ORIGINAL REPORT
EFFECTS OF SEMI-IMMERSIVE VIRTUAL REALITY AND MANIPULATION OF OPTIC FLOW SPEED ON GAIT BIOMECHANICS IN PEOPLE POST-STROKE
Emma DE KEERSMAECKER, PhD1–4, Anke VAN BLADEL, PhD4–6, Silvia ZACCARDI, MSc1,3,7, Nina LEFEBER, PhD1, Carlos RODRIGUEZ-GUERRERO, PhD8, Eric KERCKHOFS, PhD1, Bart JANSEN, PhD3,7,9 and Eva SWINNEN, PhD1–4
From the 1Rehabilitation Research Group, Vrije Universiteit Brussel, Brussels, Belgium, 2Center for Neurosciences (C4N), Brussels, Belgium, 3Brussels Human Robotic Research Center (BruBotics), Brussels, Belgium, 4Alliance research group REBI (Rehabilitation technology for people with a brain injury), Vrije Universiteit Brussel & Ghent University, Brussels, Ghent, Belgium, 5Department of Rehabilitation Sciences, Ghent University, Ghent, Belgium, 6Department of Physical and Rehabilitation Medicine, Ghent University Hospital, Ghent, Belgium, 7Department of Electronics and Informatics, Engineering Sciences, Vrije Universiteit Brussel, Brussels, Belgium, 8Department of Mechanical Engineering, KU Leuven, Heverlee Leuven, Belgium, and 9Imec, Leuven, Belgium
Objectives: To investigate how people post-stroke and healthy people experience the addition of semi-immersive virtual reality (VR) and optic flow speed manipulation while walking on a treadmill, and if optic flow speed manipulation could be used in rehabilitation to elicit changes in post-stroke gait biomechanics.
Methods: Sixteen people post-stroke and 16 healthy controls walked on a self-paced treadmill. After 2 habituation trials (without and with VR), participants walked 3 more trials under the following conditions of optic flow: matched, slow, and fast. Primary outcome measures were spatiotemporal gait parameters and lower limb kinematics. Secondary outcomes (simulator sickness and enjoyment) were assessed with the Simulator Sickness Questionnaire (SSQ) and visual analogue scales (VAS).
Results: VR did not influence the gait biomechanics, and optic flow manipulation had a limited effect. Both groups significantly increased their walking speed with the slow optic flow and decreased their speed with the fast optic flow. For the other gait parameters, only small changes were found. Only people post-stroke had a significant increase on the SSQ and the enjoyment-VAS.
Conclusion: Adding semi-immersive VR did not influence the gait pattern, was well tolerated, and enjoyable. Both groups altered their gait parameters when the optic flow speed was adjusted during the protocol. Incorporating such manipulations into treadmill training is feasible, but further research about the type of manipulation and level of immersion is needed.
LAY ABSTRACT
This study investigated the effect of virtual reality and manipulation of the speed of the virtual environment while walking on a treadmill, both in people post-stroke and healthy people. Sixteen people post-stroke and 16 healthy controls walked on a self-paced treadmill. After 2 habituation trials (without and with virtual reality), participants walked 3 more trials under the following conditions of optic flow: (i) walking with a matching virtual environment, (ii) walking with a slower virtual environment, and (iii) walking with a faster virtual environment. The addition of virtual reality did not influence the gait pattern of people post-stroke or healthy people. When the speed of the virtual environment was manipulated, people post-stroke altered their gait pattern by changing their walking speed. People increased their walking speed with a slower virtual environment, and decreased their speed with a faster virtual environment. Incorporating such manipulations for treadmill training could be feasible, but further research is needed.
Key words: virtual reality; optic flow; gait biomechanics, stroke.
Citation: J Rehabil Med 2024; 56: jrm12384. DOI: https://doi.org/10.2340/jrm.v56.12384.
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Submitted: Apr 25, 2023; Accepted: Nov 28, 2023; Published: Jan 10, 2024
Correspondence address: Eva Swinnen, Rehabilitation Research, Department of Physiotherapy, Human Physiology and Anatomy, Vrije Universiteit Brussel, Laarbeeklaan 121, BE-1090 Jette, Belgium. E-mail: eva.swinnen@vub.be
Post-stroke gait rehabilitation remains a major challenge. Two-thirds of all stroke survivors experience walking impairments, resulting in decreased activities of daily living, level of participation, and quality of life (1, 2). To improve these impairments, patients need a high-intensity, repetitive, and task-specific rehabilitation, such as treadmill training (3). However, an important downside of treadmill walking is the incorrect visual information subjects receive while walking (4).
An important source of visual information to guide locomotion is optic flow (OF), a pattern of visual motion projected onto the retina of the eye (5). Optic flow provides us with information about the direction and speed of self-motion (5, 6). However, during treadmill walking, the visual information about the direction and speed of walking is inconsistent with the proprioceptive input of the lower limbs. Subjects are walking on the treadmill, but their environment remains static.
With the use of virtual reality (VR), this mismatch can be resolved by letting patients walk in a virtual environment, and VR can provide safe and controlled environments in which patients can be challenged by conflicting sensorimotor stimulations (7). By manipulating the OF speed, through increasing or decreasing the OF speed with respect to the subject’s walking speed, each time there will be a mismatch between the OF and the proprioceptive information. As a result, people will adjust their gait pattern to reduce this discrepancy (8). Investigating the effect of manipulation of OF speed during gait helps us understand how people respond to these manipulations and how this may be useful for rehabilitation purposes.
