ORIGINAL REPORT
EFFECT OF OXYGEN THERAPY DURATION ON COGNITIVE IMPAIRMENT 12 MONTHSAFTER HOSPITALIZATION FOR SARS-COV-2 INFECTION
Amandine RAPIN, MD, PhD12, Arnaud CALMUS, MSc1,3, Charles PRADEAU, MD4, Redha TAIAR, MD, PhD5, Gaël BELASSIAN, MD1, Olivier GODEFROY, MD, PhD6, Sandy CARAZO-MENDEZ, MD1 and François C. BOYER, MD, PhD1,2
From the 1Department of Physical and Rehabilitation Medicine, hôpital Sebastopol, 2Faculty of Medicine, Reims Champagne-Ardenne University, MATIM, Reims, France VieFra, EA3797, 3Reims Champagne-Ardenne University, C2S, EA6291, Reims, 4Physical and Rehabilitation Medicine Department, Strasbourg University Hospital, Strasbourg, 5Reims Champagne-Ardenne University, MATIM, Reims and 6Functional Neuroscience and Pathologies Laboratory (UR UPJV 4559), Amiens University Hospital, Amiens, France
Objective: To identify predictors of persistent cognitive impairment at 12 months after hospitalization due to COVID-19 (SARS-CoV-2) infection.
Design: Retrospective, single-centre study.
Subjects: All consecutive patients assessed in physical and rehabilitation medicine consultations at 3 months with a neuropsychiatric testing (NPT) at 6 months.
Methods: A Mini Mental State Examination (MMSE) was performed at 3 months and NPT at 6 and 12 months, exploring global cognitive efficiency, attention and processing speed, short-term memory and executive function. Logistic regression and receiver operating characteristic curves were used to identify predictors of persistent cognitive impairment.
Results: Among 56 patients, 64.3% and 53.6% had 1 or more impaired cognitive functions at 6 and 12 months, respectively, attention and processing speed being the most represented (41.1% at 12 month). Duration of oxygen therapy (odds ratio 0.926 [0.871–0.985], p = 0.015) and MMSE score at 3 months (odds ratio 0.464 [0.276–0.783], p = 0.004) were associated with cognitive impairment at 12 months by multivariable analysis (R² 0.372–0.497).
Conclusions: Half of patients have cognitive impairment 12 months after acute SARS-CoV-2 infection requiring hospitalization. The duration of oxygen therapy in acute care could be a protective parameter. Systematic evaluation with the MMSE at 3 months after infection might be an effective tool to detect risk.
LAY ABSTRACT
Early identification of people at risk for post-COVID-19 cognitive impairment is a major issue. This study aimed to identify predictors of persistent cognitive impairment at 12 months after COVID-19 (SARS-CoV-2) infection requiring hospitalization. Fifty-six patients from a large regional hospital were assessed by neuropsychiatric testing at 6 and 12 months after infection. Global cognitive efficiency, attention and processing speed, short-term memory and executive function were assessed. Of these patients, 64.3% and 53.6% had 1 or more impaired cognitive functions at 6 and 12 months, respectively, attention and processing speed being the most represented (41.1% at 12 months). Initial duration of oxygen therapy was associated with cognitive impairment at 12 months, as a protective parameter. The score on the Mini Mental State Examination at 3 months was also associated with a persisting cognitive impairment at 12 months and could be an easy way to detect patients at risk.
Key words: COVID-19; cognitive dysfunction; oxygen inhalation therapy; follow-up studies.
Citation: J Rehabil Med 2023; 55: jrm12609. DOI: https://doi.org/10.2340/jrm.v55.12609.
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Oct 8, 2023; Published: Nov 16, 2023
*Correspondence address: Amandine Rapin, Department of Physical and Rehabilitation Medicine, hôpital Sebastopol, 48rue de Sebastopol, FR-51092, Reims, France. E-mail: arapin@chu-reims.fr
Competing interests and funding: The authors have no conflicts of interest to declare.
The National Institute for Health and Care Excellence (NICE) and the World Health Organization (WHO) have defined “long COVID” (or post-COVID condition) as a group of signs and symptoms observed during or after infection compatible with COVID-19 (SARS-CoV-2), and persisting for more than 12 weeks, and that cannot be explained by another diagnosis (1, 2). The most commonly described symptoms are fatigue, dyspnoea and cognitive dysfunction or “brain fogginess” (1).
Various cognitive domains have been found to be impaired among patients with long COVID. In a recent meta-analysis, Tavares-Junior et al. (3) reported that executive function, attentional processes and episodic memory were the most frequently affected domains. However, the pathophysiology of post-COVID cognitive dysfunction remains to be elucidated. Several hypotheses have been proposed. The neurotropism of SARS-CoV-2 is thought to be involved, with reports of the virus being found in the olfactory nerves, for instance (4–6). However, autopsy studies of cerebral lesions do not seem to be compatible with the specific changes attributable to a virus (7). Other factors have also been proposed, such as persistent low-grade inflammation (8, 9), the accumulation of proteins involved in neurodegenerative diseases (10), baseline nutritional status (11), altered cerebral glucose metabolism in the subacute phase (12), but not in chronic phase (13), or microvascular processes (14).
In a meta-analysis, Premraj et al. (15) estimated that 32% of patients still had “brain fogginess” at 3 months after acute COVID-19 infection, while Alkodaymi et al. (16) reported that 22% of patients still had difficulty concentrating at 6 months. In another meta-analysis, Zeng et al. (17) reported that 19.7% had cognitive deficits at 12 months after COVID-19 infection, although only a few studies assessed this outcome at 12 months. In a report of 1-year follow-up after discharge from hospital for COVID-19 infection among 53 patients who underwent neuropsychiatric assessments at 1 year, Ferrucci et al. (18) reported that 49.1% had deficits on 1 or more tests. Various predictors have been investigated, but no predictive factors have been identified for persistent cognitive impairment at 1 year.
Other studies have investigated the predictors of cognitive impairment late after infection, with conflicting results in terms of the link with the severity of initial infection (19, 20), the length of hospital stay (21, 22) or the initial symptoms (20, 23, 24).
Therefore, the aim of this study was to identify the predictors of persisting cognitive dysfunction at 1 year after COVID-19 infection requiring hospitalization in a population of patients who underwent neuropsychiatric assessments at 6 and 12 months after infection.
