ORIGINAL REPORT
ACCELEROMETER-MEASURED PHYSICAL ACTIVITY AT 3 MONTHS AS A PREDICTOR OF SYMPTOMS OF DEPRESSION AND ANXIETY 1 YEAR AFTER STROKE: A MULTICENTRE PROSPECTIVE COHORT STUDY IN CENTRAL NORWAY
Ailan PHAN, MSc1, Torunn ASKIM, PhD1, Stian LYDERSEN, PhD2, Bent INDREDAVIK, MD, PhD1,3,4 and Torgeir WETHAL, MD, PhD1,4
From the 1Department of Neuromedicine and Movement Science, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology, 2Regional Centre for Child and Youth Mental Health and Child Welfare, Department of Mental Health, Norwegian University of Science and Technology, 3Department of Medical Quality Registries, St Olavs Hospital, Trondheim University Hospital and 4Stroke Unit, Department of Internal Medicine, St Olavs Hospital, Trondheim University Hospital, Trondheim, Norway
Objectives: To study sedentary behaviour and physical activity at 3 months as predictors for symptoms of depression and anxiety at 1-year post-stroke.
Design: A prospective cohort study.
Patients: Patients with first-ever ischaemic stroke.
Methods: Mood was assessed 3- and 12-months post-stroke using the Hospital Anxiety and Depression Scale. Sedentary behaviour and physical activity were measured using accelerometry 3 months post-stroke.
Results: A total of 292 participants (116 (39.7%) females; mean age 71.7 (standard deviation 11.3) years) were included. At 12 months, 16.7% experienced depression and 19.5% anxiety, respectively. Adjusting for age and sex, regression analysis showed that comorbidity burden (β 0.26; 95% confidence interval (95% CI) 0.02, 0.51; p = 0.038), stroke severity (β 0.22; 95% CI 0.10, 0.35; p = 0.001), functional disability (β 0.89, 95% CI 0.49, 1.30; p = 0.000), and global cognition (β–0.15; 95% CI –0.25, –0.05; p = 0.004) predicted depression. Multi-adjusted analysis showed sedentary behaviour and physical activity did not significantly predict depression or anxiety (p > 0.05).
Conclusion: Sedentary behaviour and physical activity did not significantly predict mood after stroke. Comorbidity burden, stroke severity, functional disability, and global cognition were identified as possible predictors of depression. More research is needed to determine the impact of physical activity on depression and anxiety symptoms.
LAY ABSTRACT
Depression and anxiety are common after stroke. In the general population, physical activity is associated with improved mental health. This study found that stroke survivors spend most of their day sedentary. Physical activity was not associated with mood, but the findings of this study suggest possible relationships between stroke severity, functional disability, cognition, physical health issues, and depression. Further research is needed to investigate the relationship between physical activity and mood after a stroke.
Key words: anxiety; depression; mental health; physical activity; risk factors; stroke.
Citation: J Rehabil Med 2023; 55: jrm12309. DOI: https://doi.org/10.2340/jrm.v55.12309
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Oct 24, 2023; Published: Nov 16, 2023
Correspondence address: Ailan Phan, Department of Neuromedicine and Movement Science, Norwegian University of Science and Technology, Department of Medicine and Health Science, NO-7491 Trondheim, Norway. E-mail: ailan.phan@ntnu.no.
Competing interests and funding: The authors have no conflicts of interest to declare.
Depression and anxiety are common sequelae after stroke and have a major impact on daily -functioning and individual well-being (1, 2). Prevalence rates for depression and anxiety during the first year following stroke have been estimated as 33% (95% CI 26–39%) and 29.3% (95% CI 25–33.5%), respectively (1, 3). Engaging in physical activity (PA) is a potential modifiable risk factor for preventing and treating depression and anxiety (4, 5). In addition to playing an important role in preventing strokes and vascular events (6), PA is positively associated with quality of life (7). In healthy subjects and different patient populations, several studies have established that PA is effective in improving depression and anxiety (8). Available evidence also suggests that PA plays a protective role in preventing the onset of depression and anxiety and that even small amounts of PA can decrease the incidence of depressive episodes (9, 10). Past studies have also demonstrated an association between sedentary behaviour (SB) and increased risk of depression, even after adjustment for PA (11). However, these findings are not easily transferable to stroke survivors, who often have high levels of comorbidity and have residual functional disabilities and cognitive deficits following stroke. Reports from stroke populations imply the effects of exercise on reducing symptoms of depression and anxiety (12, 13). Nevertheless, past studies on the effects of SB and PA on mood in stroke survivors are limited by their small sample sizes and are additionally scarce and inconclusive (14). Also, past evidence on the association between PA and mood in stroke survivors has predominantly relied on PA performed in clinical settings and self-report questionnaires.
