ORIGINAL REPORT
CHANGE CHARACTERISTICS OF HEALTH-RELATED QUALITY OF LIFE AND ITS ASSOCIATION WITH POST-STROKE FATIGUE AT FOUR-YEAR FOLLOW-UP
Synne G. PEDERSEN, PhD1, Audny ANKE, PhD1-3, Mari T. LØKHOLM, Cand. Psychol1, Marianne B. HALVORSEN, PhD4, Marit KIRKEVOLD, PhD2,5, Guri HEIBERG, PhD1, Marte ØRBO, PhD6 and Oddgeir FRIBORG, PhD6
From the 1Department of Rehabilitation, University Hospital of North Norway, Tromsø, 2Institute of Health and Society, Research Centre for Habilitation and Rehabilitation Model and Services (CHARM), Faculty of Medicine, University of Oslo, Oslo, 3Faculty of Health Sciences, Department of Clinical Medicine, UiT – The Arctic University of Norway, Tromsø, 4Department of Pediatric Rehabilitation, University Hospital of North Norway, Tromsø, 5Institute of Nursing and Health Promotion, Oslo Metropolitan University, Oslo, and 6Faculty of Health Sciences, Department of Psychology, UiT – The Arctic University of Norway, Tromsø, Norway
Objective: To explore trajectories that describe change in post-stroke health-related quality of life with fatigue as outcome.
Design: Observational and prospective study.
Subjects: Stroke survivors (N = 144) with predominantly mild or moderate strokes.
Methods: The multidimensional Stroke-Specific Quality of Life scale was used at 1 and 4 years, and the Fatigue Severity Scale at 4 years post-stroke. Latent class growth analyses were used as person-oriented analyses to identify meaningful trajectories. Socio-demographic and stroke-related covariables provided customary adjustment of the outcome, as well as prediction of class membership.
Results: The latent class growth analysis models were estimated for “physical health”, “visual-language”, and “cognitive-social-mental” components of the Stroke-Specific Quality of Life scale, which extracted trajectories describing a variation in stable, deteriorating and improving functional patterns. The stable, well-functioning trajectory was most frequent across all components. More pronounced fatigue was associated with trajectories describing worse functioning, which was more prominent among females compared with males. Living alone implied more fatigue in the “cognitive-social-mental” component. Within the “visual-language” components’ trajectories, younger and older participants reported more fatigue compared with middle-aged participants.
Conclusion: Most participants belonged to the stable, well-functioning trajectories, which showed a consistently lower level of fatigue compared with the other trajectories.
LAY ABSTRACT
The long-term consequences of stroke may be highly individual and multifaceted. The question of how such individual differences may unfold and change beyond the first year after stroke may be of substantial clinical interest regarding which subgroups show more favourable and unfavourable rehabilitation trajectories. The current study explored functional trajectories from 1 to 4 years post-stroke and their association with post-stroke fatigue. A total of 144 individuals with mainly mild or moderate strokes were included. Their functions were measured with the Stroke-Specific Quality of Life scale at 1 and 4 years post-stroke, and fatigue with the Fatigue Severity Scale 4 years post-stroke. The study found that the majority of subjects belonged to the trajectories described as stable, well-functioning from 1 to 4 years post-stroke. Participants who experienced less fatigue were those who had the highest and most stable function throughout the recovery course.
Key words: fatigue; health-related quality of life; functioning; stroke; latent class growth analysis.
Citation: J Rehabil Med 2024; 56: jrm13389. DOI: https://doi.org/10.2340/jrm.v56.13389.
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Submitted: May 25, 2023; Accepted: Nov 29, 2023; Published: Jan 4, 2024
Correspondence address: Synne Garder Pedersen, Department of Rehabilitation, University Hospital of North Norway, Sykehusveien 38, NO-9038 Tromsø, Norway. E-mails: synne.garder.pedersen@unn.no; synnegp@hotmail.com
Competing interests and funding: The authors have no conflicts of interest to declare.
Dam Foundation.