OF speed and its influence on locomotion have been examined in the healthy population (9–13) and several clinical populations, such as older adults (14–16) and neurological patients (8, 17–19). Most of these studies investigated the effect of OF speed on walking speed and, in a few cases, also on other spatiotemporal gait parameters, such as cadence or step length. Based on their findings, it is suggested that OF can exert an influence on locomotion, mainly on walking speed, although there are conflicting results between populations. In general, it seems that healthy people will increase their walking speed with a slower OF and decrease their speed with a faster OF. This strategy may be altered in neurological patients, due to damage in specific brain areas (5). It is assumed that people post-stroke still have the ability to use OF information during walking, but alterations are possible and responses can be heterogeneous, depending on the location of the brain lesion (5). With the use of VR, the selective manipulation of OF speed could be used to induce desired locomotor changes, such as an increase in walking speed, and therefore has the potential to advance the field of post-stroke gait rehabilitation. However, studies about the effect of OF speed on post-stroke locomotion are still scarce, and, in general, they include limited gait-related outcomes (8, 19). Given the potential of OF speed manipulation to enhance gait training, further exploration is necessary to determine its usefulness for rehabilitation purposes.
Two key concepts of VR are immersion and sense of presence (20). Based on their level of immersion, VR devices and systems can be classified into 2 categories: (i) non-immersive, or semi-immersive, VR systems, that let the user perceive both the virtual environment, and a part of the real world (e.g. TV screens, projection screens); and (ii) fully immersive VR systems, that fully integrate the user into the virtual environment by blocking out perception of the real world (e.g. head-mounted display; HMD) (21). It is known that the level of immersion has an impact on users’ VR experience and affects their sense of presence (i.e. the feeling of being physically present in the virtual world) (22). Exposure to more immersive virtual environments will elicit stronger feelings of being physically present in the virtual world (23). This study is part of a larger trial that investigates the effect of OF speed on gait biomechanics, simulator sickness and level of enjoyment, both in a semi-immersive and fully immersive virtual environment. This paper reports only the results of manipulating the OF speed in a semi-immersive virtual environment. A second paper discusses the results of manipulating the OF speed in a fully immersive virtual environment, and compares the results of walking with different OF speeds in order to investigate the effect of the level of immersion (semi-immersive vs fully immersive) on gait biomechanics (24).
The aim of the current study is to investigate in people post-stroke and healthy people: (i) the effect on gait biomechanics, motion sickness, and enjoyment of adding semi-immersive VR while walking on a treadmill; and (ii) the effect on gait biomechanics of manipulation of OF speed. The study hypotheses are that: (i) adding semi-immersive VR will alter the gait biomechanics in both groups; and (ii) both healthy people and people post-stroke will alter their gait pattern in response to the manipulation OF speed.
METHODS
Study design
This study is part of a larger trial investigating the effect of OF speed and the level of immersion on gait biomechanics, simulator sickness, and level of enjoyment. An experimental, 2-group, single-centre trial was conducted in which people post-stroke and healthy controls performed 2 VR-enhanced treadmill walking sessions. Both sessions were identical and carried out on 2 separate days within 10 days. Only the VR system used to manipulate the OF speed differed: the semi-immersive Gait Real-time Interactive Lab (GRAIL; MOTEK Medical Bv. Houten, The Netherlands) system and the fully immersive HMD, Occulus (MetaQuest, USA). This paper discusses the results of the GRAIL session and focuses on the effect of adding semi-immersive VR and manipulation of the OF speed. The study took place at the Smart Space laboratory of the University Hospital in Ghent, Ghent, Belgium. The study was approved by the ethics committee of the University of Brussels and the University Hospital of Ghent (B1432020000120) and pre-registered at ClinicalTrials.gov (NCT04521829).
Participants
Included were chronic, ambulatory stroke patients, and age- and sex-matched healthy adults. The following inclusion criteria were used for the stroke population: (i) diagnosed with stroke, (ii) stroke onset ≥ 3 months, (iii) adult (≥18 years), (iv) ambulatory with an impaired gait pattern (Functional Ambulation Categories (FAC)-score 2, 3 or 4), (v) ability to walk on a treadmill 4 times for 8 min without bodyweight support, with use of the hand rail being allowed, (vi) ability to signal pain, fear, and discomfort, and (vii) ability to provide informed consent. People post-stroke were excluded if they had: (i) other neurological deficits leading to impaired gait (e.g. Parkinson’s disease, multiple sclerosis), (ii) comorbidities (e.g. severe osteoporosis, cardiovascular instability), (iii) visual and/or vestibular disorders that can interfere with VR (e.g. partial blindness, Meniere’s disease), (iv) severe spasticity of the lower limbs (Modified Ashworth Scale > 2), (v) acute medical illness, (vi) inability to understand and carry out instructions, and (vii) severe unilateral spatial neglect.
For the healthy participants, the following inclusion criteria were used: (i) normal or corrected-to-normal vision, and (ii) no locomotion impairments. Participants were excluded if they (i) have had significant lower extremity injuries during the last 2 years that might affect their gait, and (ii) had any type of vestibular/visual deficiency. All participants had to provide written informed consent to be included in the study.
Based on a sample size calculation (G*Power 3.1.9.4) (F-tests, repeated measures analysis of variance (ANOVA), within-between subjects) with Cohen’s f of 0.25 (moderate effect size), type 1 error probability of 0.05, power of 0.80 for 2 groups and 4 conditions, a minimum of 24 participants, divided equally in 2 groups, had to be recruited.