Several factors impacting mood following stroke have been suggested. Past studies have suggested that cognition and mood are linked (15, 16). Physical disability, stroke severity, and comorbidity burden are also probable factors associated with emotional outcomes (17–19). Clarifying how these factors affect emotional outcomes can help to identify individuals at high risk of developing post-stroke depression and anxiety. This can optimize patient follow-up to prevent and reduce symptoms. Considering that the behavioural pattern after stroke appears to be established within the first 3 months and indicates a long-term activity pattern (20), we hypothesize that there is a link between activity levels at 3 months and mood after 12 months.
In this study, the primary objective was to study SB and PA levels at 3 months as predictors for symptoms of depression and anxiety 1 year after a stroke. A secondary objective was to assess the prevalence and changes in symptoms of depression and anxiety at 3 and 12 months, while also identifying additional potential predictors linked to depression or anxiety. These factors include physical disability, stroke severity, comorbidity burden, and cognition.
METHODS
Study design
MIDNOR STROKE is a prospective longitudinal multicentre cohort study including patients in Central Norway with first-ever ischaemic stroke. Patients were recruited from stroke units at the following 8 hospitals: St Olav, Molde, Levanger, Namsos, Volda, Kristiansund, Ålesund and Orkdal, between 1 June 2015, and 1 November 2017. Together, those participating hospitals serve a catchment area of approximately 700,000 inhabitants and are the care providers for the acute treatment of all stroke patients in Central Norway. Participants had to meet the following inclusion criteria: cerebral infarction according to International Classification of Diseases 10th revision (ICD-10) clinical modification diagnosis code I63, ≥ 18 years of age, first-ever stroke, residency in Central Norway, and recruitment within 7 days of symptom onset. In the current sub-study, patients who attended 3-month follow-up at the outpatient clinic and were willing to wear the activPAL activity monitor (The activPAL3™, Model 20.2, PAL Technologies Ltd, Glasgow, UK) for 7 days were included. Patients were excluded if they had severe disabilities before stroke, defined as a Modified Rankin Scale (mRS) score of 5. Participation in the study was voluntary and by written informed consent. Patients who were not able to provide consent were recruited if informed consent was obtained from the next of kin. All participants received standard care and treatment in accordance with the national guidelines for treatment and rehabilitation of stroke (21).
Data collection
Demographic information, along with details regarding functional status, stroke severity, comorbid conditions, medications, PA, and global cognition, were collected during the index hospital stay and at 3-month follow-up assessment. Symptoms of depression and anxiety at 3- and 12 months were assessed using the self-administered questionnaire the Hospital Anxiety and Depression Scale (HADS) (22). Subjects with high scores on HADS anxiety or depression were asked if they received adequate care and referred to a physician if needed. The HADS has been validated in stroke populations and is one of the most common tools for screening depression and anxiety (23). It contains 2 subscales, 1 for anxiety and 1 for depression. The following cut-offs for scores to determine non-case level, mild, moderate, and severe symptoms of anxiety or depression were used: 0–7, 8–10, 11–14, and 15–21 for both subscales, respectively. Functional status was assessed using the mRS at base-line and 3 months (24). Functional independence and dependency were defined by mRS scores of 0–2 and 3–5, respectively. PA prior to stroke was assessed using a self-report questionnaire, the HUNT I PA questionnaire (25). Participants were asked about their activity levels in the 6 months prior to their stroke. The Charlson Comorbidity Index (CCI) (26) measured at baseline was used to determine the degree of comorbidity burden (26), while stroke severity on day 1 following hospital admission was assessed using the National Institutes of Health Stroke Scale (NIHSS) (27). Global cognition at 3 months was assessed using the Montreal Cognitive Assessment (MoCA) (28). Antidepressant medications were classified as drugs with Anatomical Therapeutic Chemical Classification system (ATC) code N06A, while anxiolytic drugs encompassed all medications with ATC code N05B.
SB and PA were measured using a thigh-worn activPAL activity monitor for up to 7 consecutive days at 3 months. The activPAL device obtains information about body position and transitions between the postures (sitting/lying or upright), walking, and walking speed in which energy expenditure measured in metabolic equivalents (METs) is inferred indirectly. Valid whole-day recordings were determined using the software PALanalysis (version v8.11.8.75; PAL Technologies Ltd, Glasgow, UK) validation algorithm (CREA algorithm v1.3), where wear time protocol was set to 24-h protocol, and by visual inspection of the daily summaries for each participant.
