Objective: To determine the efficacy of repetitive transcranial magnetic stimulation vs sham stimulation on improving lower-limb functional outcomes in individuals with neurological disorders.
Data sources: PubMed, CINAHL, Embase and Scopus databases were searched from inception to 31 March 2020 to identify papers (n = 1,198). Two researchers independently reviewed studies for eligibility. Randomized clinical trials with parallel-group design, involving individuals with neurological disorders, including lower-limb functional outcome measures and published in scientific peer-reviewed journals were included.
Data extraction: Two researchers independently screened eligible papers (n = 27) for study design, clinical population characteristics, stimulation protocol and relevant outcome measures, and assessed study quality.
Data synthesis: Studies presented a moderate risk of selection, attrition and reporting bias. An overall effect of repetitive transcranial magnetic stimulation was found for outcomes: gait (effect size [95% confidence interval; 95% CI]: 0.51 [0.29; 0.74], p = 0.003) and muscle strength (0.99 [0.40; 1.58], p = 0.001) and disorders: stroke (0.20 [0.00; 0.39], p = 0.05), Parkinson’s disease (1.01 [0.65; 1.37], p = 0.02) and spinal cord injury (0.50 [0.14; 0.85], p = 0.006), compared with sham. No effect was found for outcomes: mobility and balance.
Conclusion: Supplementary repetitive transcranial magnetic stimulation may promote rehabilitation focused on ambulation and muscle strength and overall lower-limb functional recovery in individuals with stroke, Parkinson’s disease and spinal cord injury. Further evidence is needed to extrapolate these findings.
Key words: transcranial magnetic stimulation; neurological disorders; lower extremity.
Accepted Dec 1, 2021; Epub ahead of print Dec 15, 2021
J Rehabil Med 2022; 54: jrm00256
doi: 10.2340/jrm.v53.1097
Correspondence address: Søren Krogh, Department of Neurology, Regional Hospital Viborg, Heiberg All 5A, DK-8800 Viborg, Denmark. E-mail: skjensen87@gmail.com
lay abstract
Non-invasive magnetic brain stimulation can cause beneficial changes in the central nervous system of individuals with neurological disorders, which, in turn, may have a number of therapeutic qualities. This paper summarizes current knowledge about whether the technique can be used to promote recovery of leg movement function. By searching the available literature for studies on individuals with neurological disorders that have compared the effects of magnetic brain stimulation with placebo stimulation, 27 relevant studies were identified. Combined data from these studies suggested that real stimulation, compared with placebo, had positive effects specifically for recovery of walking ability and maximal leg muscle strength, as well as for improvement in overall leg movement function in individuals with stroke, Parkinson’s disease and spinal cord injury. These findings are important for patients and therapists seeking to improve rehabilitation outcomes. This research area deserves increased scientific focus.
INTRODUCTION
Across the spectrum of neurological conditions, extensive heterogeneity exists in terms of aetiology, sequelae and clinical manifestations of disability, and, accordingly, in their management and rehabilitation approaches. Neurological disorders have common traits, however, in that they cause dynamic, plastic changes in particular neural networks. These include compensatory plastic responses, proving adaptive for the individual, but also, and to a greater extent, dysfunctional responses and deteriorative processes that contribute to further disability in the individual (1, 2). In many neurological disorders these processes contribute to impair locomotor functioning, which in turn have detrimental effects on independence and quality of life. Motor deficiencies are generally naïve to pharmacological treatment, and pharmacotherapy has other significant limitations, such as non-customizability to the individual and adverse effects, which can lead to low adherence and discontinuation (3, 4). Physical therapies, such as physiotherapy and exercise, may show some success in maintaining or promoting motor function and skills for the individual. However, successfulness of the rehabilitation effort is largely dependent on the patient’s level of corporation and on the expertise of the therapist (5); consequently, outcomes vary widely.
Neurostimulation appears as a promising tool in various neurorehabilitation settings, as it comprises a number of qualities that address many of the issues mentioned above; e.g. the ability to selectively target locomotor neural circuitries and to improve favourable, while suppressing maladaptive, patterns of neural activity. Furthermore, in particular for non-invasive brain stimulation, such as transcranial magnetic stimulation (TMS), treatment is often associated with no or only mild adverse effects (6). In TMS an electric current is discharged in an insulated coil placed on the scalp surface, which generates a brief, focal magnetic field that is capable of depolarizing neurones in the underlying cortical areas through electromagnetic induction. If the electromagnetic pulses are delivered repetitively (rTMS), it is possible to achieve persistent modulation of neuronal excitability in the targeted cortical structures (7, 8).
Since the mid-1990’s the use and efficacy of rTMS have been extensively investigated as treatment for a range of mental disorders (9), but the method has only more recently gained recognition within wider areas of neurology and neurorehabilitation. To date, a considerable amount of research has been published that investigates the effects of rTMS on chronic (10) and neuropathic (11) pain, dysphagia (12), aphasia (13), aggregate motor symptoms in Parkinson’s disease (PD) (14) and upper limb extremity function following stroke (15). Less attention has been paid to lower limb motor function, despite the fact that mobility and ambulatory function are reported as important determinants of life satisfaction in individuals with neurological disorders (16–18). One explanation for this delay in the application of rTMS for stimulation of lower limb-specific motor function may be that double-cone-shaped and Hesed coils (H-coils), which are capable of stimulating deeper cortical areas, such as the leg motor cortex, have only recently become widely available (19).
The aims of this review were therefore to: (i) perform a systematic analysis to examine study and reporting quality; and (ii) conduct a meta-analysis to evaluate the effect of rTMS on mobility- and gait-related outcome measures in individuals with neurological disorders, by summarizing evidence from published randomized trials with comparable sham-controlled group designs. Thus, the specific objectives were to: (i) systematically review studies examining rTMS on lower limb functional outcome measures (LLFO) in clinical neurorehabilitation; and (ii) compare the efficiency of real rTMS to sham stimulation in improving lower limb function.
METHODS
This review was prospectively registered (Identifier: CRD42020176837) at the International Prospective Register of Systematic Reviews (PROSPERO, Centre for Reviews and Dissemination, University of York, York, UK) and composed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 guidelines (20, 21).
Search strategy
A literature search to identify research papers examining rTMS on LLFO in individuals with neurological disorders was carried out on the following databases for the time period of inception to 31 March 2020: PubMed, CINAHL, Embase, Scopus. The title and abstract of each study were screened, and only human trials utilizing rTMS on individuals with neurological disorders were selected. The reference list of relevant papers was also examined. Key search terms were: “neurological disorder” OR “neurological disease” OR “spinal cord injury” OR “stroke” OR “multiple sclerosis” OR “cerebral palsy” OR “amyotrophic lateral sclerosis” AND “Human” for patient type; “repetitive transcranial magnetic stimulation” OR “rTMS” OR “non-invasive brain stimulation” OR “NIBS” for intervention category; and “lower limb” OR “leg” for outcome. The full search strategy is listed in Appendix SI.
Inclusion and exclusion criteria
rTMS intervention studies (minimum 5 rTMS sessions with stimulation over areas directly or indirectly involved in descending motor control) involving individuals with a neurological disorder as classified by the World Health Organization (WHO) (22), participants n ≥ 10, published in English, Danish, Swedish or Norwegian in scientific peer-reviewed journals were included in the analysis. Studies were required to include at least 1 LLFO, and only randomized controlled trials (RCTs) comparing real rTMS with sham stimulation in a comparable study population were included in the review. Acute studies, case studies, single-arm studies or reports not published in scientific peer-reviewed journals in the specified languages were excluded. RCTs involving combination treatments, where the effects of rTMS could not be isolated, and full cross-over type studies, where masking is compromised and carry-over effects are probable, were also excluded.