Apparatus
Participants walked on the GRAIL system, an integrative motion capture system consisting of 10 optical motion cameras (Vicon Inc.,Vicon Motion Systems, Yarnton, UK), a dual-belt treadmill with integrated force sensors, a 180° cylindrical projection screen, and D-Flow software (MOTEK Medical Bv) (Fig. 1). The treadmill was self-paced, meaning that participants had control over the speed of the treadmill and could change speed at will. For safety, participants wore a safety harness and the maximum walking speed was set at 2 m/s. The virtual environment, provided by MOTEK Medical Bv, represented an Italian city street from which the game elements had been removed so that participants only had to walk forward.

Fig. 1. The Gait Real-time Interactive Lab (GRAIL) system.
Experimental procedure
Both groups underwent 5 walking trials. The first trial consisted of 8 min walking without VR to familiarize with self-paced walking (25). During the second trial (habituation trial), the VR was added and participants walked for 5 min to get used to the projection screen. Thereafter, participants underwent 3 more walking trials, of 8 min each, during which the OF speed was being manipulated: 2 times slower than, equal to, and 2 times faster than their comfortable walking speed (i.e. the mean walking speed during the habituation trial). The manipulation of OF speed occurred after 1 min and lasted for the remaining 7 min. The OF speed manipulation (matched, slow, fast) was randomized using block randomization in Microsoft Excel® (Microsoft corporation, Redmond, Washington, USA). Participants were not informed about this manipulation. Between walking trials, participants had 5 min to rest and to complete the questionnaires.
Outcomes and pre-processing
The primary outcome measure was gait biomechanics, which included lower limb kinematics and spatiotemporal gait parameters (i.e. walking speed, cadence, stride time, step length, swing, stance time, and step width).
Kinematic data were recorded using a 10-camera VICON Vero 1.3 system at 100 Hz and the full-body Plug-in-Gait model provided by Vicon Motion Systems. For current analyses, only lower limb marker data were used. Sagittal kinematic marker data of the hip, knee, and ankle were processed using Vicon Nexus software (Vicon Motion Systems). Gait cycle segmentation of kinematic data and calculation of the spatiotemporal parameters (i.e. cadence, stride time, step length, swing, stance time, and step width) were performed in Python 3.7. (Anaconda Inc., Austin, TX, USA) with custom-made scripts. Walking speed was measured continuously and was derived directly from the treadmill system.
The secondary outcome measures were simulator sickness and level of enjoyment. Simulator sickness was assessed with the Simulator Sickness Questionnaire (SSQ) (26). Before and after each walking trial, participants had to indicate on a 4-point Likert scale how much 16 symptoms were affecting them at that moment. The total score (0–179.52) is the sum of the 16 items multiplied by 3.74, with higher scores indicating higher levels of simulator sickness. Level of enjoyment was assessed with 2 visual analogue scales (VAS). After walking without and with the VR, participants were asked to answer following questions: “VAS1 – Indicate on the line below how much you enjoyed walking on the treadmill under these conditions”, and “VAS2 – Indicate on the line below whether you would like to do this type of gait training during your rehabilitation” (stroke group only).
Statistical analysis
IBM SPSS Statistics version 28, custom-made scripts in Python 3.7. and Matlab (R2022s) (Mathworks, Natick, USA) were used for statistical analysis. Level of significance was set at α = 0.05. Baseline characteristics between groups were compared using an independent sample t-test and Mann–Whitney U test for respectively normally and not-normally distributed continuous variables and a χ2 test for categorical variables. A paired t-test (normally distributed data) or Wilcoxon signed-rank test (not-normally distributed data) and independent samples t-test (normally distributed data) or Mann–Whitney U test (not-normally distributed data) were used, respectively, to examine the within- and between-group differences for the questionnaires.
To investigate the effect of VR on gait biomechanics, the mean during the last 30 s of the trial without VR was compared with those obtained during the last 30 s of the habituation trial. For spatiotemporal data, linear mixed-effect models (LMM) were used. LMM were conducted with condition (no VR, with VR) and group (post-stroke, healthy) as fixed factors, accounting for the within-subject correlations. Models were built using the Akaike’s Information Criteria (AIC) in SPSS (IBM, Armonk, New York, USA). The within-subject covariance was unstructured. For kinematic data, statistical parametric mapping (SPM) was used. A SPM 2-way ANOVA was performed to examine the effect of condition (no VR, with VR) and group (post-stroke, healthy): the F-statistic (SPM(F)) was calculated at each point of the time-series. Where SPM(F) crossed a threshold equivalent to α = 0.05, post-hoc analyses were performed using SPM paired t-tests. For post-hoc comparisons, the SPM(t) statistic was calculated for each comparison. The critical threshold was set equivalent to α = 0.0253 to account for multiple comparisons. The t-statistic (SPM(t) was calculated at each point of the time-series and where SPM(t) crossed the threshold, significant differences were found.