ActivPAL events files were used for data analysis. A default setting of 10 s for a minimum sitting or upright period was used. Data were processed using a custom MATLAB script (MATLAB version R2021b, Natick, MA, US), where daytime behaviour, defined as all activity occurring from 08.00 h to 23.30 h, was extracted. Data processing included filtering out brief standing events of ≤ 3 s during a walking bout and steps ≤ 3 s in an upright position. Active behaviour was categorized using the following cut-offs for METs: light PA (LPA) 1.5–3 METs, moderate PA (MPA) 3–6 METs, and vigorous PA (VPA) > 6 METs. SB was defined as time spent sitting or lying. Only data from participants with at least 2 valid recording days were included in the analysis. We chose a minimum of 2 days of recording based on previous stroke studies and participant recording days. Although previous stroke monitoring protocols showed significant variation in activity duration (1–8 consecutive days) (20), a study by Fini et al. concluded that 2 days of measurement is sufficient for reliable measurement after stroke for many simple variables (29).
Statistical analysis
The mean time (in h) spent in SB, LPA, MPA, and VPA was computed by summation and divided by the total wear time (in days) per individual. Descriptive statistics are reported as mean and standard deviation (SD) for continuous variables, while numbers and percentages are reported for categorical variables. Confounders for PA and predictors of emotional outcome were selected a priori and based on literature and clinical judgement. A directed acyclic graph representing the assumed causal paths was also used (Fig. S1). Linear regression models were used with HADS depression or HADS anxiety scores as dependent variables, and PA variables, 1 at a time, as main covariates. The analyses were age- and sex-adjusted first and then adjusted simultaneously for the following covariates: MoCA, mRS, CCI, NIHSS at day 1, and use of antidepressive or anxiolytic drugs. For participants missing at most 2 items in the HADS depression or HADS anxiety scales, missing item scores were imputed with the mean scores on the scale for that participant. For the remaining variables, missing values were handled using available case analysis; that is, each analysis included cases with available data on the included variables.
Normality of residuals was assessed by visual inspection of Q-Q plots. Linearity was assessed by visual inspection of scatterplots for the most relevant variables. Estimates with 95% confidence intervals (95% CI) were reported where relevant. Two-sided p-values < 0.05 were considered statistically significant. However, due to multiple hypotheses, p-values between 1% and 5% should be interpreted with caution. The analyses were conducted using Rstudio: Integrated Development for R (version 3.6.3) and packages Stats (version 3.6.3) and jtools (version 2.2.1), Boston, MA URL: http://www.rstudio.com/.
RESULTS
The MIDNOR STROKE study included 794 participants. The current sub-study included 292 stroke survivors with activPAL assessments. A flow chart of participants is shown in Fig. 1.
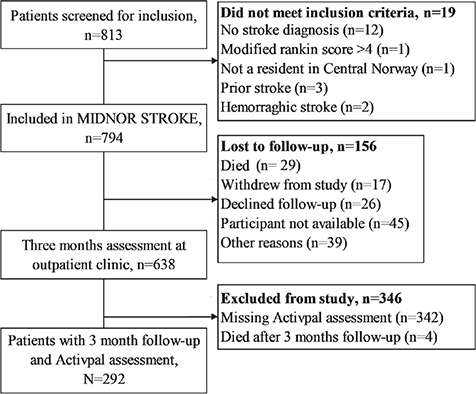
Fig. 1. Flow chart of study participants.
Baseline characteristics of the included participants and participants not included due to lost-to-follow-up and missing activity data are summarized in Table I. On average, participants included in the current analyses were younger, had less comorbidity burden (details of the included conditions are specified in Table SVII), had milder strokes, were more functionally independent at 3 months, and included a smaller percentage of females. In terms of pre-stroke activity, included participants reported higher levels of exercise in terms of frequency, intensity, and duration.
Of the 292 participants, 262 (89.7%) had 6–7 complete days of activity monitoring data, 23 (7.9%) had 5 days of data, and the remaining 7 (2.4%) had 2–4 days of consecutive data. Descriptive data on time spent in sedentary, light, and moderate PA at 3 months follow-up are shown in Table II. None of the participants engaged in vigorous PA.