METHODS
During the first wave of the COVID-19 pandemic, patients were assessed in the Physical Medicine and Rehabilitation (PMR) unit of Reims University Hospital, Reims, France, at 3 months after discharge from hospitalization due to COVID-19 infection and evaluated with a Mini-Mental State Examination (MMSE) and a Montreal Cognitive assessment (MoCA). In view of the uncertainty early in the pandemic surrounding the possible outcomes of patients after infection, systematic evaluation at 6 and 12 months was proposed.
A retrospective study was performed of consecutive patients evaluated in first consultation between 2 June 2020 and 13 November 2020. All patients provided informed consent. In accordance with French legislation (namely the Jardé law), this retrospective study was approved by the French national commission for personal data protection (Comité National de l’Information et des Libertés; CNIL) (number 2049775 v 0).
The results are reported in compliance with the Strengthening in Reporting of Observational studies in Epidemiology (STROBE) statement for cohort studies.
Study population
Inclusion criteria were: age > 18 years, SARS-CoV-2 infection confirmed by reverse transcriptase polymerase chain reaction and/or indisputable clinical and/or paraclinical arguments; need for hospitalization at the acute phase; ability to read and write French fluently; and consent to participate. Exclusion criteria were: patients under judicial or other forms of legal protection, patients with cerebral lesions or cognitive impairment before SARS-CoV-2 infection.
Study outcomes
Patient characteristics. At inclusion, socio-demographic data were recorded for all patients (age, sex, body mass, height, level of education, profession, living situation). Information about the acute phase of SARS-CoV-2 infection was collected from the patients’ digital medical files, and included: initial severity, type of oxygen therapy required (if any) at the acute phase, initial blood gas findings including partial pressure of oxygen (PaO2) and partial pressure of carbon dioxide (PaCO2); biological findings including C-reactive protein (CRP), high-sensitivity troponin, and blood albumin levels. The total duration of oxygen therapy was defined as the total number of days on which oxygen was administered to the patient, regardless of the mode of administration (invasive mechanical ventilation, high-flow nasal oxygen therapy). The study also recorded length of initial hospital stay.
Evaluation of cognitive function. The tests used to evaluate cognitive function were chosen according to the patients’ complaints and reports in the literature. The selected tests as well as the cut-offs used to interpret them are represented in Table I. The various tests used in the neuropsychological evaluation were proposed in a predefined order, which was always the same for each participant, and also the same order of performance from one session to the next. The duration of the assessment was between 60 and 75 min. All tests were scored and interpreted according to norms and recommendations of the French Working Group for reflection on cognitive evaluation (GRECO). Patients were considered to have cognitive impairment if 1 or more test was under the cut-off score, and to be “recovering” if there was lower number of cognitive deficits at 12 months than at 6 months.
The Brief Cognitive Status Examination (BCSE) is an optional cognitive examination of the Wechsler memory scale IV (WMS-IV) used in the current study. It helps to assess global cognitive functioning. The results are expressed as very low, low, borderline, low average, and average.
The Wechsler Adult Intelligence Scale IV (WAIS-IV) (25) yields total IQ score, and 4 composite indexes, among which the working memory index (WMI), and the processing speed index (PSI) were selected. A cognitive processing deficit is usually defined by a score that is more than 1 standard deviation (SD) below the population mean (26). In the current study, for this test, –1.5 SD was chosen to diagnose a deficit.
The Trail-Making Test (TMT) examines the trajectories of visual scanning and visual movement. The score is the time, in s, to complete the task, the number of errors and the number of alternation and perseveration errors. Norms with percentiles are available for the French population (27).
The Test of Attentional Performance (TAP) (28) is a standardized software package that uses simple reaction paradigms. The performance criteria are the reaction time and any mistakes. Among the 14 proposed subtests, 2 subtests were used in the current study: TAP Alertness and TAP Go/No go. Results can be expressed as reaction time, in s, or T-scores. Percentiles are also available. The TAP version 2.3.1 was used.
Self-report questionnaires. Hospital Anxiety and Depression Scale (HADS) was used to screen for symptoms of anxiety and depression. A score of 11 or greater corresponding to the likely presence of symptoms of anxiety or depression (29, 30).
Fatigue was measured using the Modified Fatigue Impact scale (MFIS) (31). A score of 38 or higher identifies fatigued individuals (32).
Patients were also asked about the presence of any cognitive disturbances.
Quality of life was assessed using the World Health Organization Quality of Life questionnaire (WHOQoL-Bref). Norms are available in the French general population (33).
Imagery. When available, results of brain magnetic resonance imaging (MRI) taken in the year after infection were collected.
Statistical analysis
Data are described as number and percentage or median and interquartiles for qualitative and quantitative variables, respectively. No imputation was applied for missing data. Results at 6 and 12 months were compared using paired tests for repeat measures, the Wilcoxon or McNemar test, as appropriate. Logistic regression was performed to identify the factors significantly associated with persistent cognitive impairment at 12 months. The multivariate model included factors that yielded a p-value < 0.05 by univariate analysis. Results are reported as odds ratios and 95% confidence intervals (95% CI). A p-value < 0.05 was considered statistically significant.
A sensitivity- and specificity-based approach with receiver operating characteristic (ROC) curves was also performed, to determine the optimal cut-off values for quantitative variables most strongly associated with 1-year cognitive impairment.
All analyses were performed using SPSS 28.0.
RESULTS
A total of 75 patients were followed up at the University Hospital of Reims, France, after the first wave of the COVID-19 pandemic. Among these, 65 underwent neuropsychiatric evaluation at 6 months after the acute infection. Nine patients did not undergo neuropsychiatric evaluation at 12 months. These 9 patients were comparable in terms of age, sex, level of education and MMSE score at inclusion to those who did undergo neuropsychiatric evaluation at 12 months. The flowchart of the study population is shown in Fig. 1. The characteristics of the 56 patients with neuropsychiatric evaluation at both 6 and 12 months are shown in Table II. The majority were men (60.7%), mean age was 65 years. All patients had been hospitalized, with (at the very least) nasal oxygen therapy, and 31 patients (55.4%) required admission to the intensive care unit (ICU) for high-flow nasal oxygen (n = 13, 23.2%) or invasive mechanical ventilation (n = 18, 32.1%).