To investigate the effect of OF speed on the gait biomechanics, 4 time-points were compared: the mean during the 30 s before the manipulation, compared with those obtained during the 30 s immediately after the manipulation, the middle 30 s, and the last 30 s of the 8-min trial. LMM were conducted for spatiotemporal parameters, with OF condition (matched, fast, slow), time (pre-manipulation, post-manipulation, middle, and end of the trial), and group (post-stroke, healthy) as fixed factors, accounting for the within-subject correlations. Models were built using the AIC, in SPSS. The within-subject covariance was unstructured. For kinematic data, a SPM 2-way repeated measures ANOVA was performed to examine the effect of time (pre-manipulation, post-manipulation, middle, and end of the trial) and group (post-stroke, healthy) in each OF condition: the SPM(F) was calculated at each point of the time-series. Where SPM(F) crossed a threshold equivalent to α = 0.05, post-hoc analyses were performed using SPM paired t-tests. For post-hoc comparisons, the SPM(t) statistic was calculated for each comparison. The critical threshold was set equivalent to α = 0.017 to account for multiple comparisons. Significant differences were recorded where the SPM(t) crossed this threshold.
RESULTS
Subjects’ characteristics
Sixteen people post-stroke and 16 age- and sex-matched healthy controls participated in this study. There was no significant difference in baseline characteristics observed between groups (Table I).
Effect of semi-immersive virtual reality on spatio-temporal gait parameters and lower limb kinematics
Spatiotemporal gait parameters. For statistical analysis of spatiotemporal parameters, a series of LMM were conducted (Table SI). The resulting LMM focusing on the effect of condition (no VR, with VR) and group (post-stroke, healthy) suggested that no significant interaction effect between condition and group, nor a main effect of condition was found for all spatiotemporal parameters (Table II).
Lower limb kinematics. SPM 2-way ANOVA analyses were performed on 15 subjects in each group, due to missing data of 1 person in the stroke group (missing data was due to 1 or more Vicon markers that fell off while the subject was walking). To maintain equal group sizes, the healthy matched participant was also removed from the analysis. The SPM 2-way ANOVA also revealed no significant interaction effect, nor a main effect of condition (Figs S1 and S2).
Effect of semi-immersive virtual reality on simulator sickness and enjoyment
Simulator sickness. A significant increase in the SSQ score was observed only in the stroke group after walking with VR (2.34 (4.71) points to 8.88 (6.81) points (mean difference (MD) 6.55 (6.90) points, p = 0.005). The healthy group had a non-significant increase from 0.94 (1.67) points to 3.27 (5.93) points (MD 2.34 (5.44) points, p = 0.084). The difference between groups was not significant (p = 0.076).
Enjoyment. The stroke group enjoyed walking on the treadmill more with the VR compared with without, as indicated by a significant increase in VAS1, from 5.38 (2.19) to 7.26 (1.87) (MD 1.88 (1.81), p = 0.002). The healthy group had a non-significant increase from 5.96 (2.56) to 6.79(2.08) (MD 0.83 (2.02), p = 0.179). The difference between groups was not significant (p = 0.186). The stroke group was positive about the implementation of VR in their gait training, as indicated by a significant increase in VAS2, from 7.03 (2.77) to 8.35 (1.57) (MD 1.32 (2.07), p = 0.041).
Effect of optic flow speed manipulation on the spatio-temporal gait parameters and lower limb kinematics
Spatiotemporal gait parameters. For the statistical analysis of spatiotemporal parameters, a series of LMM were conducted. The resulting LMM focusing on OF condition (matched, fast, slow), time (pre-manipulation, post-manipulation, middle, and end of the trial), and group (post-stroke, healthy) suggested interactions between condition and time with a main effect of group for all the spatiotemporal parameters. For none of the spatiotemporal parameters, a 3-way interaction between OF condition, time, and group was suggested (Table SII).
Table III shows the MD between OF condition and time for all spatiotemporal gait parameters. Significant interaction effects of condition and time revealed that, in both groups, only the slow OF speed led to an immediate change in walking speed. Immediately after the manipulation, participants significantly increased their walking speed and this increase in walking speed was also maintained over time. In the fast OF condition, immediately after the manipulation, participants decreased their walking speed, but this change in walking speed was not significant. When there was no manipulation and participants walked with a matched OF, participants significantly increased their walking speed towards the end of the trial.