Study selection
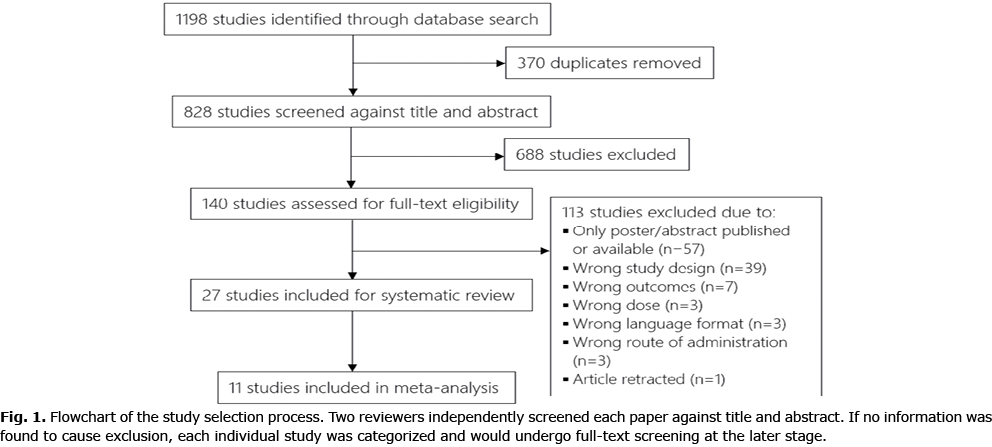
Study selection was performed using an online systematic review software (Covidence, Veritas Health Innovation, Melbourne, Australia. Available from: www.covidence.org). Two researchers (SK and ABJ) independently reviewed the entire set of studies generated from the search, and excluded duplicates and those that failed to meet the inclusion criteria (cf. Fig. 1 for a flowchart of study selection process). In case of any discrepancies a third reviewer (HK) was invited to evaluate the record.
Data extraction and quality assessment
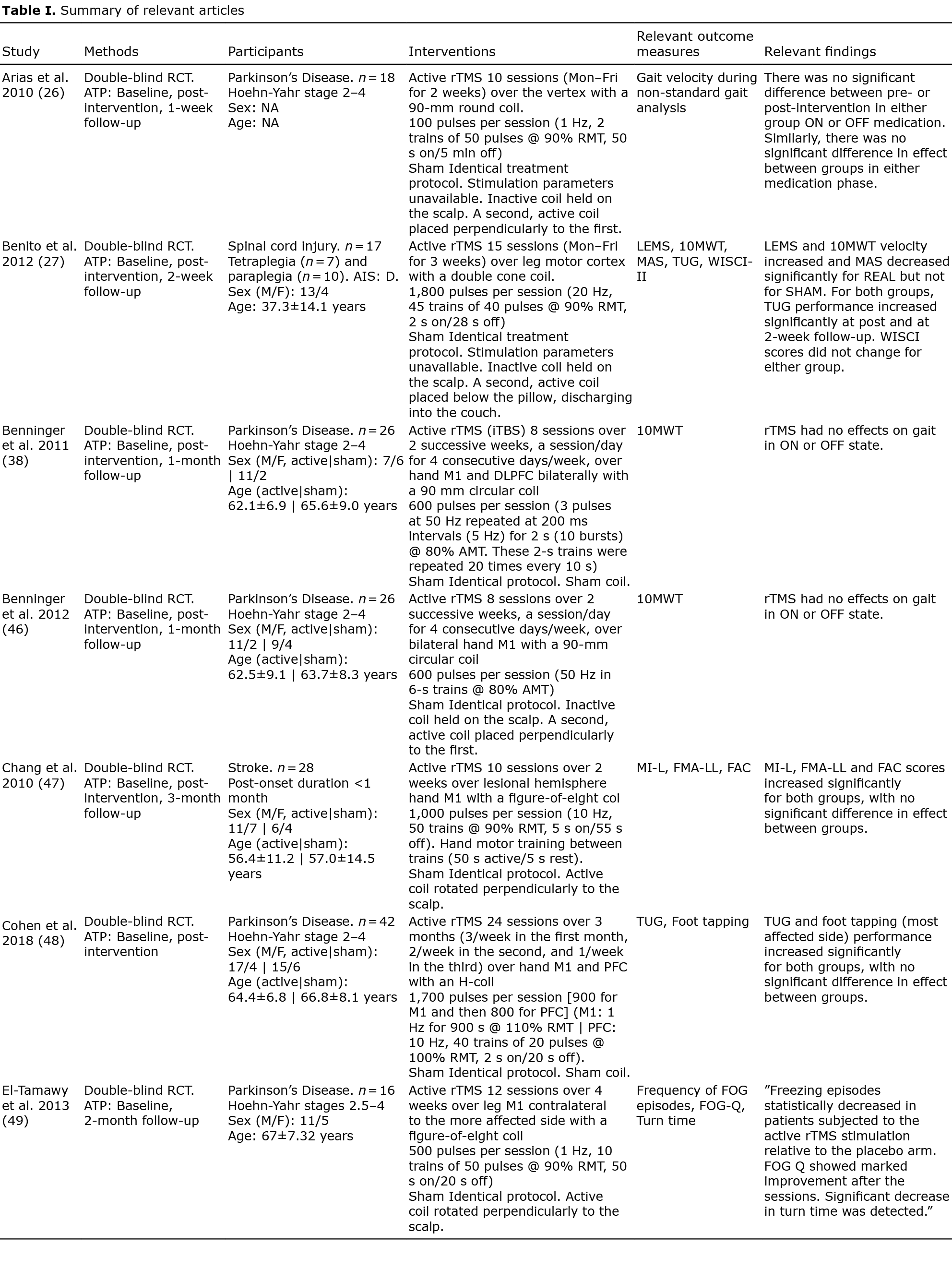
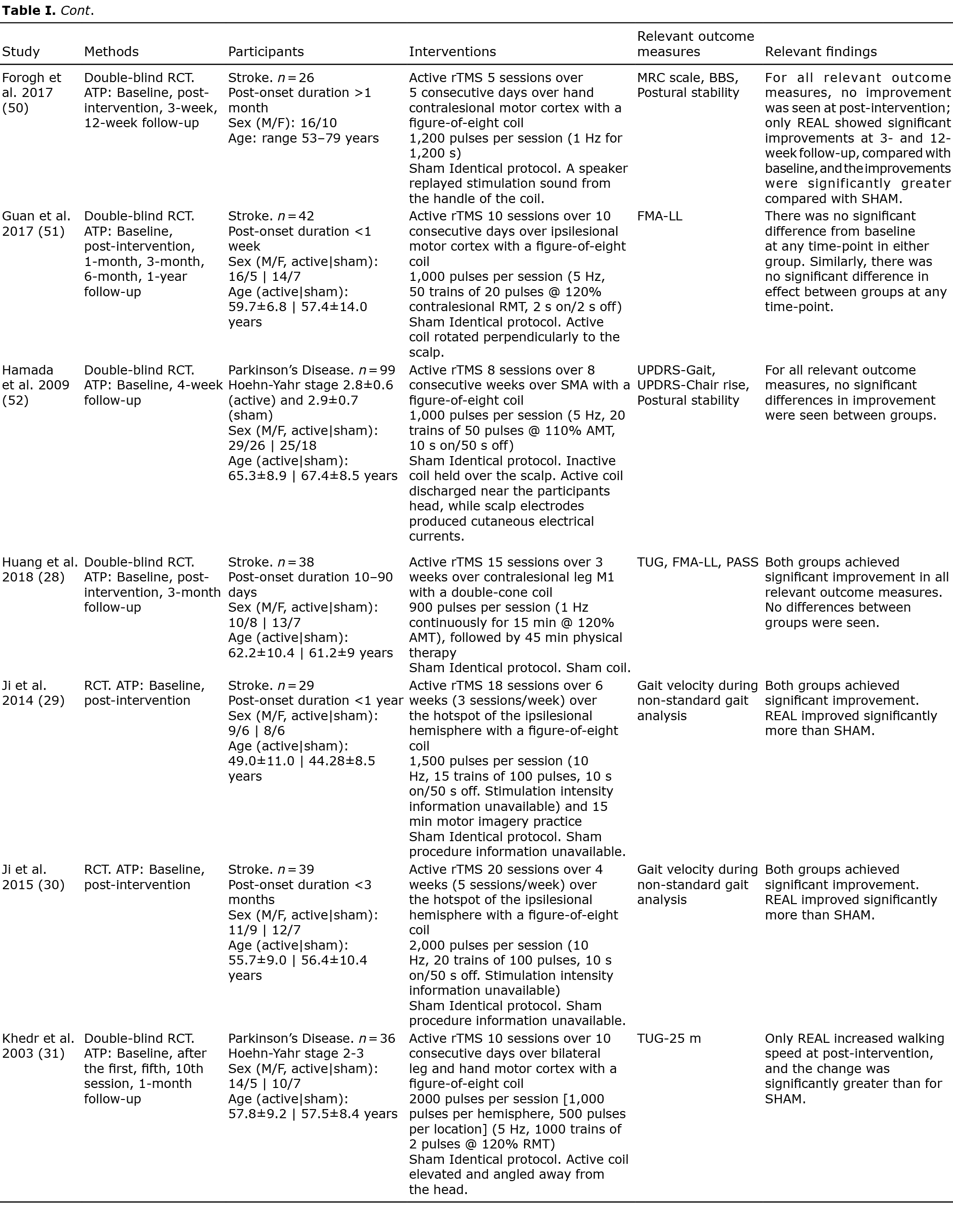
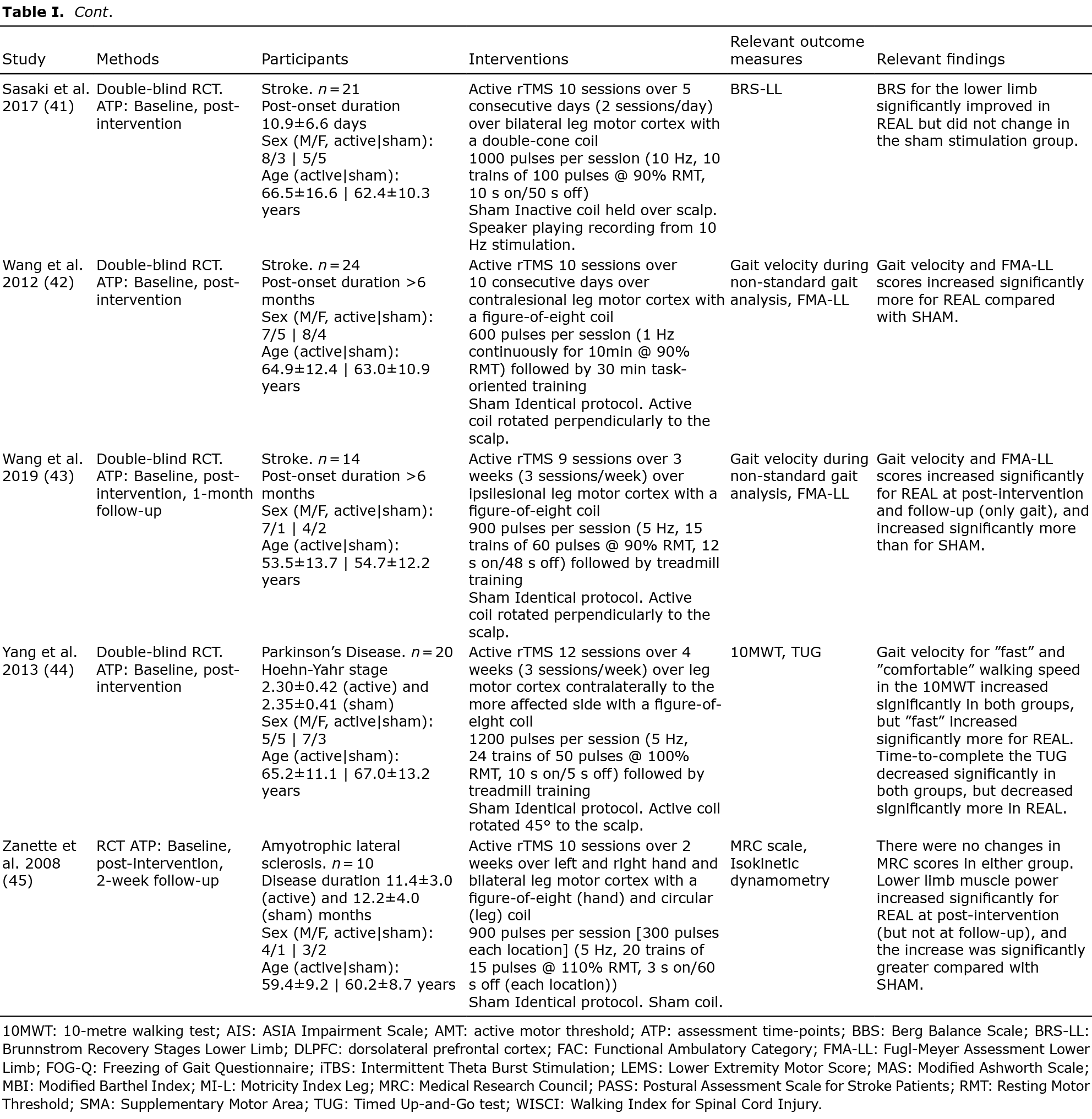
Eligible papers were independently screened by the same 2 researchers for: (i) study design, (ii) clinical population characteristics, (iii) rTMS protocol, and (iv) relevant outcome measure(s). The following data were extracted: (i) level of blinding, assessment time-points. (ii) For all studies: n, sex and age for each intervention group individually, whenever possible. For stroke and amyotrophic lateral sclerosis (ALS): time since onset; spinal cord injury (SCI): level (paraplegia/tetraplegia) and degree (American Spinal Injury Association Impairment Scale) of neurological disability; PD: degree of disability (Hoehn & Yahr stage); multiple sclerosis (MS): disease phase. (iii) Number of sessions, intervention duration, area of stimulation, coil type, total number of pulses per session, stimulation frequency and intensity, procedures for sham stimulation and, if applicable, type of task performed in combination. (iv) Relevance to lower limb function.
The quality of the included studies was evaluated using the Cochrane Collaboration’s risk-of-bias guidelines (23). The results from the 2 assessors’ evaluations were compared and any discrepancies were resolved by consensus with a third researcher.
Data synthesis and meta-analysis
