ORIGINAL REPORT
WHOLE-BODY CRYOSTIMULATION: A REHABILITATION BOOSTER
Paolo CAPODAGLIO, MD1,2, Riccardo CREMASCOLI, MD3,4, Paolo PITERÀ, MSc1,5 and Jacopo M. FONTANA, PhD1
From the 1Orthopedic Rehabilitation Unit and Research Laboratory in Biomechanics and Rehabilitation, Istituto Auxologico Italiano, IRCCS, San Giuseppe Hospital, Verbania, Italy, 2Department of Surgical Sciences, Physical Medicine and Rehabilitation, University of Turin, Turin, Italy, 3Department of Neurology and Neurorehabilitation, Istituto Auxologico Italiano, IRCCS, San Giuseppe Hospital, Verbania, Italy, 4Department of Neurosciences, University of Turin, Turin, Italy, 5Department of Food, Environmental and Nutritional Sciences (DeFENS), International Center for the Assessment of Nutritional Status (ICANS), University of Milan, Milan, Italy.
A growing body of work suggests that whole-body cryostimulation (WBC) could play a role as a promising adjuvant therapy in various conditions of rehabilitation interest. In fact, WBC is currently being used to relieve symptoms in rheumatoid arthritis, fibromyalgia, ankylosing spondylitis, depression and anxiety, multiple sclerosis, sleep disturbances, muscle soreness after strenuous physical exercise, post-Covid syndrome and obesity. WBC is not only a symptomatic physical therapy but rather represents an “adaptation therapy” because of the repeated shock-like cryogenic cold stimulus over the entire body surface that induces reactions in the autonomic, endocrine, circulatory, neuromuscular and immunological systems, resulting in an adaptation that contributes to the restoration of the homeostatic state. Therefore, based on the existing evidence, WBC can be described as follows:
- a “training method” for the autonomic nervous system;
- a novel anti-inflammatory and antioxidant treatment;
- a treatment with beneficial effects on body composition and adipose tissue.
In our opinion, the powerful effects of thermal stress on the physiological responses of the human body present unique features that could potentially be exploited to boost rehabilitation outcomes in various conditions. Therefore, we believe it is important to highlight the potential use of WBC for medical use and emphasize its relevance in the field of rehabilitation with the aim of stimulating scientific studies on the efficacy of WBC as an adjuvant treatment in various conditions of rehabilitation interest.
LAY ABSTRACT
A growing body of work suggests that whole-body cryostimulation (WBC) could play a role as a promising adjuvant therapy in various conditions of rehabilitation interest, as it can act as
- a “training method” for the autonomic nervous system;
- a novel anti-inflammatory and antioxidant treatment;
- a treatment with beneficial effects on body composition and adipose tissue.
Therefore, we want to highlight the potential use of WBC for medical use and its relevance in the field of rehabilitation with the aim of stimulating scientific studies on the efficacy of WBC as an adjuvant treatment in various conditions of rehabilitation interest.
Key words: Whole-body Cryostimulation; Rehabilitation; Low-grade inflammation; Oxidative stress; Cryotherapy.