| Condition | Time-point | Walking speed (m/s) | Cadence (stride/min) | Stride time (s) | Step length (cm) | |||||||
| MD [95% CI] | p-value | MD [95% CI] | p-value | MD [95% CI] | p-value | MD [95% CI] | p-value | MD [95% CI] | p-value | |||
| Affected leg | Unaffected leg | |||||||||||
| Matched OF | Pre | Post | 0.01 [–0.02;0.03] |
1.000 | 0.07 [–0.39;0.52] |
1.000 | 0.00 [–0.01;0.02] |
1.000 | 0.13 [–0.75;1.02] |
1.000 | 0.07 [–0.82;0.96] |
1.000 |
| Mid | 0.03 [–0.01;0.06] |
0.389 | 0.31 [–0.53;1.14] |
1.000 | –0.01 [–0.03;0.01] |
1.000 | 0.87 [–0.50;2.23] |
0.498 | 1.02 [–0.50;2.54] |
0.413 | ||
| End | 0.05 [0.01;0.10] |
0.008* | 0.70 [–0.10;1.49] |
0.117 | –0.02 [–0.04;0.00] |
0.124 | 1.70 [0.26;3.14] |
0.014* | 1.95 [0.00;3.90] |
0.050* | ||
| Fast OF | Pre | Post | –0.03 [–0.06;0.00] |
0.054 | –0.70 [–1.29;–0.12] |
0.012* | 0.02 [0.00;0.04] |
0.065 | –0.56 [–1.94;0.82] |
1.000 | –0.56 [–2.03;0.91] |
1.000 |
| Mid | 0.00 [–0.05;0.04] |
1.000 | –0.47 [–1.41;0.48] |
1.000 | 0.01 [–0.02;0.05] |
1.000 | 0.78 [–1.24;2.81] |
1.000 | 0.17 [–1.79;2.13] |
1.000 | ||
| End | 0.00 [–0.05;0.05] |
1.000 | –0.55 [–1.52;0.41] |
0.690 | 0.02 [–0.02;0.05] |
1.000 | 1.46 [–0.64;3.55] |
0.353 | 0.57 [–2.07;3.22] |
1.000 | ||
| Slow OF | Pre | Post | 0.05 [0.03;0.07] |
<0.001* | 0.94 [0.43;1.45] |
< 0.001* | –0.03 [–0.05;–0.01] |
0.007* | 1.78 [0.97;2.58] |
<0.001* | 1.62 [0.29;2.95] |
0.010* |
| Mid | 0.06 [0.01;0.11] |
0.011* | 1.00 [0.01;2.00] |
0.046* | –0.03 [–0.07;0.01] |
0.381 | 2.10 [0.17;4.02] |
0.026* | 2.22 [–0.98;5.42] |
0.362 | ||
| End | 0.06 [0.02;0.10] |
0.001* | 1.14 [0.11;2.18] |
0.024* | –0.03 [–0.07;0.00] |
0.093 | 1.81 [0.37;3.24] |
0.007* | 2.04 [–0.71;4.79] |
0.269 | ||
| Condition | Time-point | Stance time (% gait cycle) | Swing time (% gait cycle) | Step width (cm) | ||||||||||
| MD [95% CI] | p-value | MD [95% CI] | p-value | MD [95% CI] | p-value | MD [95% CI] | p-value | MD [95% CI] | p-value | MD [95% CI] | p-value | |||
| Affected leg | Unaffected leg | Affected leg | Unaffected leg | Affected leg | Unaffected leg | |||||||||
| Matched OF | Pre | Post | 0.07 [–0.18;0.31] |
1.000 | –0.02 [–0.28;0.24] |
1.000 | –0.07 [–0.31;0.18] |
1.000 | 0.02 [–0.24;0.28] |
1.000 | 0.10 [–0.54;0.73] |
1.000 | 0.08 [–0.50;0.66] |
1.000 |
| Mid | –0.13 [–0.52;0.27] |
1.000 | –0.08 [–0.43;0.27] |
1.000 | 0.13 [–0.27;0.52] |
1.000 | 0.08 [–0.27;0.43] |
1.000 | 0.14 [–0.29;0.57] |
1.000 | 0.16 [–0.26;0.58] |
1.000 | ||
| End | –0.42 [–0.97;0.14] |
0.255 | –0.37 [–0.84;0.09] |
0.174 | 0.42 [–0.14;0.97] |
0.255 | 0.37 [–0.09;0.84] |
0.174 | 0.39 [–0.27;1.05] |
0.638 | 0.40 [–0.24;1.05] |
0.538 | ||
| Fast OF | Pre | Post | 0.05 [–0.41;0.50] |
1.000 | 0.29 [–0.05;0.63] |
0.141 | –0.05 [–0.50;0.41] |
1.000 | –0.29 [–0.63;0.05] |
0.141 | 0.32 [–0.03;0.66] |
0.084 | 0.25 [–0.11;0.61] |
0.336 |
| Mid | 0.15 [–0.25;0.55] |
1.000 | 0.19 [–0.31;0.68] |
1.000 | –0.15 [–0.55;0.25] |
1.000 | –0.19 [–0.68;0.31] |
1.000 | 0.18 [–0.33;0.70] |
1.000 | 0.18 [–0.34;0.70] |
1.000 | ||
| End | –0.05 [–0.65;0.55] |
1.000 | 0.13 [–0.41;0.67] |
1.000 | 0.05 [–0.55;0.65] |
1.000 | 0.13 [–0.67;0.41] |
1.000 | 0.35 [–0.38;1.07] |
1.000 | 0.34 [–0.38;1.05] |
1.000 | ||
| Slow OF | Pre | Post | –0.54 [–0.95;–0.13] |
0.005* | –0.40 [–0.71;–0.08] |
0.007* | 0.54 [0.13;0.95] |
0.005* | 0.40 [0.08;0.71] |
0.007* | 0.23 [–0.22;0.67] |
0.973 | 0.21 [–0.23;0.65] |
1.000 |
| Mid | –0.64 [–1.52;0.24] |
0.286 | –0.35 [–0.86;0.16] |
0.369 | 0.64 [–0.24;1.52] |
0.286 | 0.35 [–0.16;0.86] |
0.369 | 0.22 [–0.52;0.96] |
1.000 | 0.16 [–0.56;0.88] |
1.000 | ||
| End | –0.52 [–1.26;0.23] |
0.353 | –0.37 [–0.72;–0.01] |
0.038* | 0.52 [–0.23;1.26] |
0.353 | 0.37 [0.01;0.72] |
0.038* | 0.28 [–0.36;0.92] |
1.000 | 0.28 [–0.33;0.90] |
1.000 | ||
| Values are reported as mean difference (MD) and 95% confidence interval (95% CI). | ||||||||||||||
| *Significant change. | ||||||||||||||
| OF: optic flow; m/s: meter per seconds; min: minute; cm: centimeter | ||||||||||||||
In the slow OF condition, the immediate increase in walking speed was accompanied by a significantly faster cadence, a shorter stride time, an increase in the affected and unaffected step length, a shorter stance time, and a longer swing time in both the affected and unaffected leg. The significant changes in cadence and affected step length were maintained over time. In the fast OF condition, only a significantly slower cadence was found immediately after the manipulation. The increase in walking speed at the end of the matched OF condition was accompanied by an increased step length of both the affected and unaffected leg.