Overall, 43 participants had missing scores for either HADS D or A assessment at 12 months. Details are provided in Table SI. Among these, 9 participants exhibited missing data on ≤ 2 items for either the HADS D or HADS A score at 12 months. For the HADS D score, mean scores were imputed for 4 participants, while for the HADS A scores, imputation was performed for 6 participants. HADS D scores were obtained from 273 and 257 participants at 3 and 12 months, respectively. After 3 and 12 months, 15.8% and 16.7% of the participants reported mild to severe symptoms of depression (Fig. 2). The mean (SD) score for HADS depression was 3.6 (3.4) at 3 months and 3.8 (3.4) at 12 months, respectively. These data revealed that 18.6% (n = 8/43) of the individuals mild to severe symptoms of depression at 3 months were using antidepressant drugs. Results showed that the majority (91.1%, n = 185/203) of participants with non-case levels of depression at 3 months maintained the same level after 12 months, while 8.9% (n = 18/203) developed mild to moderate symptoms of depression. Among individuals with symptoms of depression (> 7 HADS D points) at 3 months, 42.1% (n = 16/38) maintained the same level of symptom severity, 50.0% (n = 19/38) showed improvements in symptom severity, and 7.9% (n = 3/38) transitioned to greater severity.
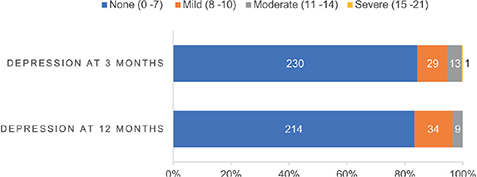
Fig. 2. Hospital Anxiety and Depression scale (HADS) depression score levels at 3 and 12 months. Numbers of participants are listed for each category.
Results of the age- and sex-adjusted analyses for depression are shown in Table III. Predictors found to be positively and significantly (p < 0.05) associated with symptoms of depression were high mRS and NIHSS scores, increased comorbidity burden, and time spent in SB. Higher scores on MoCA and time spent in light or moderate PA significantly (p < 0.01) predicted lower HADS-D scores at 12 months. Results from the multivariable analyses focusing on SB, PA, and symptoms of depression at 12 months, adjusted for all other variables, are shown in Table IV. Time spent in sedentary, light, or moderate PA was not significantly associated with depression (p > 0.05).
| Covariate | Reg. coeff. | (95% CI) | p- value |
| Charlson Comorbidity Index (score) | 0.26 | (0.02, 0.51) | 0.038 |
| NIHSS Day 1a | 0.22 | (0.10, 0.35) | 0.001 |
| MRs at 3 monthsb | 0.89 | (0.49, 1.30) | 0.000 |
| MoCA at 3 months | –0.15 | (–0.25, –0.05) | 0.004 |
| Antidepressants at 3 months | 1.43 | (0.02, 2.85) | 0.047 |
| Sedentary behaviour/day, h | 0.32 | (0.11, 0.54) | 0.003 |
| Light intensity PA/day (MET < 3), h | –0.34 | (–0.58, –0.09) | 0.007 |
| Moderate intensity PA/day (MET 3–6), h | –1.25 | (–2.16, –0.33) | 0.008 |
| an = 252, bn = 255. | |||
| Reg. coeff.: regression coefficient; NIHSS: National Institutes of Health Stroke Scale; mRS: Modified Rankin Scale; MoCA: Montreal Cognitive Assessment; MET: metabolic equivalent; 95% CI: 95% confidence interval; PA: physical activity. | |||
A total of 265 and 257 HADS anxiety scores were obtained at 3 and 12 months, respectively. Details are shown in Table SII. The number of participants with non-case level, mild, moderate, and severe symptoms of anxiety at 3 and 12 months are shown in Fig. 3. The mean (SD) score for HADS anxiety at 3 months was 4.0 (3.6) and 4.0 at 12 months (3.9). At 3 and 12 months, 45 (17.0%) and 50 (19.5%) participants reported mild to severe symptoms of anxiety, respectively.
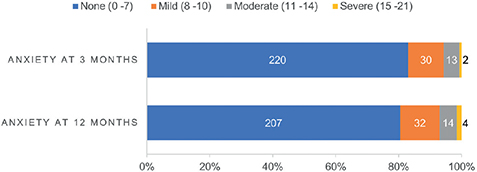
Fig. 3. Hospital Anxiety and Depression scale (HADS) anxiety score levels at 3 and 12 months. Numbers of participants are listed for each category.