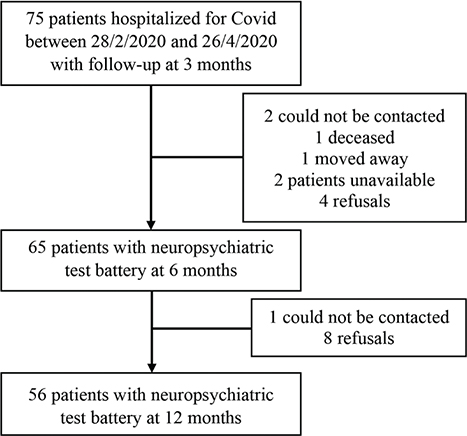
Fig. 1. Flow chart of the study population.
The results of the neuropsychiatric tests at 6 and 12 months are described in Table III. The results of the BCSE, PSI, WMI and visual reproduction tests were statistically significantly different at 12 months compared with the results observed at 6 months. The cognitive status (impaired or not) of 1 patient could not be defined at 12 months due to missing data. At 6 and 12 months, a total of 36 (64.3%) and 30 (53.6%) patients, respectively, had at least 1 cognitive deficit (Table IV). Details of function impaired are described in Table SI.
| 6 months Median [IQR] | N< threshold at 6 months n (%) | 12-month Median [IQR] | N< threshold at 12-month n (%) | p-value | |
| TMT A timea | 36.5 [27.5–49.75] | 2 (3.6) | 38 [28–47] | 1 (1.8) | 0.574 |
| TMT A errorsa | 0 [0-1] | 10 (17.9) | 0 [0–0] | 4 (4.1) | 0.146 |
| TMT Aa | 12 (21.4) | 5 (8.9) | 0.146 | ||
| TMT B time | 104 [76.5–152] | 6 (10.7) | 93 [68–151] | 5 (8.9) | 0.131 |
| TMT B errorsa | 0 [0–1] | 2 (3.6) | 0 [0–1] | 3 (5.4) | 0.725 |
| TMT B perseverance | 0 [0–1] | 8 (14.3) | 0 [0–1] | 5 (8.9) | 0.375 |
| TMT Ba | 13 (23.2) | 9 (16.1) | 0.219 | ||
| BCSEa | 49 [44–52] | 14 (25) | 52 [48–56] | 8 (14.3) | < 0.001 |
| Digit span forwarda | 5 [4–6] | 5 [5-6] | 0.171 | ||
| Digit span forward standard scorea | 9 [7–10] | 2 (3.6) | 9 [7–11] | 2 (3.6) | 0.053 |
| Digit span backwarda | 4 [3–4] | 4 [3-4] | 0.461 | ||
| Digit span backward standard scorea | 8 [7–10] | 1 (1.8) | 8 [7–10] | 1 (1.8) | 0.344 |
| Symbol digit forwardb | 5 [4–6] | 5 [4.75-6] | 0.171 | ||
| Symbol digit backwardb | 4 [4–5] | 5 [4-6] | 0.461 | ||
| Symbol digit standard scoreb | 9 [7–9] | 10 (17.9) | 10 [8–12] | 8 (14.3) | 0.004 |
| Processing speed indexb | 100 [92–111] | 6 (10.9) | 102 [89–114] | 5 (8.9) | 0.07 |
| Less than high school | 94 [86–101.5] | 97 [84–102] | |||
| High school or higher | 105 [99.2–111.7] | 109.5 [103–114.7] | |||
| University or higher | 111 [94–112.5] | 114 [91.5–120] | |||
| Working memory indexa | 89.5 [83–100] | 8 (14.3) | 94 [83–106] | 6 (10.9) | 0.004 |
| Less than high school | 88 [80–95.5] | 89.5 [80–103] | |||
| High school or higher | 89.5 [87.2–104.5] | 100 [87.2–112.5] | |||
| University or higher | 94 [86.5–112] | 100 [91–110.5] | |||
| TAP without cue (median reaction time, ms)b | 272.2 [244.3–323.2] | 8 (14.3) | 274.2 [247.1–317.4] | 7 (12.5) | 0.694 |
| TAP with cue (median reaction time, ms)b | 271.8 [242.4–344.5] | 14 (25) | 275.2 [241.5–330.9] | 12 (21.4) | 0.460 |
| Go/No-Go (median reaction time, ms)c | 403.3 [366.8–452.3] | 1 (1.8) | 407 [373.7–442.3] | 2 (3.6) | 0.783 |
| a1 missing data, b2 missing data, c5 missing data. IQR: interquartile range; TMT: Trail Making Test; BCSE: Brief Cognitive Status Examination; TAP: Test for Attentional Performance. p-value for paired samples Wilcoxon test comparing quantitative data. |
|||||
| 6 months n (%) | 12 months n (%) | p-value | |
| Attention and processing speed* | 26 (46.5) | 23 (41.1) | 0.003 |
| Short-term memory* | 11 (19.6) | 10 (17.9) | < 0.001 |
| Executive function and working memory* | 12 (21.4) | 11 (19.6) | 0.001 |
| Overall efficiency* | 14 (25) | 8 (14.3) | < 0.001 |
| 1 or more impairment* | 36 (64.3) | 30 (53.6) | < 0.001 |
| 1 function impaired | 19 (33.9) | 16 (28.6) | 0.358 |
| 2 functions impaired | 9 (16.1) | 8 (14.3) | 0.604 |
| 3 functions impaired | 8 (14.3) | 6 (10.7) | < 0.001 |
| A function (among the 3 functions described in Table I) is considered to be impaired when 1 or more of the tests of this function is below the lower limit. *1 missing data. | |||
Twenty patients underwent a brain MRI scan at 1 year after acute infection. Among these, 10 had normal findings, 4 patients had white matter lesions (2 with Fazekas score 1, and 2 with Fazekas score 2), 3 patients had medial temporal atrophy (2 patients with Scheltens score of 1, and 1 patient with Scheltens score of 2), and 3 patients had both (Fazekas score 1 associated with Scheltens score 1, 2, or 3). Finally, 1 patient also had hyperintensity in the olfactory grooves. There was no association between the presence of any MRI anomalies and cognitive impairment (p = 0.670).