Individuals with stroke report poorer health and function than the general population (1, 2), and physical (3), cognitive (4), and emotional (5) health consequences are common. Social reintegration may be affected (6), as well as reduced health-related quality of life (HRQoL) (7, 8). The individual differences in how these health consequences may unfold during the rehabilitation period and beyond the first year may be of substantial clinical interest for understanding long-term favourable and unfavourable rehabilitation trajectories.
Few studies have examined sub-groupings of individual differences in long-term change patterns within the rehabilitation area. No studies have examined it specifically for the multidimensional Stroke-Specific Quality of Life (SS-QOL) scale (9), a stroke-specific HRQoL instrument that covers multiple functional domains (i.e. physical, social, emotional, mental, and linguistic functioning) (10, 11). Studies of HRQoL trajectories after stroke (12–14), subarachnoid haemorrhage (15), or traumatic brain injury (16), have extracted 3–4 trajectories, of which the more favourable recovery trajectories have constituted from 50% to 80–90% of the sample. Higher age and lower education were associated with poorer trajectories in 1 study (12), but not in the other studies. Regarding stroke-related factors, more pronounced baseline stroke severity, disability (14, 15), and comorbidity (12, 14), have been related to poorer trajectories.
Post-stroke fatigue (PSF) is a pervasive symptom that may persist for several years after the stroke (11, 17). However, evidence is lacking in how fatigue may be expressed differently within subgroups characterizing various functional HRQoL trajectories, such as stability, improvement, or deterioration. PSF has been regarded as a negative factor for the rehabilitation and recovery process following stroke (17, 18), and most stroke survivors consider fatigue as the worst symptom to manage (19).
The current study aimed to describe post-stroke outcomes by using latent class growth analysis (LCGA) modelling techniques to uncover how individuals cluster together and form distinct patterns, or latent classes, which describe long-term functional changes. In contrast to conventional variable-centred methods, such as regression analyses, that fit the same parameter for all participants, mixture models, such as LCGA, search for response patterns that are shared within the latent classes or subsets of the data, but which are qualitatively different between the classes (20); hence, the labelling person-oriented analyses. LCGA thus enables empirical identification of sub-group patterns describing various levels of stability and change. This study therefore applied LCGA to determine the number of distinct functional trajectories, and what these trajectories might imply in terms of PSF at 4-year follow-up. As previous studies of PSF point to demographic, comorbidity, and stroke-related factors as relevant covariables (21–23) these were included in the analysis to explore if they could predict the latent class trajectories.
The aims of this study were to: (i) explore the number of trajectories that may account for changes in SS-QOL scores from 1 to 4 years post-stroke; (ii) characterize these trajectories and their prevalence; (iii) understand the trajectories’ effects in terms of PSF at 4-year follow-up and examine if the included covariables are related to the extracted trajectories.
METHODS
Design
The study is an observational prospective study with fatigue data collected at 4 years post-stroke, and functional data collected at 1 and 4 years post-stroke. The study is registered as NCT 03639259 in ClinicalTrials.gov.
Participants
Recruitment of participants was from the Norwegian arm of the study “Rehabilitation, function, and quality of life after stroke in North Norway and Central Denmark – the NorDenStroke study” (7, 8). Persons with verified cerebral stroke were recruited from 1 of 3 stroke units at the University Hospital of North Norway (UNN HF), between March 2014 and December 2015 (7). Persons with stroke related to brain malignancy, subarachnoid haemorrhage, or brain trauma were excluded.