Lower limb kinematics. The SPM 2-way repeated measures ANOVA was performed on 11 subjects in each group due to missing data (4 stroke patients, 1 healthy participant, missing data was due to 1 or more Vicon markers that fell off while the subject was walking). To maintain equal group sizes, the matched participants were removed from the analyses. The SPM analyses revealed a significant interaction effect for the ankle joint in the matched and fast condition. A significant main effect of time was found for the knee and hip (both affected and unaffected side post-stroke) in the matched and slow condition and for the ankle (unaffected side post-stroke), knee (affected side post-stroke), and hip (unaffected side post-stroke) in the fast condition (Figs S3–S8). In the post-hoc SPM t-tests, the critical threshold was only exceeded in the fast condition when comparing pre- and post-manipulation both at the knee and hip joint of the healthy group. After the fast OF manipulation, a decrease in knee flexion with a maximum of 1.2° was found between 66% and 72% of the gait cycle. At the hip, a decrease in hip flexion with a maximum of 0.77° was found between 0–5% and 96–100% of the gait cycle (Fig. 2). In the other post-hoc pairwise comparisons, the critical threshold was never exceeded.
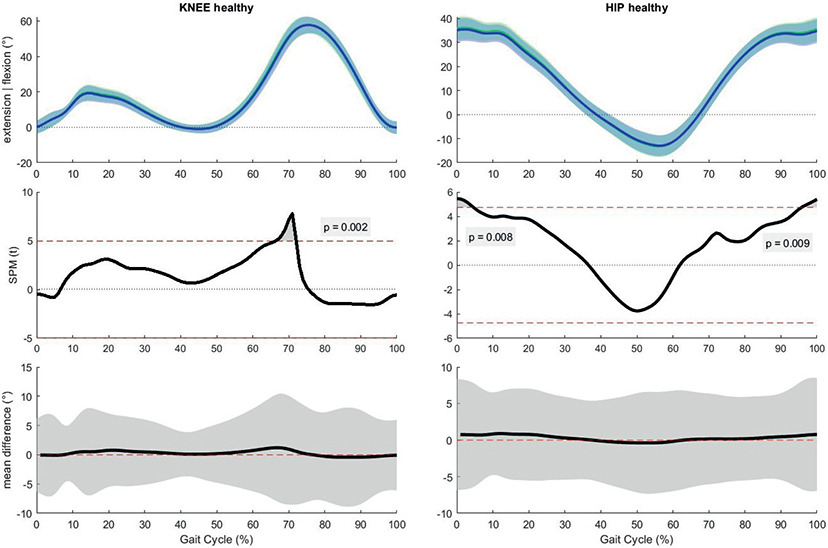
Fig. 2. Results of the post-hoc analyses, paired samples t-test for the fast condition in the healthy group. Horizontal axis is percentage gait cycle. First row is mean joint angles ±1 standard deviation for healthy people pre-manipulation (green) and post-manipulation (blue). Second row shows the statistical parametric mapping t-statistic (SPM(t)) value throughout the gait cycle. The dashed red line is equivalent to α=0.02. Third row shows mean difference with 95% confidence interval between pre- and post-manipulation.
DISCUSSION
This study investigated the effect of adding semi-immersive VR and manipulating the OF speed when walking on a self-paced treadmill on spatiotemporal gait parameters, lower limb kinematics, simulator sickness, and enjoyment in people post-stroke and healthy people.
Although it is suggested in the literature that the addition of semi-immersive VR during (self-paced) treadmill walking leads to a more conservative and more cautious gait pattern with shorter stride length and time, increased step width, and reduced hip and knee range of motion (27, 28), the current results stipulated that adding semi-immersive VR during self-paced treadmill walking did not influence the gait pattern in healthy people and people post-stroke. No statistically significant changes in spatiotemporal parameters and lower limb kinematics were found when VR was added. However, there were some differences between studies that may explain this discrepancy. The most important difference is that people did not walk on a self-paced treadmill. The type of treadmill (fixed speed or self-paced speed) could influence the way people adjust their gait pattern due to the VR. Furthermore, in the current study, adding semi-immersive VR elicited only minor symptoms of simulator sickness. All participants were able to complete the walking session and showed no signs of severe simulator sickness. Even though people post-stroke showed a significant increase in SSQ score, very low total scores were reported in both groups. It seems that walking in a semi-immersive virtual environment did not elicit major side-effects.
The virtual environment in this study represented an endless animated city street and contained no moving objects or game elements. Participants had no assignments in the virtual environment and only had to walk forward. It is possible that in more challenging environments alterations in the gait pattern may occur. Nevertheless, results of the VAS questions showed that patients enjoyed walking with the VR and would like to use VR in their gait rehabilitation. These results are promising and support the use of VR for post-stroke gait rehabilitation. However, more research about implementing valuable principles in the virtual environment that could positively influence the gait pattern and could be used during gait training is needed.