Table V compares patients with vs those without persistent cognitive deficit at 1 year. Multivariate logistic regression analysis was performed including all variables with a p-value < 0.05 by univariate analysis. The duration of oxygen therapy in the acute phase and the MMSE score at 3 months after infection were found to be significantly associated with the presence of persistent cognitive impairment at 1 year (odds ratio 0.926 [0.871–0.985], p = 0.015 and 0.464 [0.276–0.783], p = 0.004, respectively, R² 0.372 Cox and Snell, 0.497 Nagelkerke).
| No deficit at 12 months n = 25 | ≥1 deficit at 12 months n = 30 | p-value | |
| Patient characteristics, n (%) | |||
| Age > 65 years | 14 (56) | 14 (47) | 0.491 |
| Women | 9 (36) | 13 (43) | 0.580 |
| Obesity | 11 (44) | 12 (40) | 0.765 |
| Diabetes | 4 (16) | 7 (23) | 0.498 |
| Hypertension | 11 (44) | 14 (47) | 0.843 |
| > 2 comorbidities | 14 (56) | 17 (57) | 0.960 |
| Level of education | |||
| Less than high school | 13 (52) | 15 (50) | 1 |
| High school diploma | 6 (20) | 6 (20) | |
| University or higher | 8 (32) | 9 (30) | |
| Acute symptoms | |||
| Loss of smell, n (%) | 8 (33) | 10 (33) | 1 |
| Asthenia, n (%) | 18 (75) | 22 (73) | 0.890 |
| PaO2 at admission (mmHg), median [IQR] | 74 [65–80] | 76.5 [64–86] | 0.291 |
| PaCO2 at admission (mmHg), median [IQR] | 34.5 [32–37.7] | 34.5 [30–37] | 0.665 |
| Weight loss (kg), median [IQR] | 9 [6–16] | 6 [2.7–15.2] | 0.094 |
| Peak CRP (mg/L), median [IQR] | 158 [115–239] | 188 [88–188] | 0.838 |
| Troponin at admission (ng/L), median [IQR] | 21 [7.5–46.5] | 13 [8–18] | 0.290 |
| Acute management | |||
| Need for ICU admission, n (%) | 19 (76) | 12 (40) | 0.007 |
| Orotracheal intubation, n (%) | 12 (48) | 6 (20) | 0.028 |
| Duration of ventilation, days, median [IQR] | 17 [9.2–38.2] | 9 [7–14] | 0.007 |
| Corticosteroids, n (%) | 20 (80) | 18 (60) | 0.110 |
| Evaluation at 3 months, median [IQR] | |||
| MoCA | 25 [25–27] | 23.5 [19.5–26] | 0.01 |
| MMSE | 29 [28–29] | 26.5 [24.7–28] | < 0.001 |
| Complaints at 12 months | |||
| Complained of cognitive impairment, n (%) | 14 (56) | 17 (57) | 0.906 |
| Complained of fatigue, n (%) | 5 (20) | 1 (3) | 0.082 |
| Complained of mood disturbance, n (%) | 8 (25) | 12 (40) | 0.539 |
| HADS-Anxiety*, median [IQR] | 4 [2–8] | 6 [4–7.5] | 0.250 |
| HADS-Depression*, median [IQR] | 4 [1.5–7.5] | 6 [2–10.5] | 0.325 |
| MFIS*, median [IQR] | 35 [11.5–58.5] | 46 [23.5–61] | 0.45 |
| Cognitive | 3 [0–5] | 4 [1.5–6] | 0.201 |
| Physical | 23 [4–28] | 21 [14.5–29] | 0.869 |
| Social | 17 [4–24] | 20 [6.5–28.5] | 0.255 |
| WHOQoL – BREF, median [IQR] | |||
| Physical* | 64.3 [53.6–82.1] | 60.7 [39.3–67.9] | 0.161 |
| Psychological* | 66.7 [58.3–81.2] | 62.5 [54.2–72.9] | 0.243 |
| Social* | 66.7 [54.2–83.3] | 66.7 [54.2–79.2] | 0.630 |
| Environmental* | 78.1 [68.7–90.6] | 75 [64.1–87.5] | 0.418 |
| IQR: interquartile range; PaO2: partial pressure of oxygen; PaCO2: partial pressure of carbon dioxide; CRP: C-reactive protein; ICU: intensive care unit; MoCA: Montreal Cognitive Assessment; MMSE: Mini mental State Examination; HADS: Hospital Anxiety and Depression Scale; MFIS: Modified Fatigue Impact Scale; WHOQoL: World Health Organization Quality Of Life. *1 missing data. | |||
The ROC curve for oxygen therapy in acute phase is shown in Fig. 2 (area under the curve (AUC) 0.7 [0.55–0.85], p = 0.015). Oxygen therapy lasting < 10 days in hospitalized patients had a sensitivity of 71% and specificity of 52% for the identification of patients who would have persistent cognitive impairment at 1 year.
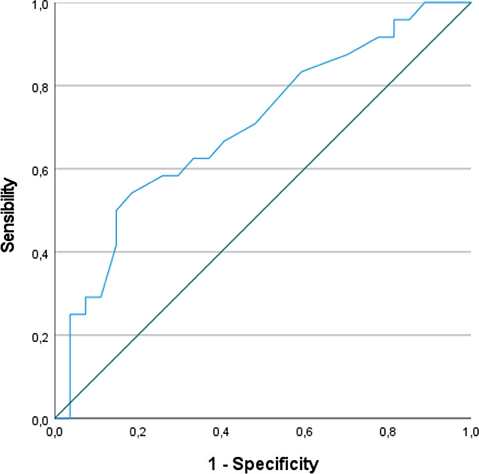
Fig. 2. Receiver operating characteristic (ROC) curve showing the sensitivity and specificity of duration of ventilation to predict persistence of cognitive deficit at 12 months.
The ROC curve for MMSE score at 3 months is shown in Fig. 3 (AUC 0.766 [0.643–0.889], p = 0.0007). An MMSE score at 3 months < 25 points had 100% sensitivity, but 23.3% specificity for the detection of patients who would have persistent cognitive impairment at 1 year. A threshold of < 28 points had 76% sensitivity and 63% specificity.
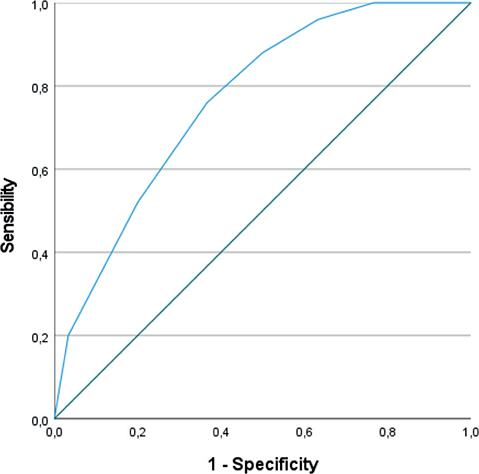
Fig. 3. Receiver operating characteristic (ROC) curve showing the sensitivity and specificity of the Mini Mental State Examination (MMSE) score at 3 months to predict persistence of cognitive deficit at 12 months.