A meta-analysis was performed using the following comparison: pre-post change following real rTMS vs change following sham stimulation. Data were obtained in the correct format, which allowed for inclusion in the data synthesis, from 11 out of the 27 included studies, either directly from studies or via private correspondence with authors. Due to considerable heterogeneity in study designs with regard to follow-up, the first assessment available post-intervention was chosen as follow-up. First, data were sorted and analysed based on outcome: gait, mobility, muscle strength, spasticity and balance, to provide an overview of the effects of rTMS on each of the categories. Subsequently, a secondary analysis, based on patient category, was performed to assess any potential differences in neurophysiological adaptations to rTMS between populations. Due to limited data regarding maximal muscle strength, the focus of the meta-analysis was on clinical outcome measures.
Data extracted at the group level were expressed in the form of mean of difference, standard deviation (SD) and sample size. When insufficient or wrongly formatted data were provided, data were extrapolated from figures when possible; otherwise, authors were contacted and asked to provide the data. In cases of non-parametric reporting of data, data were converted using basic properties of the log normal distribution. In instances where a more negative change value was favourable, e.g. a reduction in time to complete a test, data were simply multiplied by –1. The meta-analysis was conducted using a designated review manager software (RevMan 5.4, The Cochrane Collaboration, London, UK. Available from: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman). Converted data were pooled and calculated using standardized mean differences and 95% CI; thus, a single effect size (Hedges’ g [95% confidence interval; 95% CI]) for real rTMS was expressed for each overall category and each of its sub-categories. A fixed-effect model was applied to determine heterogeneity between studies, using I2 statistics. Effect sizes were interpreted using Cohen’s guidelines (24) (0.2 = small effect, 0.5 = moderate effect, 0.8 = large effect, > 0.8 = very large effect), while heterogeneity was interpreted using Cochrane’s guidelines (25) (I2 = 0% to 40%: might not be important; I2 = 30% to 60%: may represent moderate heterogeneity; I2 = 50% to 90%: may represent substantial heterogeneity; I2 = 75% to 100%: considerable heterogeneity).