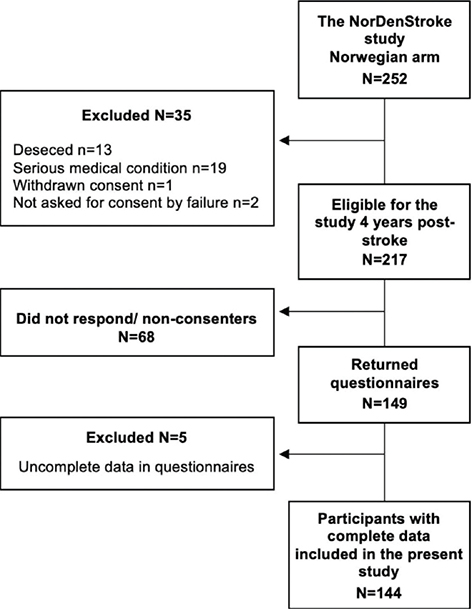
Included stroke survivors fulfilled the inclusion criteria of the National Norwegian Stroke Register, defined clinically according to the World Health Organization’s (WHO’s) definition as acute ischaemic or haemorrhagic stroke (I.63 and I.61, respectively) in individuals aged 18 years or above (International Classification of Diseases – 10th edition). For the current study, stroke survivors who had completed questionnaires in the NorDenStroke study 1 year post-stroke were included (n = 217). A drop-out analysis compared the 149 participants with the 68 stroke survivors who did not respond, or consent, to the fatigue follow-up study when invited to participate 4 years post-stroke. The participants who did not respond, or consent, (n = 68) were significantly older (p = 0.001) than the participants who agreed to the fatigue follow-up study (n = 149) (mean age = 72 (standard deviation (SD) = 10.7) vs 67 (SD = 11.0) years). However, the groups did not differ in sex, stroke type or stroke severity. A flowchart following the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) criteria is shown in Fig. 1.
Data collection procedures at 4-year follow-up
A health professional informed eligible participants about the study and asked for written consent. After new written consent was obtained, questionnaires were sent by post. In case of missing data in the questionnaires, participants received a follow-up telephone call and were encouraged to answer any missing questions. Five participants were unable to complete the questionnaires due to declining health and were consequently excluded.
Variables
Information on stroke-related factors and medical information had previously been collected from the National Norwegian Stroke Register, or the participant’s medical files. At baseline, data on age, sex, stroke subtype, and stroke severity were obtained from the National Norwegian Stroke Registry. Information on marital status (married/cohabiting or single), and work status (working/studying) were obtained from questionnaires 1 year post-stroke. Information on comorbidity was collected from the medical files 4 years post-stroke.
The Norwegian version (24) of the Fatigue Severity Scale (FSS) (25) was used to measure fatigue 4 years post-stroke. The FSS has been used widely to assess fatigue in population-based stroke research (26), and has shown excellent internal consistency (Cronbach’s alpha = 0.92), and good test-retest reliability (intraclass correlation coefficient = 0.742) (27). The FSS questionnaire encompasses 9 items investigating fatigue in daily life across domains of: daily activity, social participation, sleep, and motivation. The items of the FSS are graded on a 7-point Likert scale (higher scores indicating more fatigue), ranging from 1 (no problem) to 7 (a significant problem). Based on all 9 items, a global average score is calculated (24). A cut-off score of ≥ 5 is recommended for defining severe fatigue (24).
Functional-related consequences were collected with the Norwegian version of the SS-QOL scale (9) at 2 time-points: 1 year and 4 years post-stroke. The SS-QOL is a multidimensional stroke-specific HRQoL instrument, which predominantly assesses the functional impact of stroke. The scale consists of 49 items across 12 domains: mobility, energy, upper extremity function, work and productivity, mood, self-care, social roles, family roles, vision, language, thinking, and personality. Each domain is measured by 3–6 items using a 5-point (1–5) Likert scale, where higher scores indicate better function. An average index score for each domain allows for comparison between domains and is useful for identifying specific capacities affected by stroke (9). Previous studies have extracted components of the SS-QOL scale with principal component analysis (7, 28). Several studies have documented the reliability of the SS-QOL domains and overall scores, confirming an acceptable internal consistency of the domains (9, 10, 29). The test-retest reliability coefficient has been documented as generally good (Spearman’s rho = 0.65–0.99) (10, 29).