Among participants with HADS anxiety scores available at both 3 and 12 months, data revealed that 89.7% (n = 175/195) of those initially displaying non-case levels of anxiety at 3 months remained at the same level after 12 months. While 10.2% (n = 20/195) developed mild to severe symptoms of anxiety. Among individuals who reported mild to severe symptoms of anxiety (> 7 symptoms) at 3 months (n = 38), 50.0% (n = 19/38) reported the same level of symptom severity at 12 months follow-up. In addition, 47.4% (n = 18/38) showed symptom improvement, while 2.6% (n = 1/38) developed worsening anxiety at 12 months (see details in Table SII). At 3- and 12-months, 22.4% (n = 62/277) and 24.4% (n = 63/258) of participants, respectively, reported mild to severe symptoms of depression or anxiety (see details in Tables SIII and SIV). Comorbid symptoms of mild to severe depression and anxiety were present in 10.0% (n = 26/261) and 11.7% (n = 30/256) of the study population at 3 and 12 months, respectively (see details in Tables SV and SVI).
Results from the age- and sex-adjusted variables for symptoms of anxiety are shown in Table V. None of the selected predictors were significantly associated with anxiety at 12 months (p > 0.05). In our multivariable regression models for anxiety at 12 months, time spent in sedentary, light, or moderate PA didnot significantly predict symptoms of anxiety (Table VI) (p > 0.05).
| Covariate | Reg. coeff. | (95% CI) | p-value |
| Charlson Comorbidity Index (score) | 0.25 | (–0.04, 0.54) | 0.090 |
| NIHSS Day 1a | 0.07 | (–0.08, 0.21) | 0.365 |
| MRs at 3 monthsb | 0.43 | (–0.05, 0.90) | 0.081 |
| MoCA at 3 months | –0.08 | (–0.20, 0.03) | 0.164 |
| Antidepressants at 3 months | 0.77 | (–2.07, 3.62) | 0.591 |
| Sedentary behaviour/day, h | 0.10 | (–0.14, 0.35) | 0.412 |
| Light intensity PA/day (MET < 3), h | –0.09 | (–0.37, 0.20) | 0.543 |
| Moderate intensity PA/day (MET 3–6), h | –0.68 | (–1.75, 0.39) | 0.215 |
| an = 252, bn = 255. | |||
| Reg. coeff.: regression coefficient; 95% CI: 95% confidence interval; NIHSS: National Institutes of Health Stroke Scale; mRS: Modified Rankin Scale; MoCA: Montreal Cognitive Assessment; MET: metabolic equivalent; PA: physical activity. | |||
DISCUSSION
This study demonstrated that approximately 1 in 4 stroke survivors experienced depression or anxiety 1-year post-stroke. Symptom burden appears to persist from 3 months and extend into the chronic stages of stroke. To the best of our knowledge, this is the first study to examine the relationship between objectively measured PA at 3 months in stroke survivors and symptoms of depression and anxiety 12 months post-stroke. The current multivariable analysis showed no statistically significant relationship between SB, PA, and symptoms of depression or anxiety post-stroke. In the current age- and sex-adjusted analyses, stroke severity, physical function, comorbidity burden, and cognitive function were predictive of symptoms of depression at 12 months. None of the selected predictors showed significant associations with symptoms of anxiety at 12 months.
Studies reporting prevalence rates post-stroke vary greatly (3, 30). The prevalence rates of depression and anxiety at 3 and 12 months post-stroke in the current study are lower than the estimated pooled prevalence rates for depression and anxiety reported by Hackett and Pickles (3) and Knapp et al. (30). Nonetheless, the current data align with the prevalence rates for depression and anxiety reported among individuals with heart diseases, stroke, and diabetes mellitus in the HUNT4 study population (31). The lower prevalence rates in the current study population can be explained by the fact that our sub-study consisted largely of individuals with milder strokes, lower degrees of functional disability, and a higher proportion of males. These factors have been shown to predict lower levels of post-stroke depression and anxiety (17, 18). Furthermore, past estimates vary largely due to differences in time of assessment, study setting, heterogeneity of study population, and different caseness thresholds for rating scales, as well as assessment methods (2, 3, 30). Prior investigations into mood trajectories post-stroke have demonstrated that individuals with depression or anxiety are at high risk of either remaining stable over time or developing more severe symptoms (2, 32, 33). The current findings provide additional support to the existing evidence of depression and anxiety persistently extending into the chronic phases of stroke, even after mild ischaemic stroke. Considering that the majority of individuals with non-case levels of anxiety or depression maintained their status over the course of 12 months, the current study findings could suggest the presence of resilient stroke survivors who maintain a stable positive mood.