RESULTS
Study selection and study characteristics
A flowchart depicting the trial selection procedure is shown in Fig. 1. The search retrieved 1,198 studies; after removing duplicates, 828 studies were screened against title and abstract, and of these, 688 studies were excluded. A total of 140 studies were reviewed for eligibility through full-text screening, of which 113 studies were excluded due to various reasons (cf. Fig. 1). The remaining 27 studies (26, 27, 36–45, 28, 46–52, 29–35) were included in the systematic review.
Table I presents a summary of the findings. Across the included studies, the following disorders were represented: stroke (30, 33, 49, 50, 34, 36–38, 40, 42, 43, 48) (40.7%), PD (26, 28, 51, 29, 31, 32, 35, 39, 44–46) (40.7%), SCI (27, 41) (7.4%), MS (47) (3.7%) and ALS (52) (3.7%). For intervention parameters, 19 studies (27, 28, 43–48, 50, 52, 29, 30, 34, 35, 37–39, 41) (70.4%) applied high-frequency stimulation (≥ 5 Hz), 7 studies (26, 32, 33, 36, 40, 42, 49) (25.9%) applied low-frequency stimulation (≤1 Hz) while a single study (31) (3.7%) used a hybrid stimulation design. A total of 23 disparate effect measures were identified as relevant outcome parameters. Twenty-one studies (77.8%) included at least one outcome measure directly related to ambulation, 13 studies (48.1%) included at least one outcome measure related to mobility, 6 studies (22.2%) included a balance/postural stability outcome, while 4 studies (14.8%) included a lower limb muscle strength outcome and 2 studies (7.4%) included a spasticity outcome.

Risk of bias assessment
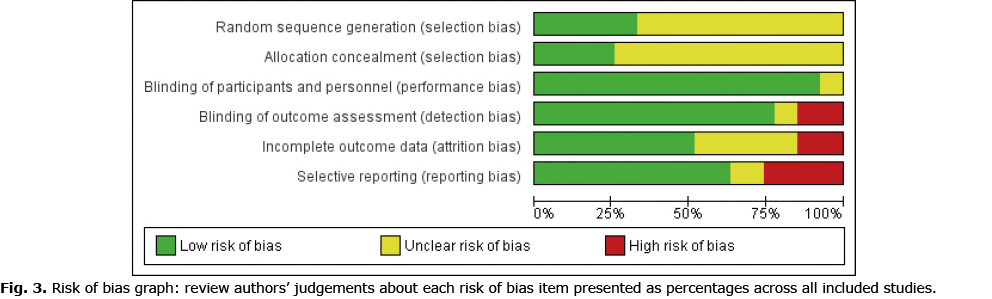
Figs 2 and 3 provide an overview of the risk of bias in the included trials. Overall, a low risk of bias was found for reporting related to blinding. All studies blinded participants to allocation, as per inclusion criteria, and only 2 studies failed to report of blinding of intervention personnel (37, 38). In 6 studies (22.2%), blinding of outcome assessors were either not performed or not concisely reported (33, 37, 38, 44, 45, 52). A majority of studies (66.7%) failed to adequately report randomization sequence generation and allocation concealment procedures. Typically, while information was provided that participant allocation was performed using a randomized sequence method, it was not specified how the specific randomization sequence was generated. In addition, most studies (74.1%) did not specify how allocation concealment was achieved. Therefore, a moderate to high risk of selection bias was deemed to be present in the presented findings. Approximately half (51.9%) of the studies sufficiently disclosed data related to drop-outs and loss-to-follow-ups, while most studies (63.0%) provided detailed results, which appeared free of potential reporting bias.
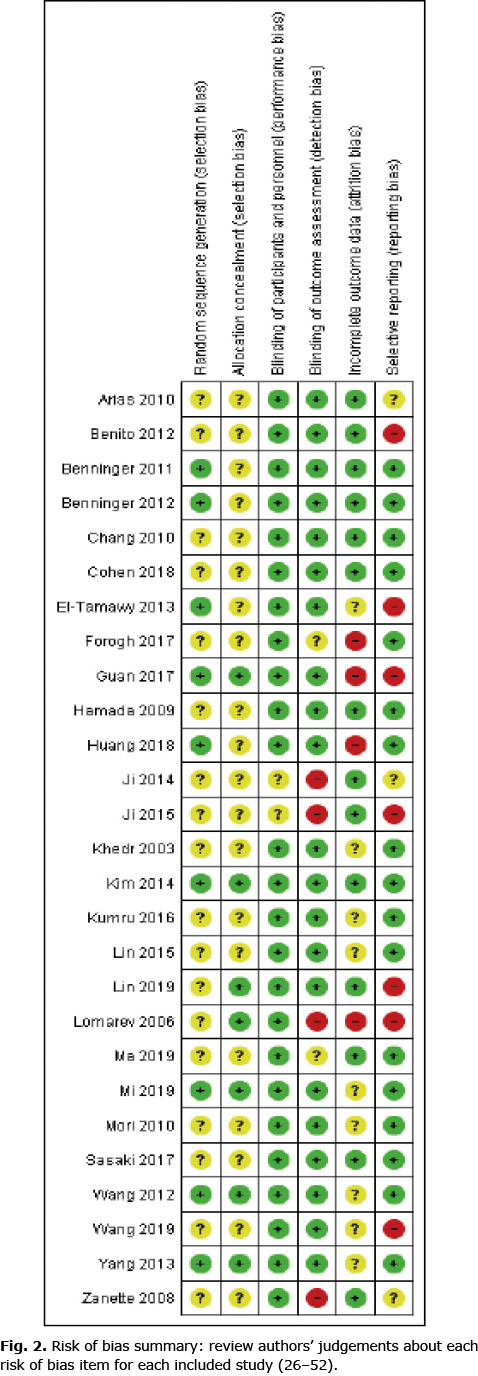
Effect on selected outcome parameters
A qualitative summary of the findings for each individual outcome measure at each follow-up period is provided in Table I.