Among the 36 patients with at least 1 cognitive deficit at 6 months, 18 patients (50%) were shown to be recovering, i.e. they showed a reduction in the number of cognitive deficits at 12 months. These recovering patients had more frequently been in ICU (12 (66.7%) vs 5 (29.4%), p = 0.028), and more frequently had prone positioning (7 (38.9%) vs 1 (5.9%), p = 0.041). Median MMSE score at 3 months in recovering patients was 28 [26-29] vs 25 [23–28] for the 18 patients who were not found to be recovering (p = 0.014).
DISCUSSION
This study sought to identify the characteristics that could enable early detection of patients likely to have persistent cognitive impairment at 1 year after hospitalization for acute SARS-CoV-2 infection. More than half the patients hospitalized for COVID-19 had at least 1 persisting deficit at 1 year. The only acute predictive factor identified in the current study was the duration of oxygen therapy, with a protective effect of a longer exposition. Ferrucci et al. (18) used a methodology similar to ours, and found an association between the level of initial hypoxemia and cognitive deficits at 6 months. The duration of oxygen therapy, representing the therapeutic response to hypoxaemia, seems to protect against the risk of long-term cognitive impairment.
Pathophysiology of post-COVID cognitive impairment is not clear, but seems to be polyfactorial, involving hypometabolism (12), persistent low-noise inflammation (8, 9), immune dysfunction (34), microangiopathies (14) and hypoxaemia. Acute hypoxaemia is 1 of the aetiological mechanisms thought to be implicated in the persistence of neurological deficits in patients who have been hospitalized for acute SARS-CoV-2 infection (35). The appropriate duration and dose of oxygen therapy (of any form) could therefore be the best available protection against the consequences of hypoxaemia. These findings are in line with those of Alemanno et al. (20), who found that patients who had undergone orotracheal intubation had higher MMSE and MoCA scores than those with other types of ventilation, or no ventilation. A link between hypoxaemia and cognitive impairment has been reported in other nosological settings (36), providing additional support for this hypothesis. Finally, Adingupu et al. (37) used the frequency-domain near-infrared spectroscopy (NIRS) to measure cerebral tissue oxygen saturation in patient at 8 month post-SARS-CoV-2 infection. They found correlation between persisting hypoxia, supposed to be related to inflammation, and brain fog and fatigue The use of NIRS in clinical practice seems thus to be an interesting work track.
The results of the cerebral MRIs collected retrospectively in this study did not show any abnormality in the patients with the disease. Douaud et al. (38) compared pre- and post-COVID MRIs, showing a decrease in grey matter in the orbitofrontal cortex and parahippocampal gyrus, traces of tissue damage in the piriform cortex and an overall decrease in brain size. Cerebral MRI alone therefore seems more contributory in the event of a possible comparison with the previous state. The search for hypometabolism by 18F-FDG PET could be more sensitive (12, 39).
The current study did not find any association between the severity of the initial infection, and the presence of long-term cognitive impairment, and indeed, conflicting findings have been reported in this regard. Some studies have reported that initial severity, with need for ICU admission, was associated with persistent cognitive deficit at 4 (40, 41), 6 (42, 43) and 12 months (44). Others, on the contrary, have failed to show any such association at 1 (22), 2 (45), 3 (20, 46), or 8 months (47). The multicentre, prospective “Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation” (PHOSP-COVID) cohort study (48) reported similar findings to ours, since the authors also failed to observe any relationship between initial severity of physical or mental symptoms, persistent low-grade inflammation, the severity of acute illness and the occurrence of cognitive impairment. Conversely, cognitive impairments appeared to be more prevalent and persistent in moderate forms of disease among patients who did not require hospitalization (48).
Similarly, no association was found between the intensity of initial inflammation, and the persistence of cognitive deficit, which is congruent with the find-ings reported by Woo et al (49) in 18 young patients followed-up at 20–105 days (median 85 days) after recovery from mild to moderate disease. Mendez et al. (45) reported comparable findings at 2 months and Miskowiak et al. (50) at 3–4 months after acute illness. In the PHOSP-COVID cohort mentioned above (48); the authors purported that persistent systemic inflammation could be implicated in patient recovery, while Zhou et al. (22) reported a correlation between cognitive impairment and persistent inflammation at 3 weeks after acute infection.
The current study did not find any association between persisting cognitive impairment at 1 year and loss of taste or smell as symptoms of the initial infection. This point remains debated in the literature, with conflicting results reported at various follow-up time-points (20, 24, 51, 52). However, Dias de Melo et al. (53) recently found independence between anosmia and neuroinvasion by SARS-CoV2 in an animal model.
The absence of any established characteristics or associations that could help to quantify the risk of persisting cognitive deficit means that personalized follow-up should be systematic for all patients after COVID-19 infection (48), using screening tools that are capable of distinguishing patients at risk of long-term sequelae. Few authors have investigated the predictors of long-term cognitive impairment late after COVID-19. Aiello et al. (54) compared the MMSE and MoCA scores to screen for severe cognitive impairment, but at 2 to 3 months after COVID-19 infection. Both tools were found to be valid for this purpose, with a slightly higher sensitivity observed with the MoCA (54). Our finding that MMSE score at 3 months after infection is related to the persistence of deficits at 12 months is original and noteworthy. Although the MMSE is reputed to have less discriminant capacity for the identification of mild cognitive impairment (55), it appears to be a valid and useful tool in patients post-COVID (53). Moreover, it is easy to use in routine practice, with a short administration time (56), which also makes it attractive for use in primary care (57).
The need for systematic screening is reinforced by the fact that patients are largely unaware of their cognitive deficits. Indeed, the current data show no association between the presence of persisting cognitive impairment at 12 months, and self-reported cognitive complaints by the patients. This phenomenon has previously been described in several studies (49, 58, 59), and reflects the fact that there is a pervasive lack of awareness of these symptoms among COVID-19 patients. Nevertheless, the impact of cognitive dysfunction on social participation underpins the need to perform careful screening and provide personalized follow-up.