In line with prior studies, the current data show that stroke survivors are predominantly sedentary and engage in little moderate to vigorous PA (34, 35). The current findings are similar to those of the study of Wondergem et al. (36), who reported SB and PA levels from free-living stroke survivors in the first 2 months post-stroke. Compared with the few other studies that have objectively assessed levels of SB and PA (35, 37) among stroke survivors, the current study population appears to be more physically active, on average, in terms of less time spent sedentary and more time spent in moderate PA. After adjustments for probable factors affecting PA post-stroke, this study found no significant relationship between SB, PA, and mood. Considering that a quarter of the current study participants reported no or infrequent exercise, the observed lack of effect might stem from low baseline PA levels. Despite substantial evidence supporting the inverse relationship between PA and depression (4), the influence of PA on mood over time and the ideal duration for engaging in PA remains unclear. It is unknown whether the current study population achieved the optimal daily mean amount of PA and intensity necessary to impact mood. One recent cross-sectional study has reported an association between World Health Organization (WHO)-recommended PA levels and a lower risk of depression in stroke survivors (38). The evidence is, however, limited by the nature of the self-reported PA data and lacks adjustments for important factors such as stroke severity and time since stroke. Even less work has been done on the relationship between PA and anxiety post-stroke. A few studies have investigated the relationship; however, data are inconclusive (12, 39), and summarized data from a Cochrane review show that data have been inconsistent due to major bias and confounding issues (14). Furthermore, reports on the relationship between PA levels and mood post-stroke in community-dwelling survivors appear to be lacking.
This study identified cognitive impairment, comorbidity burden, stroke severity and physical disability as possible predictors of depression at 12 months. These findings are supported by several studies (17, 40–42). While several lines of evidence support that individuals with depression and anxiety spend more time in SB (43–45), the direction of the association remains unclear. Considering that we have not taken the prior history of mood (pre-stroke) into account, our findings are limited in terms of interpreting the PA levels at 3 months in relation to symptoms of depression and anxiety occurring post-stroke.
Unlike research concerning post-stroke depression, studies investigating predictors of anxiety have not been as comprehensive, and findings vary due to the wide range of assessments of post-stroke anxiety, time elapsed after stroke, and the heterogeneity of study samples (18). While a systematic review and meta-analysis have identified stroke severity and cognitive impairment as the main predictors of post-stroke anxiety (18), the current study failed to demonstrate any relationship between stroke severity, cognitive impairment, and post-stroke anxiety symptoms. Prior findings have, however, been based on a few studies only, and authors of the systematic review conclude that past studies lack methodological and statistical rigour.
This study has several strengths and limitations. To the best of our knowledge, this is the first study to explore the relationship between PA and post-stroke mood using accelerometers. A major strength is that this allowed for sustained monitoring of activity levels among stroke survivors in their free-living environments. A limitation is that we only assessed accumulated time spent in PA and SB, this may explain why the current results did not confirm previous research showing that breaking up SB or engaging in prolong PA were associated with mood (38). We also lacked data on vigorous PA and changes in activity patterns from 3 to 12 months. Moreover, we have not considered probable factors such as self-efficacy and social support, which could influence PA and subsequently affect mood. A major limitation is the substantial number of participants missing activPAL assessment. Our data suggests that participants with no activPal recording had more severe strokes and displayed a higher degree of disability. This implies that the current study sample possibly represents a comparatively healthier stroke population, engaged in higher activity levels on average. In addition, while the HADS self-report questionnaire is commonly used and validated in stroke populations, it is important to recognize that self-report assessments often overestimate the prevalence rates of subject with symptoms of depression or anxiety compared to interview-based methods. Moreover, the limited impact of PA on mood in our study may be due to the small sample size of participants with depression, anxiety or milder symptoms.
In conclusion, the current study showed that depression and anxiety are prevalent post-stroke. Symptom burden appears to persist from 3 to 12 months. After adjusting for age and sex, stroke severity, comorbidity burden functional disability, and cognition predicted symptoms of depression at 12 months. No statistically significant relationship between PA and post-stroke mood was observed. Considering that stroke survivors are highly sedentary, there may be a large treatment potential in increasing PA levels. Further studies are needed to determine the impact of PA on symptoms of depression or anxiety.
ACKNOWLEDGEMENTS
We thank the participants, study nurses, physicians, and other colleagues at the collaborating hospitals: Namsos, Ålesund, Volda, Kristiansund, Orkdal, Levanger, Molde, and St Olav, who participated in the study. We thank Xiang Chun Tan for her help in processing the ActivPAL data and for valuable input on data quality.
The MIDNOR STROKE study was funded by The Liaison Committee for Education, Research, and Innovation in Central Norway and the Foundation DAM. The funders had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data.
This study was conducted in compliance with ethics standards as outlined in the Declaration of Helsinki. This study was approved by the Regional Committee of Medical and Health Research Ethics Western Norway (2015/453/REK midt).