Citation: JRM-CC 2022; 5: jrmcc00086. DOI: http://dx.doi.org/10.2340/jrmcc.v5.2810
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jul 15, 2022; Published: Sep 19, 2022
Correspondence address: Capodaglio Paolo, Orthopedic Rehabilitation Unit and Research Laboratory in Biomechanics and Rehabilitation, Istituto Auxologico Italiano, IRCCS, San Giuseppe Hospital, Verbania, Italy. E-mail: p.capodaglio@auxologico.it
Competing interests and funding: This research has been funded by O2H s.r.l. and partially supported by the Italian Ministry of Health.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
About 40 years have elapsed since the first successful exposure of the entire body of rheumatoid arthritis patients to extreme cold for a short period of time (2–3 min at temperatures ranging from −110°C to −140°C). Since then, whole-body cryostimulation (WBC) has found widespread use in sports medicine. This trend was a reflection of the scientific evidence gathered mainly in this specific literature. However, with the growing knowledge of WBC’s mechanisms of action, related effects and advances in technology that can nowadays tame the thermal stress by providing a controlled and homogeneous “dose of cold” over the patient’s body surface, the spectrum of diseases that can be effectively treated using WBC has expanded considerably. WBC is currently being used to relieve symptoms in rheumatoid arthritis (1), fibromyalgia (1–3), ankylosing spondylitis (4), depression and anxiety (5), multiple sclerosis (6), sleep disturbances (7), muscle soreness after strenuous physical exercise (8), post-Covid syndrome (9, 10) and obesity (11, 12). In our opinion, the powerful effects of thermal stress on the physiological responses of the human body present unique features that could potentially be exploited to boost rehabilitation outcomes in various conditions. WBC is not just a symptomatic physical therapy, but rather represents an “adaptation therapy” because of the repeated shock-like cryogenic cold stimulus over the entire body surface that induces reactions in the autonomic, endocrine, circulatory, neuromuscular and immunological systems, resulting in an adaptation that contributes to the restoration of the homeostatic state (Fig. 1). Based on the existing evidence, WBC can be described as follows:
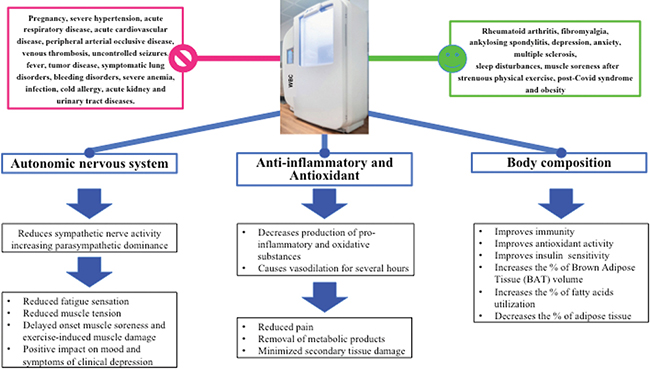
Fig. 1. Working mechanisms of whole-body cryostimulation and its indications and contraindications.
- a “training method” for the autonomic nervous system (13): the afferent signals from the peripheral receptors converge in the medial preoptic region of the hypothalamus, from which efferent signals cause reflex cutaneous vasoconstriction, leading to a shift in blood volume towards the core that results in an increased central pressure. This effect is responsible for reducing sympathetic nerve activity through baroreflex activation and shifting autonomic control of heart rate towards parasympathetic dominance. Remote from cold stimulation, an increase in parasympathetic cardiac control occurs (13) even overnight (14). These changes affect fatigue sensation, reduce muscle tension and facilitate post-exercise recovery by reducing the sensations of delayed onset muscle soreness and exercise-induced muscle damage. Lower fatigue sensation is linked to improved mood and sleep quality (14), with a positive impact on symptoms of clinical depression (5).
- a new anti-inflammatory and antioxidant treatment: repeated exposure to WBC decreases the production of pro-inflammatory and oxidative substances, while anti-inflammatory and anti-oxidative compounds are produced in larger amounts (15), thus minimizing secondary tissue damage. Shortly after exposure to cold, vasodilation occurs, allowing for a considerable change in blood flow that can last several hours if followed by exercise, resulting in the removal of metabolic products (16).
- a treatment that delivers beneficial effects on body composition and adipose tissue (12): the anti-inflammatory and anti-oxidative actions improve immunity, antioxidant activity and insulin sensitivity, as well as increase the percentage of brown adipose tissue volume and fatty acid utilization (17), decreasing the percentage of adipose tissue (18).
