doi: 10.1016/s0003-9993(96)90188-4. PMID: 8976307.
- Stoll T, Huber E, Seifert B, Michel BA, Stucki G. Maximal isometric muscle strength: normative values and gender-specific relation to age. Clin Rheumatol 2000; 19 (2): 105–113. doi: 10.1007/s100670050026. PMID: 10791620.
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 1999; 70 (2): 113-119. doi: 10.1080/02701367.1999.10608028. PMID: 10380242.
- Anton HA, Miller WC, Townson AF. Measuring fatigue in persons with spinal cord injury. Arch Phys Med Rehabil 2008; 89 (3): 538–542. doi: 10.1016/j.apmr.2007.11.009. PMID: 18295634; PMCID: PMC3595300.
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46 (10): 1121–1123. doi: 10.1001/archneur.1989.00520460115022. PMID: 2803071.
- Horn KK, Jennings S, Richardson G, Vliet DV, Hefford C, Abbott JH. The patient-specific functional scale: psychometrics, clinimetrics, and application as a clinical outcome measure. J Orthop Sports Phys Ther 2012; 42 (1): 30–42. doi: 10.2519/jospt.2012.3727. Epub 2011 Oct 25. PMID: 22031594.
- Binkley JM, Stratford PW, Lott SA, Riddle DL. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys Ther 1999; 79 (4): 371–383. PMID: 10201543.
- Franchignoni F, Vercelli S, Giordano A, Sartorio F, Bravini E, Ferriero G. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J Orthop Sports Phys Ther 2014; 44 (1): 30–39. doi: 10.2519/jospt.2014.4893. Epub 2013 Oct 30. PMID: 24175606.
- Nashner LM. Chapter 13: Computerized dynamic posturography. In: Jacobson GP, Newman CW, Kartush JM, editors. Handbook of balance function testing. San Diego, CA: Singular Publishing Group; 1997.
- Anagnostou E, Zachou A, Breza M, Kladi A, Karadima G, Koutsis G. Disentangling balance impairments in spinal and bulbar muscular atrophy. Neurosci Lett 2019; 705: 94–98. doi: 10.1016/j.neulet.2019.04.044. Epub 2019 Apr 23. PMID: 31026532.
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al.; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43 (7): 1334–1359. doi: 10.1249/MSS.0b013e318213fefb. PMID: 21694556.
- Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The Physical Activity Guidelines for Americans. JAMA 2018; 320 (19): 2020–2028. doi: 10.1001/jama.2018.14854. PMID: 30418471.
- Robertson RJ, Goss FL, Rutkowski J, Lenz B, Dixon C, Timmer J, et al. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med Sci Sports Exerc 2003; 35 (2): 333–341. doi: 10.1249/01.MSS.0000048831.15016.2A. PMID: 12569225.
- Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther 2011; 41 (5): 319–327. doi: 10.2519/jospt.2011.3515. Epub 2011 Feb 18. PMID: 21335930.
- Milner-Brown HS, Miller RG. Muscle strengthening through high-resistance weight training in patients with neuromuscular disorders. Arch Phys Med Rehabil. 1988; 69 (1): 14–19. PMID: 3337636.
- Ribeiro F, Teixeira F, Brochado G, Oliveira J. Impact of low cost strength training of dorsi- and plantar flexors on balance and functional mobility in institutionalized elderly people. Geriatr Gerontol Int 2009; 9 (1): 75–80. doi: 10.1111/j.1447-0594.2008.00500.x. PMID: 19260983.
- Holviala J, Häkkinen A, Alen M, Sallinen J, Kraemer W, Häkkinen K. Effects of prolonged and maintenance strength training on force production, walking, and balance in aging women and men. Scand J Med Sci Sports. 2014; 24 (1): 224–233. doi: 10.1111/j.1600-0838.2012.01470.x. Epub 2012 Apr 29. PMID: 22540957.
- Jan MH, Lin JJ, Liau JJ, Lin YF, Lin DH. Investigation of clinical effects of high- and low-resistance training for patients with knee osteoarthritis: a randomized controlled trial. Phys Ther 2008; 88 (4): 427–436. doi: 10.2522/ptj.20060300. Epub 2008 Jan 24. PMID: 18218827.
- Spector SA, Lemmer JT, Koffman BM, Fleisher TA, Feuerstein IM, Hurley BF, et al. Safety and efficacy of strength training in patients with sporadic inclusion body myositis. Muscle Nerve 1997; 20 (10): 1242–1248. doi: 10.1002/(sici)1097-4598(199710)20:10<1242::aid-mus6>3.0.co;2-c. PMID: 9324080.