Statistical analyses
The analyses were conducted in SPSS Statistics (version 28) and Mplus (version 8) (30). Descriptive data are described as means/medians, SDs, and interquartile range (IQR). Significance testing of differences between simple means and categorical frequencies was conducted using t-tests and χ2 tests (or Fisher’s exact for small N). A principal component analysis (PCA) of the 12 SS-QOL subscale scores was conducted to identify fewer overarching domain scores that could adequately represent all 12 subscales and be used in the subsequent latent class analyses. Principal components with eigenvalues > 1 were extracted. The item-factor correlation tables were promax rotated (kappa = 4) to allow for component correlations as SS-QOL subscale scores are correlated.
Repeated health data may be analysed using latent class growth techniques for describing developmental change over a time period (31). The relative standing of an individual at each time-point may be modelled as a mathematical function of an underlying growth process describing the process via 2 latent variables: “the intercept”, describing participants’ initial status, and “the slope”, describing their rate of change across time. Their mean parameters describe the mean initial status and mean rate of change, whereas the variance parameter describes the distribution around these 2 means. To overcome the classical limitation of fitting each participant to the same growth process, a latent class model is put on top of the growth model to allow for separate growth parameters, i.e. intercepts and slopes, within each latent class, thus estimating the resulting latent trajectories as homogeneously as possible for the subjects within a class, and as different as possible from trajectories belonging to other classes. A latent class threshold parameter is added for the estimation of the proportion of participants belonging to the various classes. The growth variance parameters are usually fixed to zero across all classes in LCGA for model identification purposes. However, the current study allowed for a common/single intercept variance, as it did not cause convergence problems, improved model fit substantially, and accounted for individual differences around the intercept more realistically. Fig. 2 shows the conceptual model.
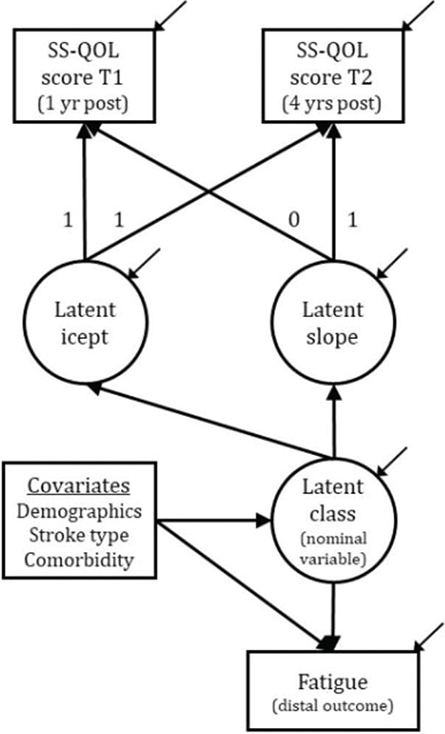
Fig. 2. The Conceptual Latent Class Growth Analysis Model. SS-QOL: Stroke-Specific Quality of Life; yr: year; T1: Time 1; T2: Time 2
Beyond the extraction of an optimum number of latent classes, distal outcome variables of clinical interest may be added to the model to understand what the “effects” of belonging to a particular class might be. Covariables are usually added to provide customary adjustment of the outcome, but with the additional advantage of predicting latent class membership. Neighbouring classes of low sample sizes were combined to improve the statistical sensitivity by constraining the predictive parameters as equal. In the current study, fatigue was added as an outcome, whereas sex, age, cohabitation and work status (as demographics), stroke characteristics (stroke type and severity) and comorbidity as stroke-related covariables. These covariables were also used for predicting class membership. For parsimonious reasons, covariables that did not contribute significantly (p < 0.05) either for adjustment or class prediction, were removed.
The full-information maximum likelihood method with robust errors (MLR) was used to estimate the log-likelihood function. In deciding the appropriate number of latent classes, we primarily consulted the Bayesian Information Criterion (BIC) (32) and the Bootstrapped Likelihood Ratio test (BLRT) (33). The BIC is a deviance index that is adjusted for sample size and model complexity. The BLRT uses random bootstraps in the estimation of whether a k-class model fits significantly better than the k-1 class (simpler) model. Entropy is a precision index that indicates how well the case classifications work on a scale from 0 (poor) to 1 (perfect). Simulation studies suggest that the BIC and BLRT perform best in deciding the number of classes or profiles to extract (34). In addition, we factored in interpretability (differentiation of the classes) and model parsimoniousness.