In the current study there were no differences in quality of life scores between patients with, and those without cognitive deficit at 12 months, which is contrary to some previous reports (45, 50, 60), but in line with others (58). This could be explained by the study population. Indeed, Voruz et al. (58) included patients with no known cognitive complaints. The current study also evaluated patients systematically, and independently of any reported cognitive complaints, which could explain why this study and that of Voruz observed no association between quality of life and cognitive function. It is also noteworthy that there was no correlation in the current study between cognitive impairment and the presence of symptoms of anxiety or depression, as assessed by the HADS scale, or fatigue assessed by the MFIS. This point is also debated in the literature, with some authors finding an association between cognitive impairments and psychiatric disorders (23, 37, 53, 54), whereas others did not find any such relationship (49, 61). There is potential for bias, such as the presence of anxiety or depressive symptoms after SARS-CoV-2 independently of any confirmed cognitive dysfunction (40). The association between cognitive deficits and perceived fatigue has not been widely investigated, but existing studies have found conflicting results (49, 58, 62). Further studies are necessary to elucidate the interplay between all these symptoms.
Finally, when cognitive deficits are diagnosed, identifying patients with the potential to recover is a worthy and important research perspective. In this study, it appears that, among the patients with cognitive deficits at 6 months, those who were in ICU and had prone positioning at the acute phrase appear to recover best, with regression of the cognitive symptoms. This is coherent with the pathophysiological hypothesis that hypoxaemia during the initial acute illness is implicated in the onset of cognitive deficits, and underlines the importance of initiating efficacious curative therapy at the acute phase. This raises the question of hypoxaemia monitoring and the acute management of hypoxaemia in patients who are cared for at home during acute infection.
Strengths and limitations
This study has some limitations; first, the absence of a control group and the small number of patients, with single-centre recruitment of patients who all required hospitalization and rehabilitation, yielding a selected population of patients with severe infection. Numerous studies have reported the existence of cognitive impairments, including in patients who did not require hospitalization, at 3–12 months after COVID-19 infection (40, 52, 62–64). Another limitation is the lack of information on functional status and the daily living impact of cognitive impairment according to neuro-psychological test. In view of the aim of the study, it would be relevant to complete the evaluations with ecological tests.
This study also shows a number of strengths. First, the results of the neuropsychiatric assessments were compared with norms for the general population established in non-pandemic times. We also sought to limit the number of false-positive results, by using robust cut-off, contrary to some other studies that used more permissive thresholds (50, 60). Moreover, we used validated neuropsychiatric tests, performed by a single, experienced examiner at both 6 and 12 months, and no subjective self-report questionnaires. There is wide diversity in the tools used in the literature (65), and consequently, wide variation in the cognitive deficits described. Consensus is needed on the battery of tests to be performed in these patients, which would be of value for both research and clinical practice, to harmonize data and enable comparisons and conclusions to be drawn about the prevalence and typology of cognitive impairment post-COVID-19.
Conclusion
After hospitalization due to SARS-CoV-2 infection, half of the patients have persisting cognitive impairment at 12 months on neuropsychological test. The persistence of cognitive impairment at 12 months was not found to be related to the initial symptoms, particularly the severity of the acute illness. The total duration of oxygen therapy (regardless of the type) was found to be associated with persisting cognitive impairment at 12 months. ROC curve analysis showed that a threshold of ≥10 days of oxygen therapy was associated with a protective effect. There is an unmet need for a consensual screening approach to detect cognitive impairment in these patients. Performing systematic screening, notably using the MMSE at 3 months after acute COVID-19 infection, could help to identify those at risk of having persisting cognitive impairment at 12 months.
ACKNOWLEDGEMENTS
The authors thank all participants and all patient organizations for contributing to this study and for sharing their experiences, and Fiona Ecarnot, PhD (University Hospital Besancon, France) for her work in the article translation and the Reims University Hospital for the funding.
REFERENCES
- Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 2022; 22: e102–e107. doi: 10.1016/S1473-3099(21)00703-9.
- Shah W, Hillman T, Playford ED, Hishmeh L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ 2021; 372: n136. doi: 10.1136/bmj.n136
- Tavares-Júnior JWL, de Souza ACC, Borges JWP, Oliveira DN, Siqueira-Neto JI, Sobreira-Neto MA, et al. COVID-19 associated cognitive impairment: a systematic review. Cortex J Devoted Study Nerv Syst Behav 2022; 152: 77–97. DOI: 10.1016/j.cortex.2022.04.006
- Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci 2021; 24: 168–175. DOI: 10.1038/s41593-020-00758-5
- Lemprière S. SARS-CoV-2 detected in olfactory neurons. Nat Rev Neurol 2021; 17: 63. DOI: 10.1038/s41582-020-00449-6
- Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol 2020; 19: 919–929. DOI: 10.1016/S1474-4422(20)30308-2
- Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, et al. Neuropathological Features of Covid-19. N Engl J Med 2020; 383: 989–992. DOI: 10.1056/NEJMc2019373