Clinical trial registration: URL: https://clinicaltrials.gov. Unique identifier: NCT03962127 (23/05/2019).
REFERENCES
- Rafsten L, Danielsson A, Sunnerhagen KS. Anxiety after stroke: a systematic review and meta-analysis. J Rehabil Med 2018; 50: 769–780.
- Mitchell AJ, Sheth B, Gill J, Yadegarfar M, Stubbs B, Yadegarfar M, et al. Prevalence and predictors of post-stroke mood disorders: a meta-analysis and meta-regression of depression, anxiety and adjustment disorder. Gen Hosp Psychiatry 2017; 47: 48–60.
- Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke 2014; 9: 1017–1025.
- Harvey SB, Øverland S, Hatch SL, Wessely S, Mykletun A, Hotopf M. Exercise and the prevention of depression: results of the Hunt cohort study. Am J Psychiatry 2018; 175: 28–36.
- Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, Vandelanotte C. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev 2015; 9: 366–378.
- Garcia L, Pearce M, Abbas A, Mok A, Strain T, Ali S, et al. Non-occupational physical activity and risk of cardiovascular disease, cancer and mortality outcomes: a dose–response meta-analysis of large prospective studies. Br J Sports Med 2023; 57: 979–989.
- Vagetti GC, Barbosa Filho VC, Moreira NB, Oliveira V de, Mazzardo O, Campos W de. Association between physical activity and quality of life in the elderly: a systematic review, 2000-2012. Rev Bras Psiquiatr 2014; 36: 76–88.
- Singh B, Olds T, Curtis R, Dumuid D, Virgara R, Watson A, et al. Effectiveness of physical activity interventions for improving depression, anxiety and distress: an overview of systematic reviews. Br J Sports Med 2023; 57: 1203–1209.
- McDowell CP, Dishman RK, Gordon BR, Herring MP. Physical activity and anxiety: a systematic review and meta-analysis of prospective cohort studies. Am J Prev Med 2019; 57: 545–556.
- Gianfredi V, Blandi L, Cacitti S, Minelli M, Signorelli C, Amerio A, et al. Depression and objectively measured physical activity: a systematic review and meta-analysis. Int J Environ Res Public Health 2020; 17: 3738.
- Eriksson M, Nääs S, Berginström N, Nordström P, Hansson P, Nordström A. Sedentary behavior as a potential risk factor for depression among 70-year-olds. J Affect Disord 2020; 263: 605–608.
- Aidar FJ, Jacó de Oliveira R, Gama de Matos D, Chilibeck PD, de Souza RF, Carneiro AL, et al. A randomized trial of the effects of an aquatic exercise program on depression, anxiety levels, and functional capacity of people who suffered an ischemic stroke. J Sports Med Phys Fitness 2018; 58: 1171–1177.
- Lennon O, Carey A, Gaffney N, Stephenson J, Blake C. A pilot randomized controlled trial to evaluate the benefit of the cardiac rehabilitation paradigm for the non-acute ischaemic stroke population. Clin Rehabil 2008; 22: 125–133.
- Saunders DH, Sanderson M, Hayes S, Johnson L, Kramer S, Carter DD, et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev 20202; 3: CD003316.
- Kim J, Yim J. Effects of an exercise protocol for improving handgrip strength and walking speed on cognitive function in patients with chronic stroke. Med Sci Monit Int Med J Exp Clin Res 2017; 23: 5402–5409.
- Barker-Collo SL. Depression and anxiety 3 months post stroke: prevalence and correlates. Arch Clin Neuropsychol 2007; 22: 519–531.
- Kutlubaev MA, Hackett ML. Part II: Predictors of depression after stroke and impact of depression on stroke outcome: an updated systematic review of observational studies. Int J Stroke 2014; 9: 1026–1036.
- Menlove L, Crayton E, Kneebone I, Allen-Crooks R, Otto E, Harder H. Predictors of anxiety after stroke: a systematic review of observational studies. J Stroke Cerebrovasc Dis 2015; 24: 1107–1117.
- Jørgensen TSH, Wium-Andersen IK, Wium-Andersen MK, Jørgensen MB, Prescott E, Maartensson S, et al. Incidence of depression after stroke, and associated risk factors and mortality outcomes, in a large cohort of Danish patients. JAMA Psychiatry 2016; 73: 1032–1040.
- Fini NA, Holland AE, Keating J, Simek J, Bernhardt J. How physically active are people following stroke? Systematic review and quantitative synthesis. Phys Ther 2017; 97: 707–717.