Regarding the second research question, manipulation of OF speed was shown to have only a limited effect on the spatiotemporal gait parameters and lower limb kinematics. Both groups increased their walking speed with an OF that was twice as slow as their comfortable walking speed and they had the tendency to decrease their speed with an OF that was twice as fast. These results are in line with previous research that reported a negative correlation between walking speed and OF speed in healthy people (9–13) and suggest that people post-stroke use the same strategy to respond to OF speed manipulations. However, the differences in walking speed in the current study did not reach the minimal clinically important difference of 0.10 m/s (29). The OF speed manipulation also had a limited, not clinically relevant, effect on the spatiotemporal parameters and lower limb kinematics. However, there are some aspects that could influence the effect of OF speed on locomotion that need further investigation.
First, the level of immersion can impact the user’s VR experience by influencing the sense of presence (i.e. the feeling of being physically present in the virtual world), with stronger feelings of “being physically present” during exposure to more immersive virtual environments (22, 23). The current study used the semi-immersive GRAIL projection screen to manipulate the OF speed. Consequently, participants were still aware of their real environment and thus also of the real OF. This might explain why OF speed manipulations were less noticeable for the participants. Perhaps using a fully immersive virtual environment would elicit a greater effect on locomotion. As mentioned earlier, this paper discusses the results of the first part of a larger study. The second part compared the results of the semi-immersive GRAIL session with the fully immersive HMD session (24). To the best of our knowledge, this is the first study to investigate the effect of immersion on OF speed.
Secondly, the type of manipulation (constant vs intermittent) could also play an important role. In the current study, a single manipulation of a constant OF speed was used and participants were not informed about the manipulation. Although this type of manipulation (slower or faster OF) had only a small effect on the gait parameters, the results of this study demonstrate that a one-time manipulation of a constant OF speed could be used during gait training to unconsciously motivate patients to change their walking speed. The choice for a constant speed manipulation was based on existing literature (9, 10, 12, 13, 15, 18). The study of Lamontagne et al. (8) reported that constant OF speeds elicited larger variations in walking speed compared with continuously changing OF speeds, both in healthy people and people post-stroke. It is suggested that constant OF speeds are easier to perceive and integrate than continuously changing OF speeds and could therefore elicit a greater effect (8). Nevertheless, previous results also indicate that the effect of OF speed manipulation is rather short lasting and that a few seconds after the manipulation, participants return to their comfortable walking speed. More research about different types of manipulation, such as multiple intermittent manipulations of a constant OF speed over a longer period, is therefore needed.
Other factors that could have influenced the current results are stroke severity, onset, and location. This study included 16 chronic, ambulatory stroke patients who walked independently, but still experienced some difficulties with stairs or uneven surfaces (indicated by a score of 4 out of 5 on the Functional Ambulation Categories (FAC)). The protocol was demanding for the participants. As a result, participants with lower FAC scores did not participate. Regarding the stroke onset, there was a lot more heterogeneity between patients. The mean time since stroke was 44.24 months, but ranged from 3.4 to 202.5 months (16.8 years). Future studies should investigate whether stroke severity and onset could influence the effect of OF speed.
Previous research revealed key brain areas that are involved in the perception and use of OF during locomotion (30). It has been shown that there is a cortical network that responds to OF and involves visual areas (middle temporal cortex, V6), multisensory areas (ventral intra-parietal area, cingulate sulcus visual area, precuneus motion area), and vestibular areas (putative area 2v, parieto-insular vestibular cortex) (30). When the stroke is located in one of these brain areas, the perception of OF can be affected, and patients could react differently to the OF speed manipulations (5). It is therefore advisable for future research to include the location of the stroke as a patient characteristic.
The current study demonstrated that adding semi-immersive VR to self-paced treadmill walking is safe and enjoyable, therefore supporting its use for post-stroke gait rehabilitation in ambulatory people with an impaired gait pattern. Despite the limited effect OF speed had on locomotion, the results of this study showed that people post-stroke respond to OF speed manipulations, providing a rationale to incorporate these manipulations in a VR-enhanced training. However, before OF speed manipulations can be implemented optimally in such a therapy, further work is needed to determine the most effective type of OF speed manipulation, as well as to investigate the carry-over effects to overground walking.
In conclusion, the addition of semi-immersive VR while walking on the self-paced treadmill of the GRAIL system did not influence the gait pattern in healthy people and people post-stroke compared with walking without VR. Optic flow speed manipulation appears to have a limited effect on the gait pattern in both groups. A negative relationship between OF speed and walking speed was observed in both groups. However, changes in all gait parameters were very small and may be clinically irrelevant. Nevertheless, walking with the semi-immersive VR and manipulating the OF speed did not elicit simulator sickness, was well tolerated, and enjoyable. Further research is needed to investigate whether different types of OF speed manipulation and higher levels of immersion could elicit a greater effect on locomotion.
ACKNOWLEDGEMENTS
The authors thank the participants for taking part in this trial, Smart Space lab for use of the GRAIL system, and the Support for Quantitative and Qualitative Research (SQUARE) of the Vrije Universiteit Brussel for their support with data analysis. With support from the University Foundation of Belgium (Universitaire Stichting van België).
Emma De Keersmaecker is a Strategic Basic Research fellow funded by the Research Foundation – Flanders (FWO) (1S58419N)
REFERENCES
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics – 2017 update: a report from the American Heart Association. Circulation 2017; 135: e146–e603.
- Dobkin BH. Clinical practice. Rehabilitation after stroke. N Engl J Med 2005; 352: 1677–1684.