Gait
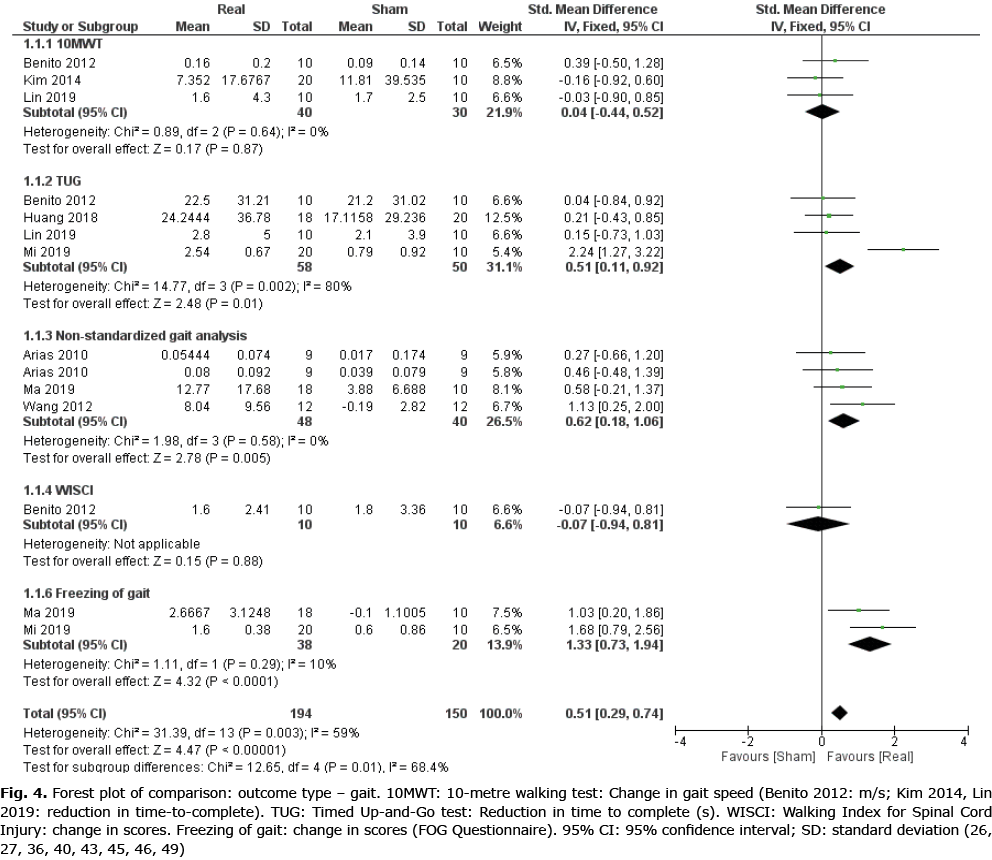
Data from 8 RCTs (26, 27, 36, 40, 43, 45, 46, 49) (14 comparisons, n = 344) were included for meta-analysis to evaluate the effect of real rTMS on gait function (Fig. 4). A moderate effect on overall gait function, which was found to be moderately to substantially heterogeneous, was found for real rTMS compared with sham stimulation (effect size (ES) 0.51 [0.29; 0.74], p < 0.00001, I2 = 59%). For sub-groups, a very large and homogenous effect of real rTMS was found for self-reported scores of freezing-of-gait (ES: 1.33 [0.73; 1.94], p < 0.00001, I2 = 10%) while a moderate effect was found for time-to-complete the Timed Up-and-Go (TUG) test (ES: 0.51 [0.11; 0.92], p = 0.01, I2 = 80%) and for gait improvement assessed with non-standardized gait tests (ES: 0.62 [0.18; 1.06], p = 0.005, I2 = 0%). No effect was found in 10-metre walking test (10MWT) performance or for Walking Index for Spinal Cord Injury II (WISCI II) scores. A test for homogeneity revealed significant and substantial heterogeneity between subgroups (I2 = 68.4%, p = 0.01).
Mobility
Data from 5 RCTs (34, 36, 42, 43, 49) (5 comparisons, n = 155) were obtained in order to evaluate the effect of real rTMS on improvements in mobility. Only data from studies using lower limb sub-scores of the Fugl-Meyer Assessment, a stroke-specific, performance-based motor recovery index, were available. Here, no effect of real rTMS was found (ES: 0.19 [–0.13; 0.51], p = 0.24, I2 = 9%) (Fig. 5).

Maximal muscle strength
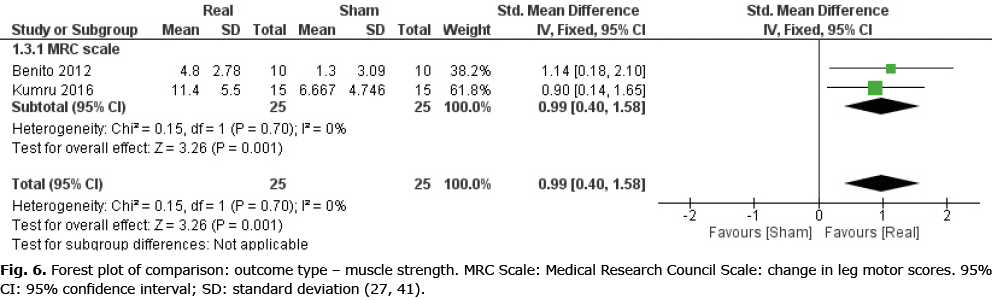
Data were obtained from 2 studies (27, 41) (2 comparisons, n = 50), both o-f which had individuals with SCI as their study population, to investigate the effect of real rTMS on recovery of lower limb muscle strength (Fig. 6). A large and homogenous effect of real rTMS on the SCI-specific variant of the Medical Research Council (MRC) scale for lower limb muscle strength (LEMS, Lower Extremity Motor Score) was found ((ES: 0.99 [0.40; 1.58], p = 0.001, I2 = 9%).
Spasticity
Data from a single study (27) (n = 20) investigating the effect of rTMS on spasticity (Modified Ashworth Scale; MAS) was obtained (ES: 0.56 [–0.34; 1.46], p = 0.22). Therefore, meta-analysis could not be performed.
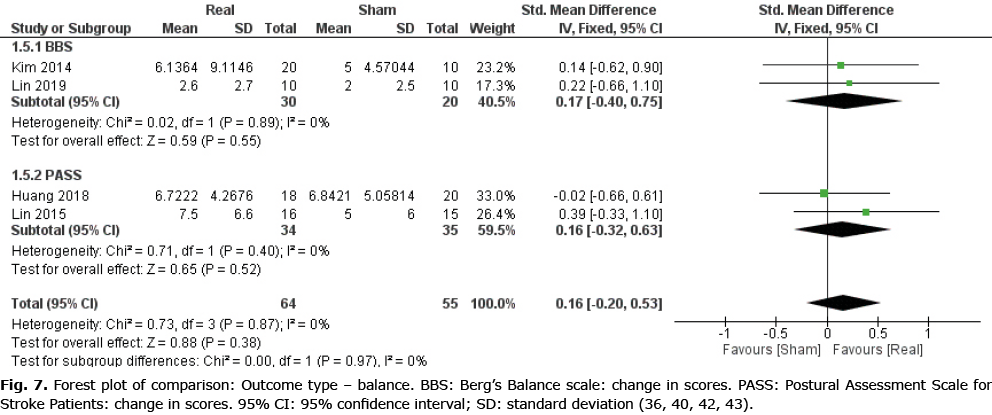
Balance
Data from 4 RCTs (36, 40, 42, 43) (4 comparisons, n = 119) were obtained in order to evaluate the effect of real rTMS on outcomes related to balance. No clear effect of real rTMS was found for this category (ES: 0.16 [–0.20; 0.53], p = 0.38, I2 = 0%) (Fig. 7).