- Walitt B, Johnson TP. The pathogenesis of neurologic symptoms of the postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. Curr Opin Neurol 2022; 35: 384–391. DOI: 10.1097/WCO.0000000000001051
- Phetsouphanh C, Darley DR, Wilson DB, Howe A, Munier CML, Patel SK, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol 2022; 23: 210–216. DOI: 10.1038/s41590-021-01113-x
- Käufer C, Schreiber CS, Hartke A-S, Denden I, Stanelle-Bertram S, Beck S, et al. Microgliosis and neuronal proteinopathy in brain persist beyond viral clearance in SARS-CoV-2 hamster model. EBioMedicine 2022; 79: 103999. DOI: 10.1016/j.ebiom.2022.103999
- Klimkiewicz J, Pankowski D, Wytrychiewicz-Pankowska K, Klimkiewicz A, Siwik P, Klimczuk J, et al. Analysis of the relationship among cognitive impairment, nutritional indexes and the clinical course among COVID-19 patients discharged from hospital-preliminary report. Nutrients 2022; 14: 1580. DOI: 10.3390/nu14081580
- Hosp JA, Dressing A, Blazhenets G, Bormann T, Rau A, Schwabenland M, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain J Neurol 2021; 144: 1263–1276. DOI: 10.1093/brain/awab009
- Dressing A, Bormann T, Blazhenets G, Schroeter N, Walter LI, Thurow J, et al. Neuropsychological profiles and cerebral glucose metabolism in neurocognitive Long COVID-syndrome. J Nucl Med 2022; 63: 1058–1063. DOI: 10.2967/jnumed.121.262677
- Lee M-H, Perl DP, Nair G, Li W, Maric D, Murray H, et al. Microvascular injury in the brains of patients with Covid-19. N Engl J Med 2021; 384: 481–483. DOI: 10.1056/NEJMc2033369
- Premraj L, Kannapadi NV, Briggs J, Seal SM, Battaglini D, Fanning J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci 2022; 434: 120162. DOI: 10.1016/j.jns.2022.120162
- Alkodaymi MS, Omrani OA, Fawzy NA, Shaar BA, Almamlouk R, Riaz M, et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 2022; 28: 657–666. DOI: 10.1016/j.cmi.2022.01.014
- Zeng N, Zhao YM, Yan W, Li C, Lu QD, Liu L, et al. A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: call for research priority and action. Mol Psychiatry 2023; 28: 423–433. doi: 10.1038/s41380-022-01614-7
- Ferrucci R, Dini M, Rosci C, Capozza A, Groppo E, Reitano MR, et al. One-year cognitive follow-up of COVID-19 hospitalized patients. Eur J Neurol 2022; 29: 2006–2014. DOI: 10.1111/ene.15324
- Hampshire A, Trender W, Chamberlain SR, Jolly AE, Grant JE, Patrick F, et al. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine 2021: 39: 101044. DOI: 10.1016/j.eclinm.2021.101044
- Alemanno F, Houdayer E, Parma A, Spina A, Del Forno A, Scatolini A, et al. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: a COVID-rehabilitation unit experience. PloS One 2021; 16: e0246590. DOI: 10.1371/journal.pone.0246590
- Negrini F, Ferrario I, Mazziotti D, Berchicci M, Bonazzi M, de Sire A, et al. Neuropsychological features of severe hospitalized coronavirus disease 2019 patients at clinical stability and clues for postacute rehabilitation. Arch Phys Med Rehabil 2021; 102: 155–158. DOI: 10.1016/j.apmr.2020.09.376
- Zhou H, Lu S, Chen J, Wei N, Wang D, Lyu H, et al. The landscape of cognitive function in recovered COVID-19 patients. J Psychiatr Res 2020; 129: 98–102. DOI: 10.1016/j.jpsychires.2020.06.022
- Beaud V, Crottaz-Herbette S, Dunet V, Vaucher J, Bernard-Valnet R, Du Pasquier R, et al. Pattern of cognitive deficits in severe COVID-19. J Neurol Neurosurg Psychiatry 2021; 92: 567–568. DOI: 10.1136/jnnp-2020-325173
- Almeria M, Cejudo JC, Sotoca J, Deus J, Krupinski J. Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain Behav Immun - Health 2020; 9: 100163. DOI: 10.1016/j.bbih.2020.100163
- Wechsler, D. Wechsler adult intelligence scale – Fourth Edition (WAIS–IV). NCS Pearson, France; 2008.
- Beaujean AA, Benson NF, McGill RJ, Dombrowski SC. A misuse of IQ scores: using the dual discrepancy/consistency model for identifying specific learning disabilities. J Intell 2018; 6: 36. DOI: 10.3390/jintelligence6030036
- Ouvrard C, Berr C, Meillon C, Ribet C, Goldberg M, Zins M, et al. Norms for standard neuropsychological tests from the French CONSTANCES cohort. Eur J Neurol 2019; 26: 786–793. DOI: 10.1111/ene.13890
- Fimm B, Zimmermann P. Testbatterie zur Aufmerksamkeitsprüfung (TAP), Version 2.1. 2008. Psytest, Herzogenrath.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361–370. DOI: 10.1111/j.1600-0447.1983.tb09716.x
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002; 52: 69–77. DOI: 10.1016/s0022-3999(01)00296-3
- Learmonth YC, Dlugonski D, Pilutti LA, Sandroff BM, Klaren R, Motl RW. Psychometric properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J Neurol Sci 2013; 331: 102–107. DOI: 10.1016/j.jns.2013.05.023
- Larson RD. Psychometric properties of the modified fatigue impact scale. Int J MS Care 2013; 15: 15–20. DOI: 10.7224/1537-2073.2012-019
- Baumann C, Erpelding M-L, Régat S, Collin J-F, Briançon S. The WHOQOL-BREF questionnaire: French adult population norms for the physical health, psychological health and social relationship dimensions. Rev Epidemiol Sante Publique 2010; 58: 33–39. DOI: 10.1016/j.respe.2009.10.009
- Yachou Y, El Idrissi A, Belapasov V, Ait Benali S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 2020; 41: 2657–2669. DOI: 10.1007/s10072-020-04575-3
- Miners S, Kehoe PG, Love S. Cognitive impact of COVID-19: looking beyond the short term. Alzheimers Res Ther 2020; 12(1): 170. DOI: 10.1186/s13195-020-00744-w
- McMorris T, Hale BJ, Barwood M, Costello J, Corbett J. Effect of acute hypoxia on cognition: a systematic review and meta-regression analysis. Neurosci Biobehav Rev 2017; 74: 225–232. DOI: 10.1016/j.neubiorev.2017.01.019
- Adingupu DD, Soroush A, Hansen A, Twomey R, Dunn JF. Brain hypoxia, neurocognitive impairment, and quality of life in people post-COVID-19. J Neurol 2023; 270: 3303–3314. DOI: 10.1007/s00415-023-11767-2
- Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022; 604: 697–707. DOI: 10.1038/s41586-022-04569-5
- Guedj E, Campion JY, Dudouet P, Kaphan E, Bregeon F, Tissot-Dupont H, et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging 2021; 48: 2823–2833. DOI: 10.1007/s00259-021-05215-4