- The Norwegian Directorate of health. Norwegian guidelines on management and rehabilitation of stroke. [Date accessed: 20.01.2023] Available from: https://www.helsedirektoratet.no/retningslinjer/hjerneslag.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand, 1983; 67: 361–370.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res 2002; 52: 69–77.
- Sulter G, Steen C, De Keyser J. Use of the Barthel Index and Modified Rankin Scale in Acute Stroke trials. Stroke 1999; 3: 1538–1541.
- Kurtze N, Rangul V, Hustvedt BE, Flanders WD. Reliability and validity of self-reported physical activity in the Nord-Trøndelag Health Study (HUNT 2). Eur J Epidemiol 2007; 22: 379–387.
- Goldstein LB, Samsa GP, Matchar DB, Horner RD. Charlson Index Comorbidity adjustment for ischemic stroke outcome studies. Stroke 2004; 35: 1941–1945.
- Brott T, Adams Jr. HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. National Institutes of Health Stroke Scale. Stroke 1989
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699.
- Fini NA, Burge AT, Bernhardt J, Holland AE. Two days of measurement provides reliable estimates of physical activity poststroke: an observational study. Arch Phys Med Rehabil 2019; 100: 883–890.
- Knapp P, Dunn-Roberts A, Sahib N, Cook L, Astin F, Kontou E, et al. Frequency of anxiety after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke 2020; 15: 244–255.
- Bojanić I, Sund ER, Sletvold H, Bjerkeset O. Prevalence trends of depression and anxiety symptoms in adults with cardiovascular diseases and diabetes 1995–2019: The HUNT studies, Norway. BMC Psychol 2021; 9: 130.
- Ayis SA, Ayerbe L, Crichton SL, Rudd AG, Wolfe CDA. The natural history of depression and trajectories of symptoms long term after stroke: the prospective south London stroke register. J Affect Disord 2016; 194: 65–71.
- White JH, Attia J, Sturm J, Carter G, Magin P. Predictors of depression and anxiety in community dwelling stroke survivors: a cohort study. Disabil Rehabil 2014; 36: 1975–1982.
- Tieges Z, Mead G, Allerhand M, Duncan F, van Wijck F, Fitzsimons C, et al. Sedentary behavior in the first year after stroke: a longitudinal cohort study with objective measures. Arch Phys Med Rehabil 2015; 96: 15–23.
- Duran AT, Pascual CB, Goldsmith J, Howard VJ, Hutto B, Colabianchi N, et al. Objectively measured physical activity and sedentary time among adults with and without stroke: a national cohort study. Stroke 2021; 52: 729–732.
- Wondergem R, Pisters MF, Heijmans MW, Wouters EJM, Bie RA de, Veenhof C, et al. Movement behavior remains stable in stroke survivors within the first two months after returning home. PLOS One 2020; 15: e0229587.
- Rand D, Eng JJ, Tang PF, Jeng JS, Hung C. How active are people with stroke? Stroke 2009; 40: 163–168.
- Apriliyasari RW, Budi IS, Tan MP, Tsai PS. Physical activity and depression in Indonesian adults with stroke: a nationwide survey. J Nurs Scholarsh 2023; 5: 356–364.
- Bateman A, Culpan FJ, Pickering AD, Powell JH, Scott OM, Greenwood RJ. The effect of aerobic training on rehabilitation outcomes after recent severe brain injury: a randomized controlled evaluation. Arch Phys Med Rehabil 2001; 82: 174–182.
- Ayerbe L, Ayis S, Wolfe CDA, Rudd AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry 2013; 202: 14–21.
- Bartoli F, Pompili M, Lillia N, Crocamo C, Salemi G, Clerici M, et al. Rates and correlates of suicidal ideation among stroke survivors: a meta-analysis. J Neurol Neurosurg Psychiatry 2017; 88: 498–504.
- Prior PL, Suskin N. Exercise for stroke prevention. Stroke Vasc Neurol 2018; 3: 59–68.
- Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES, et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am JPsychiatry 2018; 175: 631–648.
- Ayerbe L, Ayis S, Rudd AG, Heuschmann PU, Wolfe CDA. Natural history, predictors, and associations of depression 5 years after stroke. Stroke 2011; 42: 1907–1911.
- del Pozo Cruz B, Alfonso-Rosa RM, McGregor D, Chastin SF, Palarea-Albaladejo J, del Pozo Cruz J. Sedentary behaviour is associated with depression symptoms: Compositional data analysis from a representative sample of 3233 US adults and older adults assessedwithaccelerometers. J Affect Disord 2020; 265: 59–62.