- Beyaert C, Vasa R, Frykberg GE. Gait post-stroke: pathophysiology and rehabilitation strategies. Neurophysiol Clin 2015; 45: 335–355.
- Kang HK, Kim Y, Chung Y, Hwang S. Effects of treadmill training with optic flow on balance and gait in individuals following stroke: randomized controlled trials. Clin Rehabil 2012; 26: 246–255.
- Keshner EA, Lamontagne A. The untapped potential of virtual reality in rehabilitation of balance and gait in neurological disorders. Front Virtual Real 2021;2: 641650.
- Prokop T, Schubert M, Berger W. Visual influence on human locomotion – modulation to changes in optic flow. Exp Brain Res 1997; 114: 63–70.
- Adamovich SV, Fluet GG, Tunik E, Merians AS. Sensorimotor training in virtual reality: a review. Neurorehabil 2009; 25: 29–44.
- Lamontagne A, Fung J, McFadyen BJ, Faubert J. Modulation of walking speed by changing optic flow in persons with stroke. J Neuroeng Rehabil 2007; 4: 22.
- Katsavelis D, Mukherjee M, Decker L, Stergiou N. The effect of virtual reality on gait variability. Nonlinear Dynamics Psychol Life Sci 2010; 143: 239–256.
- Mohler BJ, Thompson WB, Creem-Regehr SH, Pick HL Jr, Warren WH Jr. Visual flow influences gait transition speed and preferred walking speed. Exp Brain Res 2007; 181: 221–228.
- O’Connor SM, Donelan JM. Fast visual prediction and slow optimization of preferred walking speed. J Neurophysiol 2012; 107: 2549–2559.
- Powell WA, Hand S, Stevens B, Simmonds M. Optic flow in a virtual environment: Sustained influence on speed of locomotion. Cyberpsychol Behavior 2006; 9: 710.
- Salinas MM, Wilken JM, Dingwell JB. How humans use visual optic flow to regulate stepping during walking. Gait Posture 2017; 57: 15–20.
- Konczak J. Effects of optic flow on the kinematics of human gait – a comparison of young and older adults. J Mot Behav 1994; 26: 225–236.
- Chou YH, Wagenaar RC, Saltzman E, Giphart JE, Young D, Davidsdottir R, et al. Effects of optic flow speed and lateral flow asymmetry on locomotion in younger and older adults: a virtual reality study. J Gerontol B Psychol Sci Soc Sci 2009; 64: 222–231.
- Osaba MY, Martelli D, Prado A, Agrawal SK, Lalwani AK. Age-related differences in gait adaptations during overground walking with and without visual perturbations using a virtual reality headset. Sci Rep 2020; 10: 15376.
- Schubert M, Prokop T, Brocke F, Berger W. Visual kinesthesia and locomotion in Parkinson’s disease. Mov Disord 2005; 20: 141–150.
- Lim H. Effect of the modulation of optic flow speed on gait parameters in children with hemiplegic cerebral palsy. J Phys Ther Sci 2014; 26: 145–148.
- Kang HK, Kim Y, Chung Y, Hwang S. Effects of treadmill training with optic flow on balance and gait in individuals following stroke: randomized controlled trials. Clin Rehabil 2012; 26: 246–255.
- Caldas OI, Sanchez N, Mauledoux M, Avilés OF, Rodriguez-Guerrero C. Leading presence-based strategies to manipulate user experience in virtual reality environments. Virtual Reality 2022; 26: 1507–1518.
- Vinas-Diz S, Sobrido-Prieto M. Virtual reality for therapeutic purposes in stroke: a systematic review. Neurologia 2016; 31: 255–277.
- Rose T, Nam CS, Chen KB. Immersion of virtual reality for rehabilitation – review. Appl Ergon 2018; 69: 153–161.
- Tieri G, Morone G, Paolucci S, Iosa M. Virtual reality in cognitive and motor rehabilitation: facts, fiction and fallacies. Expert Rev Med Devices 2018; 15: 107–117.
- De Keersmaecker E, Van Bladel A, Zaccardi S, Lefeber N, Rodriguez-Guerrero C, Kerckhofs E, et al. Virtual reality-enhanced walking in people post-stroke: effect of optic flow speed and level of immersion on the gait biomechanics. J Neuroeng Rehabil 2023; 20: 124.
- Meyer C, Killeen T, Easthope CS, Curt A, Bolliger M, Linnebank M, et al. Familiarization with treadmill walking: how much is enough? Sci Rep 2019; 9: 5232.
- Kennedy RS, Lane NE, Berbaum KS, Lilienthal MG. Simulator sickness questionnaire: an enhanced method of quantifying simulator sickness. Int J Aviat Psychol 1993; 3: 203–220.
- Sloot LH, van der Krogt MM, Harlaar J. Effects of adding a virtual reality environment to different modes of treadmill walking. Gait Posture 2014; 39: 939–945.
- Hollman JH, Brey RH, Robb RA, Bang TJ, Kaufman KR. Spatiotemporal gait deviations in a virtual reality environment. Gait Posture 2006; 23: 441–444.
- Bohannon RW, Glenney SS. Minimal clinically important difference for change in comfortable gait speed of adults with pathology: a systematic review. J Eval Clin Pract 2014; 20: 295–300.
- Uesaki M, Ashida H. Optic-flow selective cortical sensory regions associated with self-reported states of vection. Front Psychol 2015; 6: 775.