Disorder type
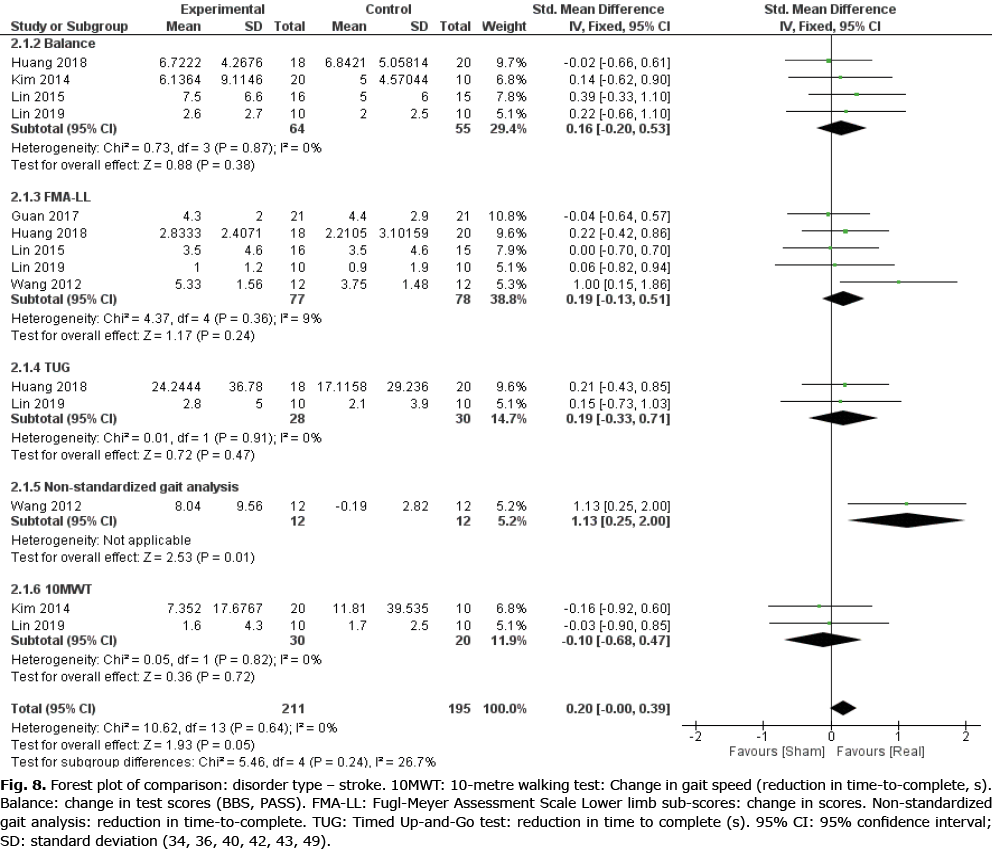
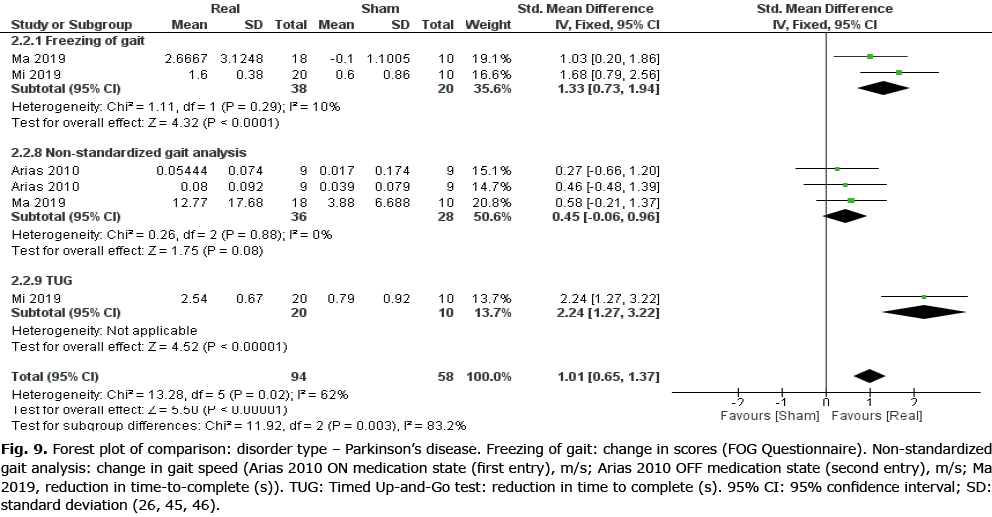
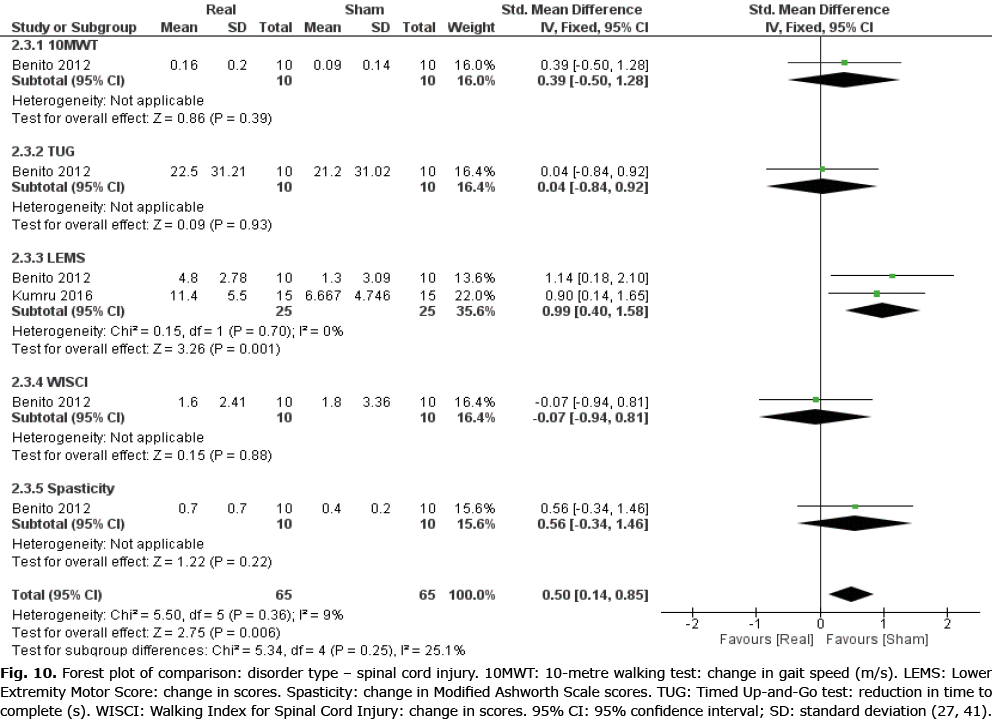
A secondary meta–analysis was performed on the identified outcome parameters in order to evaluate whether the adaptive effects of rTMS differed among the included disorders. The results are shown in Figs 8–10. A small and homogeneous increase in overall lower limb function was found for real rTMS compared with sham in individuals with stroke (14 comparisons, n = 406, ES: 0.20 [0.00; 0.39], p = 0.05, I2 = 26.7%) (Fig. 8), while a very large, but moderately to substantially heterogeneous effect was observed in individuals with PD (6 comparisons, n = 152, ES: 1.01 [0.65; 1.37], p<0.00001, I2 = 62%) (Fig. 9). In addition, a moderate and homogenous overall effect on LLFO was found following real rTMS in individuals with SCI (6 comparisons, n = 130, ES: 0.50 [0.14; 0.85], p = 0.006, I2 = 9%) (Fig. 10). No data could be obtained from studies investigating MS or ALS.
Risk of bias due to missing results
Table II gives an overview of the number of comparisons included in the present meta-analysis in relation to the total number of comparisons identified through study screening. In the primary analyses, data could not be obtained for more than half of the identified comparisons in all subgroups, except for “Balance” (inclusion ratio (IR): 0.33–0.57). As for the secondary analyses, data completeness was moderate for the Stroke (IR 0.56), low for the PD (IR 0.29) and high for the SCI (IR 0.75) subgroups. Therefore, with the exception of the SCI subgroup, a considerable risk of bias due to non-reporting exists for the results from the data syntheses.

DISCUSSION
This study reviewed 27 RCTs examining the effects of rTMS vs sham stimulation on lower limb functional outcome measures in individuals with neurological disorders. The included studies were heterogeneous, had small to medium sample sizes and presented a moderate risk of selection, attrition and reporting bias. A statistically significant effect of real rTMS compared with sham stimulation was found for outcome parameters: gait and muscle strength and for disorder types: stroke, PD and SCI. No effect of real rTMS was found for outcomes related to mobility or balance compared with sham stimulation, while meta-analysis of outcome parameters: spasticity and disorders: MS and ALS could not be performed due to a lack of data. A considerable risk of bias due to non-reporting was present in all categories except for disorder: SCI.
The findings of a positive effect of rTMS on gait function and lower limb muscle strength are in line with results from a recent review on rTMS in stroke survivors, where the authors reported improvements in walking speed, lower limb body function and FMA-LL scores following rTMS compared with control interventions (53). Notably, improvements were also found for outcomes related to lower limb activities and participation following rTMS, indicating that rTMS-induced improvements in lower limb function can lead to meaningful functional recovery in individuals with neurological impairment. In addition, another recent review investigated the effect of rTMS on ambulatory function in PD (54). Here, walking speed was reported to improve following rTMS interventions compared with sham stimulation, while a trend for improved freezing-of-gait (questionnaire scores) was found following rTMS compared with control interventions (54).