Impaired autonomic nervous system regulation is frequently observed in patients with cardiovascular diseases (19), subacute stroke (20), Parkinson’s disease (21), multiple sclerosis (22), obstructive pulmonary conditions (23) and obesity (11). Autonomic dysfunction is associated with poor functional outcome in patients with subacute stroke and is associated with fatigue in patients with multiple sclerosis. Sympathetic activity in obstructive pulmonary conditions may be affected by recurrent hypoxemia, hypercapnia, increased intrathoracic pressure swings because of airway obstruction, increased respiratory effort, systemic inflammation and beta-sympathomimetics. Treatments aimed at restoring sympathovagal balance towards a reduction in resting sympathetic activity, such as exercise training, muscle stretching and breathing relaxation techniques, should be considered in the rehabilitation of these patients. We now know that WBC can rapidly produce the same effects. Repeated exposures to WBC represent an allostatic stimulus that trains the autonomic nervous system to higher levels of performance. Although the physiological effects of WBC show univocal positive results and are already supported by a sturdy bulk of evidence, clinical studies still suffer from limited sample sizes and methodological issues, so evidence of the clinical benefits of WBC remains preliminary. However, especially because of its rapid anti-inflammatory, exercise-mimicking effects, implementing its use in patients who show poor adherence to rehabilitation protocols because of pain and inflammation may contribute to faster achievement of rehabilitation goals. Its reported effects on mood, sleep and fatigue may contribute to reducing eventual barriers in compliance with rehabilitation programmes in various neurological and frailty conditions. From the safety perspective, absolute contraindications to WBC and guidelines are regularly integrated with the latest findings in the literature. We believe it is about time to highlight to rehabilitation professionals the potential for the medical use of WBC and its relevance in the field of rehabilitation, as a non-medical use of WBC in spas or wellness centres could deliver dangerous scientific shortcuts to the general public. Given the time and resource constraints that we commonly experience in our rehabilitation settings, adjuvant treatments that are expected to boost rehabilitation outcomes should be welcomed in our practice and validated on larger populations. Rather, the current inconclusive, but less and less so, clinical evidence should stimulate scientific studies on the efficacy of WBC as an adjuvant treatment in various conditions of rehabilitation interest.
REFERENCES
- Hirvonen HE, Mikkelsson MK, Kautiainen H, Pohjolainen TH, Leirisalo-Repo M. Effectiveness of different cryotherapies on pain and disease activity in active rheumatoid arthritis. A randomised single blinded controlled trial. Clin Exp Rheumatol 2006; 24: 295–301. PMID: 16870097.
- Bettoni L, Bonomi FG, Zani V, Manisco L, Indelicato A, Lanteri P, et al. Effects of 15 consecutive cryotherapy sessions on the clinical output of fibromyalgic patients. Clin Rheumatol 2013; 32: 1337–1345. https://doi.org/10.1007/s10067-013-2280-9
- Fontana JM, Gobbi M, Piterà P, Giusti EM, Capodaglio P. Whole-body cryostimulation in fibromyalgia: a scoping review. Appl Sci 2022; 12: 4794. https://doi.org/10.3390/app12094794
- Stanek A, Cholewka A, Gadula J, Drzazga Z, Sieron A, Sieron-Stoltny K. Can whole-body cryotherapy with subsequent kinesiotherapy procedures in closed type cryogenic chamber improve BASDAI, BASFI, and some spine mobility parameters and decrease pain intensity in patients with ankylosing spondylitis? Biomed Res Int 2015; 2015: 404259. https://doi.org/10.1155/2015/404259
- Rymaszewska J, Ramsey D, Chładzińska-Kiejna S. Whole-body cryotherapy as adjunct treatment of depressive and anxiety disorders. Arch Immunol Ther Exp (Warsz) 2008; 56: 63–68. https://doi.org/10.1007/s00005-008-0006-5