- Mattioli F, Stampatori C, Righetti F, Sala E, Tomasi C, De Palma G. Neurological and cognitive sequelae of Covid-19: a four month follow-up. J Neurol 2021; 268: 4422–4428. DOI: 10.1007/s00415-021-10579-6
- Dondaine T, Ruthmann F, Vuotto F, Carton L, Gelé P, Faure K, et al. Long-term cognitive impairments following COVID-19: a possible impact of hypoxia. J Neurol 2022; 269: 3982–3989. DOI: 10.1007/s00415-022-11077-z
- García-Sánchez C, Calabria M, Grunden N, Pons C, Arroyo JA, Gómez-Anson B, et al. Neuropsychological deficits in patients with cognitive complaints after COVID-19. Brain Behav 2022; 12: e2508. DOI: 10.1002/brb3.2508
- Ollila H, Pihlaja R, Koskinen S, Tuulio-Henriksson A, Salmela V, Tiainen M, et al. Long-term cognitive functioning is impaired in ICU-treated COVID-19 patients: a comprehensive controlled neuropsychological study. Crit Care Lond Engl 2022; 26: 223. DOI: 10.1186/s13054-022-04092-z
- Liu Y-H, Chen Y, Wang Q-H, Wang L-R, Jiang L, Yang Y, et al. One-year trajectory of cognitive changes in older survivors of Covid-19 in Wuhan, China: a longitudinal cohort study. JAMA Neurol 2022; 79: 509–517. DOI: 10.1001/jamaneurol.2022.0461
- Méndez R, Balanzá-Martínez V, Luperdi SC, Estrada I, Latorre A, González-Jiménez P, et al. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J Intern Med 2021; 290: 621–631. DOI: 10.1111/joim.13262
- van den Borst B, Peters JB, Brink M, Schoon Y, Bleeker-Rovers CP, Schers H, et al. Comprehensive health assessment 3 months after recovery from acute coronavirus disease 2019 (COVID-19). Clin Infect Dis Off Publ Infect Dis Soc Am 2021; 73: e1089–1098. DOI: 10.1093/cid/ciaa1750
- Braga LW, Oliveira SB, Moreira AS, Pereira ME, Carneiro VS, Serio AS, et al. Neuropsychological manifestations of long COVID in hospitalized and non-hospitalized Brazilian Patients. NeuroRehabilitation 2022; 50: 391–400. DOI: 10.3233/NRE-228020
- Evans RA, McAuley H, Harrison EM, Shikotra A, Singapuri A, Sereno M, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med 2021; 9: 1275–1287. DOI: 10.1016/S2213-2600(21)00383-0
- Woo MS, Malsy J, Pöttgen J, Seddiq Zai S, Ufer F, Hadjilaou A, et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun 2020; 2: fcaa205. DOI: 10.1093/braincomms/fcaa205
- Miskowiak KW, Johnsen S, Sattler SM, Nielsen S, Kunalan K, Rungby J, et al. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol 2021; 46: 39–48. DOI: 10.1016/j.euroneuro.2021.03.019
- Cecchetti G, Agosta F, Canu E, Basaia S, Barbieri A, Cardamone R, et al. Cognitive, EEG, and MRI features of COVID-19 survivors: a 10-month study. J Neurol 2022; 269: 3400–3012. DOI: 10.1007/s00415-022-11047-5
- Rank A, Tzortzini A, Kling E, Schmid C, Claus R, Löll E, et al. One year after mild COVID-19: The majority of patients maintain specific immunity, but one in four still suffer from long-term symptoms. J Clin Med 2021; 10: 3305. DOI: 10.3390/jcm10153305
- de Melo GD, Perraud V, Alvarez F, Vieites-Prado A, Kim S, Kergoat L, et al. Neuroinvasion and anosmia are independent phenomena upon infection with SARS-CoV-2 and its variants. Nat Commun 2023; 14: 4485. DOI: 10.1038/s41467-023-40228-7
- Aiello EN, Fiabane E, Manera MR, Radici A, Grossi F, Ottonello M, et al. Screening for cognitive sequelae of SARS-CoV-2 infection: a comparison between the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA). Neurol Sci 2022; 43: 81–84. DOI: 10.1007/s10072-021-05630-3
- Pinto TCC, Machado L, Bulgacov TM, Rodrigues-Júnior AL, Costa MLG, Ximenes RCC, et al. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int Psychogeriatr 2019; 31: 491–504. DOI: 10.1017/S1041610218001370
- Lees RA, Hendry BA K, Broomfield N, Stott D, Larner AJ, Quinn TJ. Cognitive assessment in stroke: feasibility and test properties using differing approaches to scoring of incomplete items. Int J Geriatr Psychiatry 2017; 32: 1072–1078. DOI: 10.1002/gps.4568
- Olazarán J, Hoyos-Alonso MC, del Ser T, Garrido Barral A, Conde-Sala JL, Bermejo-Pareja F, et al. Practical application of brief cognitive tests. Neurol Barc Spain 2016; 31: 183–194. DOI: 10.1016/j.nrl.2015.07.009
- Voruz P, Cionca A, Jacot de Alcântara I, Nuber-Champier A, Allali G, Benzakour L, et al. Functional connectivity underlying cognitive and psychiatric symptoms in post-COVID-19 syndrome: is anosognosia a key determinant? Brain Commun 2022; 4: fcac057. DOI: 10.1093/braincomms/fcac057
- Amalakanti S, Arepalli KVR, Jillella JP. Cognitive assessment in asymptomatic COVID-19 subjects. Virusdisease 2021; 32: 146–149. DOI: 10.1007/s13337-021-00663-w
- Miskowiak KW, Fugledalen L, Jespersen AE, Sattler SM, Podlekareva D, Rungby J, et al. Trajectory of cognitive impairments over 1 year after COVID-19 hospitalisation: pattern, severity, and functional implications. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol 2022; 59: 82–92. DOI: 10.1016/j.euroneuro.2022.04.004
- Delgado-Alonso C, Valles-Salgado M, Delgado-Álvarez A, Yus M, Gómez-Ruiz N, Jorquera M, et al. Cognitive dysfunction associated with COVID-19: a comprehensive neuropsychological study. J Psychiatr Res 2022; 150: 40–46. DOI: 10.1016/j.jpsychires.2022.03.033
- Ortelli P, Ferrazzoli D, Sebastianelli L, Engl M, Romanello R, Nardone R, et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. J Neurol Sci 2020; 420: 117271. DOI: 10.1016/j.jns.2020.117271
- Stavem K, Einvik G, Tholin B, Ghanima W, Hessen E, Lundqvist C. Cognitive function in non-hospitalized patients 8-13 months after acute COVID-19 infection: a cohort study in Norway. PloS One 2022; 17: e0273352. DOI: 10.1371/journal.pone.0273352
- Del Brutto OH, Wu S, Mera RM, Costa AF, Recalde BY, Issa NP. Cognitive decline among individuals with history of mild symptomatic SARS-CoV-2 infection: a longitudinal prospective study nested to a population cohort. Eur J Neurol 2021; 28: 3245–3253. DOI: 10.1111/ene.14775
- Biagianti B, Di Liberto A, Nicolò Edoardo A, Lisi I, Nobilia L, de Ferrabonc GD, et al. Cognitive assessment in SARS-CoV-2 patients: a systematic review. Front Aging Neurosci 2022; 14: 909661. DOI: 10.3389/fnagi.2022.909661