The neurophysiological basis for the beneficial adaptations observed following systematic rTMS has not yet been clearly identified. A moderate body of evidence suggests that long-term rTMS can alter synaptic plasticity within the corticospinal tract, i.e. by potentiating excitatory post-synaptic potentials, which is thought to occur through a regulation of Ca2+, NO and glutamate availability and sensitivity (8, 55). Therefore, and particularly for individuals with CNS impairment, it is possible that initiation and control of volitional movement are promoted by rTMS due to an increased strength of the descending corticospinal volleys projecting onto the spinal motoneurones.
However, the findings compiled in the current study are associated with a number of potential limitations. Firstly, extensive heterogeneity was observed for several outcome parameters, indicating that treatment effects varied between the included studies. This may in part be explained by heterogeneous study protocols (study population, length of intervention, total number of sessions, follow-up) and stimulation paradigms (frequency, area of stimulation, total number of pulses per session), although it appears that substantial differences were also present in response to treatment between individuals within the same patient category. This is supported by the large variance observed in the change score values, but also by recent findings in patients with depression (56) and consciousness disorder (57), where electroencephalography was applied to clearly discriminate rTMS responders from non-responders within their study population. This observation underlines a need for further investigations of the inter-individual variability in response to rTMS treatment in individuals with neurological motor impairments. Identifying clinical and non-clinical predictors of responsiveness to rTMS treatment holds particular importance, as it could increase clinical efficacy by personalizing and adapting rTMS protocols to optimize the rehabilitation of neurological patients.
Limitations in the present findings are also present due to considerable non-reporting, as over half of the identified comparisons were unavailable for analyses. Missing results are problematic in all data syntheses, because available results may differ systematically from missing results (25); therefore, it is possible that the treatment effects reported in the present study are either under- or over-estimated. Furthermore, the observed rTMS treatment effects were based on analyses of pre- to post-intervention data. We chose to analyse post-intervention changes instead of follow-up changes due to considerations about data availability and underlying homogeneity of the findings (because the included studies employed disparate follow-up time-points). However, it is possible that an analysis on changes from pre-intervention to last available follow-up would have yielded different results. A post-hoc qualitative analysis of studies employing follow-up beyond the rTMS intervention revealed that the improvements induced by real rTMS at post-intervention were generally maintained during follow-up, except for 1 study (52). An additional study reported that improvements were seen only in real rTMS during follow-up assessments, compared with sham stimulation (33).
In conclusion, the findings of the current study suggest that implementing rTMS as an adjunct therapy to neurorehabilitation may be a viable strategy to promote ambulation and lower limb muscle strength. Specifically for rehabilitation of individuals with stroke, PD and SCI, rTMS appears to enhance recovery of overall lower limb motor function. However, treatment effects vary widely between the included studies and the results are synthesized from heterogeneous studies, which employed moderate sample sizes and presented with risk of bias. In addition, the results may be influenced by bias due to considerable non-reporting. Therefore, further data from large RCTs are needed in order to provide unambiguous recommendations for the clinical use of rTMS in neurorehabilitation. In addition, further studies on the use of rTMS on individuals with other neurological conditions are needed before these findings can be extrapolated to other patient groups.
ACKNOWLEDGEMENTS
The authors thank the original study researchers who kindly provided us with relevant data in relation to the current study.
Funding. This study was carried out with internal research funding from the Department of Neurology, Regional Hospital Viborg.
The authors have no conflicts of interest to declare.
REFERENCES
- Alia C, Spalletti C, Lai S, Panarese A, Lamola G, Bertolucci F, et al. Neuroplastic changes following brain ischemia and their contribution to stroke recovery: Novel approaches in neurorehabilitation. Front Cell Neurosci 2017; 11: 1–22.
- Thickbroom GW, Mastaglia FL. Plasticity in neurological disorders and challenges for noninvasive brain stimulation (NBS). J Neuroeng Rehabil 2009; 6: 2–6.
- Halpern R, Agarwal S, Dembek C, Borton L, Lopez-Bresnahan M. Comparison of adherence and persistence among multiple sclerosis patients treated with disease-modifying therapies: a retrospective administrative claims analysis. Patient Prefer Adherence 2011; 5: 73–84.
- Straka I, Minár M, Gažová A, Valkovič P, Kyselovič J. Clinical aspects of adherence to pharmacotherapy in Parkinson disease: a PRISMA-compliant systematic review. Medicine (Baltimore) 2018; 97: e10962.
- Carr J, Shepherd R. The changing face of neurological rehabilitation. Rev Bras Fisioter 2006; 10: 147–156.
- Maizey L, Allen CPG, Dervinis M, Verbruggen F, Varnava A, Kozlov M, et al. Comparative incidence rates of mild adverse effects to transcranial magnetic stimulation. Clin Neurophysiol 2013; 124: 536–544.
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature 2000; 406: 147–150.
- Klomjai W, Katz R, Lackmy-Vallée A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med 2015; 58: 208–213.
- Slotema CW, Blom JD, Hoek HW, Sommer IEC. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry 2010; 71: 873–884.
- Giannoni-Luza S, Pacheco-Barrios K, Cardenas-Rojas A, Mejia-Pando PF, Luna-Cuadros MA, Barouh JL, et al. Non-invasive motor cortex stimulation effects on quantitative sensory testing (QST) in healthy and chronic pain subjects. Pain 2020; 161: 1955–1975.
- Leung A, Donohue M, Xu R, Lee R, Lefaucheur JP, Khedr EM, et al. rTMS for suppressing neuropathic pain: a meta-analysis. J Pain 2009; 10: 1205–1216.
- Papadopoulou SL, Ploumis A, Exarchakos G, Theodorou SJ, Beris A, Fotopoulos AD. Versatility of repetitive transcranial magnetic stimulation in the treatment of poststroke dysphagia. J Neurosci Rural Pract 2018; 9: 391–396.
- Naeser MA, Martin PI, Treglia E, Ho M, Kaplan E, Bashir S, et al. Research with rTMS in the treatment of aphasia. Restor Neurol Neurosci 2010; 28: 511–529.
- Benninger DH, Hallett M. Non-invasive brain stimulation for Parkinson’s disease: Current concepts and outlook 2015. NeuroRehabilitation 2015; 37: 11–24.
- van Lieshout ECC, van der Worp HB, Visser-Meily JMA, Dijkhuizen RM. Timing of repetitive transcranial magnetic stimulation onset for upper limb function after stroke: a systematic review and meta-analysis. Front Neurol 2019; 10: 1269.
- Broe GA, Jorm AF, Creasey H, Grayson D, Edelbrock D, Waite LM, et al. Impact of chronic systemic and neurological disorders on disability, depression and life satisfaction. Int J Geriatr Psychiatry 1998; 13: 667–173.
- Putzke JD, Richards JS, Hicken BL, DeVivo MJ. Predictors of life satisfaction: A spinal cord injury cohort study. Arch Phys Med Rehabil 2002; 83: 555–561.
- Soh S-E, McGinley JL, Watts JJ, Iansek R, Murphy AT, Menz HB, et al. Determinants of health-related quality of life in people with Parkinson’s disease: a path analysis. Qual Life Res 2013; 22: 1543–1553.
- Zangen A, Roth Y, Voller B, Hallett M. Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol 2005; 116: 775–779.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med 2021; e1003583
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol 2021; 134: 103–112.
- WHO. Neurological disorders: public health challenges. Geneva (Switzerland). WHO Library Cataloguing 2006.
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928.
- Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press; 2013.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. 2nd edition. Chichester, UK: John Wiley & Sons; 2019.