- Miller E, Kostka J, Włodarczyk T, Dugué B. Whole-body cryostimulation (cryotherapy) provides benefits for fatigue and functional status in multiple sclerosis patients. A case-control study. Acta Neurol Scand 2016; 134: 420–426. https://doi.org/10.1111/ane.12557
- Bouzigon R, Ravier G, Dugue B, Grappe F. The use of whole-body cryostimulation to improve the quality of sleep in athletes during high level standard competitions. Br J Sports Med 2014; 48: 572–572. https://doi.org/10.1136/bjsports-2014-093494.33
- Rose C, Edwards KM, Siegler J, Graham K, Caillaud C. Whole-body cryotherapy as a recovery technique after exercise: a review of the literature. Int J Sports Med 2017; 38: 1049–60. https://doi.org/10.1055/s-0043-114861
- Gobbi M, Trotti G, Tanzi M, Kasap F, Piterà P, Capodaglio P. Post-Covid symptoms and whole-body cryotherapy: a case report. J Rehabil Med Clin Commun 2022; 5: 1000075. https://doi.org/10.2340/20030711-1000075
- Piterà P, Gobbi M, Fontana JM, Cattaldo S, Massucci M, Capodaglio P. Whole-body cryostimulation: a rehabilitation booster in post-COVID patients? A case series. Appl Sci 2022; 12: 4830. https://doi.org/10.3390/app12104830
- Fontana JM, Bozgeyik S, Gobbi M, Piterà P, Giusti EM, Dugué B, et al. Whole-body cryostimulation in obesity. A scoping review. J Therm Biol 2022; 106: 103250. https://doi.org/10.1016/j.jtherbio.2022.103250
- Lombardi G, Ziemann E, Banfi G. Whole-body cryotherapy in athletes: from therapy to stimulation. An updated review of the literature. Front Physiol 2017; 8: 258. https://doi.org/10.3389/fphys.2017.00258
- Louis J, Theurot D, Filliard JR, Volondat M, Dugué B, Dupuy O. The use of whole-body cryotherapy: time- and dose-response investigation on circulating blood catecholamines and heart rate variability. Eur J Appl Physiol 2020; 120: 1733–1743. https://doi.org/10.1007/s00421-020-04406-5
- Douzi W, Dupuy O, Tanneau M, Boucard G, Bouzigon R, Dugué B. 3-min whole body cryotherapy/cryostimulation after training in the evening improves sleep quality in physically active men. Eur J Sport Sci 2019; 19: 860–867. https://doi.org/10.1080/17461391.2018.1551937
- Pilch W, Wyrostek J, Piotrowska A, Czerwińska-Ledwig O, Zuziak R, Sadowska-Krępa E, et al. Blood pro-oxidant/antioxidant balance in young men with class II obesity after 20 sessions of whole body cryostimulation: a preliminary study. Redox Rep 2021; 26: 10–17. https://doi.org/10.1080/13510002.2021.1881328
- Bouzigon R, Dupuy O, Tiemessen I, De Nardi M, Bernard JP, Mihailovic T, et al. Cryostimulation for post-exercise recovery in athletes: a consensus and position paper. Front Sports Act Living 2021; 3: 688828. https://doi.org/10.3389/fspor.2021.688828
- Hanssen MJW, van der Lans AAJJ, Brans B, Hoeks J, Jardon KMC, Schaart G, et al. Short-term cold acclimation recruits brown adipose tissue in obese humans. Diabetes 2016; 65: 1179–1189. https://doi.org/10.2337/db15-1372
- Wiecek M, Szymura J, Sproull J, Szygula Z. Whole-body cryotherapy is an effective method of reducing abdominal obesity in menopausal women with metabolic syndrome. J Clin Med 2020; 9: 2797. https://doi.org/10.3390/jcm9092797
- Besnier F, Labrunée M, Pathak A, Pavy-Le Traon A, Galès C, Sénard JM, et al. Exercise training-induced modification in autonomic nervous system: an update for cardiac patients. Ann Phys Rehabil Med 2017; 60: 27–35. https://doi.org/10.1016/j.rehab.2016.07.002
- Scherbakov N, Barkhudaryan A, Ebner N, von Haehling S, Anker SD, Joebges M, et al. Early rehabilitation after stroke: relationship between the heart rate variability and functional outcome. ESC Heart Fail 2020; 7: 2983–2991. https://doi.org/10.1002/ehf2.12917
- Li Y, Wang J, Li X, Jing W, Omorodion I, Liu L. Association between heart rate variability and Parkinson’s disease: a meta-analysis. Curr Pharm Des 2021; 27: 2056–2067. https://doi.org/10.2174/1871527319666200905122222
- Keselbrener L, Akselrod S, Ahiron A, Eldar M, Barak Y, Rotstein Z. Is fatigue in patients with multiple sclerosis related to autonomic dysfunction? Clin Auton Res 2000; 10: 169–175. https://doi.org/10.1007/BF02291352
- van Gestel AJR, Steier J. Autonomic dysfunction in patients with chronic obstructive pulmonary disease (COPD). J Thorac Dis 2010; 2: 215–222. https://doi.org/10.3978/j.issn.2072-1439.2010.02.04.5