- Arias P, Vivas J, Grieve KL, Cudeiro J. Controlled trial on the effect of 10 days low-frequency repetitive transcranial magnetic stimulation (rTMS) on motor signs in Parkinson’s disease. Mov Disord 2010; 25: 1830–1838.
- Benito J, Kumru H, Murillo N, Costa U, Medina J, Tormos JM, et al. Motor and gait improvement in patients with incomplete spinal cord injury induced by high-frequency repetitive transcranial magnetic stimulation. Top Spinal Cord Inj Rehabil 2012; 18: 106–112.
- Benninger DH, Berman BD, Houdayer E, Pal N, Luckenbaugh DA, Schneider L, et al. Intermittent theta-burst transcranial magnetic stimulation for treatment of Parkinson disease. Neurology 2011; 76: 601–609.
- Benninger DH, Iseki K, Kranick S, Luckenbaugh DA, Houdayer E, Hallett M. Controlled study of 50-Hz repetitive transcranial magnetic stimulation for the treatment of parkinson disease. Neurorehabil Neural Repair 2012; 26: 1096–1105.
- Chang WH, Kim YH, Bang OY, Kim ST, Park YH, Lee PKW. Long-term effects of RTMS on motor recovery in patients after subacute stroke. J Rehabil Med 2010; 42: 758–764.
- Cohen OS, Rigbi A, Yahalom G, Warman-Alaluf N, Nitsan Z, Zangen A, et al. Repetitive deep TMS for Parkinson disease: a 3-month double-blind, randomized sham-controlled study. J Clin Neurophysiol 2018; 35: 159–165.
- El-Tamawy MS, Shehata HS, Shalaby NM, Nawito A, Esmail EH. Can repetitive transcranial magnetic stimulation help on-freezers with Parkinson’s disease? Egypt J Neurol Psychiatry Neurosurg 2013; 50: 355–360.
- Forogh B, Ahadi T, Nazari M, Sajadi S, Latif LA, Akhavan Hejazi SM, et al. The effect of repetitive transcranial magnetic stimulation on postural stability after acute stroke: a clinical trial. Basic Clin Neurosci 2017; 8: 405–412.
- Guan YZ, Li J, Zhang XW, Wu S, Du H, Cui LY, et al. Effectiveness of repetitive transcranial magnetic stimulation (rTMS) after acute stroke: a one-year longitudinal randomized trial. CNS Neurosci Ther 2017; 23: 940–946.
- Hamada M, Ugawa Y, Tsuji S. High-frequency rTMS over the supplementary motor area improves bradykinesia in Parkinson’s disease: subanalysis of double-blind sham-controlled study. J Neurol Sci 2009; 287: 143–146.
- Huang YZ, Lin LF, Chang KH, Hu CJ, Liou TH, Lin YN. Priming with 1-Hz repetitive transcranial magnetic stimulation over contralesional leg motor cortex does not increase the rate of regaining ambulation within 3 months of stroke: a randomized controlled trial. Am J Phys Med Rehabil 2018; 97: 339–345.
- Ji S-G, Cha H-G, Kim K-J, Kim M-K. Effects of motor imagery practice in conjunction with repetitive transcranial magnetic stimulation on stroke patients. J Magn 2014; 19: 181–184.
- Ji S-G, Kim M-K. The effects of repetitive transcranial magnetic stimulation on the gait of acute stroke patients. J Magn 2015; 20: 129–132.
- Khedr EM, Farweez HM, Islam H. Therapeutic effect of repetitive transcranial magnetic stimulation on motor function in Parkinson’s disease patients. Eur J Neurol 2003; 10: 567–572.
- Kim WS, Jung SH, Oh MK, Min YS, Lim JY, Paik NJ. Effect of repetitive transcranial magnetic stimulation over the cerebellum on patients with ataxia after posterior circulation stroke: a pilot study. J Rehabil Med 2014; 46: 418–423.
- Kumru H, Benito-Penalva J, Valls-Sole J, Murillo N, Tormos JM, Flores C, et al. Placebo-controlled study of rTMS combined with Lokomat® gait training for treatment in subjects with motor incomplete spinal cord injury. Exp Brain Res 2016; 234: 3447–3455.
- Lin YN, Hu CJ, Chi JY, Lin LF, Yen TH, Lin YK, et al. Effects of repetitive transcranial magnetic stimulation of the unaffected hemisphere leg motor area in patients with subacute stroke and substantial leg impairment: a pilot study. J Rehabil Med 2015; 47: 305–310.
- Lin LF, Chang KH, Huang YZ, Lai CH, Liou TH, Lin YN. Simultaneous stimulation in bilateral leg motor areas with intermittent theta burst stimulation to improve functional performance after stroke: a feasibility pilot study. Eur J Phys Rehabil Med 2019; 55: 162–168.
- Lomarev MP, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann EM, Hallett M. Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Mov Disord 2006; 21: 325–331.
- Ma J, Gao L, Mi T, Sun J, Chan P, Wu T. Repetitive transcranial magnetic stimulation does not improve the sequence effect in freezing of gait. Parkinsons Dis 2019; 2196195.
- Mi TM, Garg S, Ba F, Liu AP, Wu T, Gao LL, et al. High-frequency rTMS over the supplementary motor area improves freezing of gait in Parkinson’s disease: a randomized controlled trial. Park Relat Disord 2019; 68: 85–90.
- Mori F, Codecà C, Kusayanagi H, Monteleone F, Boffa L, Rimano A, et al. Effects of intermittent theta burst stimulation on spasticity in patients with multiple sclerosis. Eur J Neurol 2010; 17: 295–300.
- Sasaki N, Abo M, Hara T, Yamada N, Niimi M, Kakuda W. High-frequency rTMS on leg motor area in the early phase of stroke. Acta Neurol Belg 2017; 117: 189–194.
- Wang RY, Tseng HY, Liao KK, Wang CJ, Lai KL, Yang YR. RTMS combined with task-oriented training to improve symmetry of interhemispheric corticomotor excitability and gait performance after stroke: a randomized trial. Neurorehabil Neural Repair 2012; 26: 222–230.
- Wang RY, Wang FY, Huang SF, Yang YR. High-frequency repetitive transcranial magnetic stimulation enhanced treadmill training effects on gait performance in individuals with chronic stroke: a double-blinded randomized controlled pilot trial. Gait Posture 2019; 68: 382–387.
- Yang YR, Tseng CY, Chiou SY, Liao KK, Cheng SJ, Lai KL, et al. Combination of rTMS and treadmill training modulates corticomotor inhibition and improves walking in parkinson disease: a randomized trial. Neurorehabil Neural Repair 2013; 27: 79–86.
- Zanette G, Forgione A, Manganotti P, Fiaschi A, Tamburin S. The effect of repetitive transcranial magnetic stimulation on motor performance, fatigue and quality of life in amyotrophic lateral sclerosis. J Neurol Sci 2008; 270: 18–22.
- Tung YC, Lai CH, Liao C De, Huang SW, Liou TH, Chen HC. Repetitive transcranial magnetic stimulation of lower limb motor function in patients with stroke: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil 2019; 33: 1102–1012.
- Xie YJ, Gao Q, He CQ, Bian R. Effect of repetitive transcranial magnetic stimulation on gait and freezing of gait in Parkinson disease: a systematic review and meta-analysis. Arch Phys Med Rehabil 2020; 101: 130–140.
- Hoogendam JM, Ramakers GMJ, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 2010; 3: 95–118.
- Bailey NW, Hoy KE, Rogasch NC, Thomson RH, McQueen S, Elliot D, et al. Differentiating responders and non-responders to rTMS treatment for depression after one week using resting EEG connectivity measures. J Affect Disord 2019; 242: 68–79.
- He R, Fan J, Wang H, Zhong Y, Ma J. Differentiating responders and non-responders to rTMS treatment for disorder of consciousness using EEG after-effects. Front Neurol 2020; 11: 583268.