RESULTS
Description of the sample
A total of 144 stroke survivors with complete questionnaire data (SS-QOL and FSS) 1 and 4 years post-stroke were included as participants in the study. Table I shows the demographic and stroke characteristics of the participants. Most of the participants (n = 130, 90%) had an ischaemic stroke, and mild stroke impairment (n = 91, 63%) measured with the Scandinavian Stroke Scale at baseline. Females were significantly associated with fatigue (p ≤ 0.001). The mean age at the time of stroke onset was 67.3 years; however, age was not significantly associated with fatigue. Neither was comorbidity that included heart disease (n = 74), cancer (n = 23), intestinal disease (n = 3), metabolic disease (n = 6), migraine (n = 5), epilepsy (n = 6), and rheumatism (n = 9). Working participants and those living with a significant other 1-year post-stroke reported significantly less fatigue at follow-up than non-working participants (p = 0.022) and those who were unmarried or had no cohabitant (p = 0.003).
Principal component analysis of the Stroke-Specific Quality of Life
The PCA extracted 3 components (eigenvalue (λ) > 1). The first (λ = 6.71) and the second (λ = 1.81) represented a cognitive-social-mental (CSM) functioning component (i.e. social roles, mood, personality, energy, family roles, and thinking; loadings = 0.91, 0.91, 0.86, 0.79, 0.78 and 0.62), and physical health (PH) functioning component (self-care, mobility, upper extremity function and work/productivity; loadings = 0.98, 0.93, 0.91 and 0.82), respectively. The third (λ = 1.01) represented a visual-language (VL) functioning component (vision and language; loadings = 0.92 and 0.74). See Fig. 3 illustrating SS-QOL with domains and components.
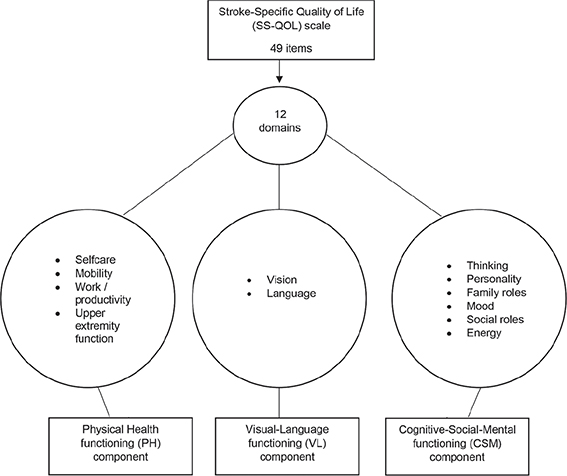
Fig. 3. Illustration of the Stroke-Specific Quality of Life (SS-QOL) scale with extracted components.
Latent class growth analyses and class descriptions
LCGA were based on summed SS-QOL domain scores according to the PCA and rescaled to a 0–100 range. LCGA models were examined for the PH, the VL, and the CSM components. An increasing number of latent classes, or trajectories, were extracted until model fit stopped improving or the estimation was unsuccessful (Table SI).
Physical health trajectories
We extracted 6 PH trajectories since the 7-class model was defined by a single case and the improvement in BIC decreased markedly after 6 classes. See Fig. 4 for the mean scores of the 6 trajectories. Despite a heterogeneous composition of trajectories, most participants (class #1, ~74%) had a stable and high level of physical functioning. The second trajectory (#2, ~11% participants) exhibited a somewhat lower yet relatively well-off level of functioning, which significantly declined 3 years later (Mchange = –11.5, p < 0.001). The remaining participants belonged to trajectories characterized by stability, but of lower functional levels (#3, #5, #6). A minor group (#4, 3.5% participants) manifested a marked functional decline (Mchange = –30.8, p < 0.001).
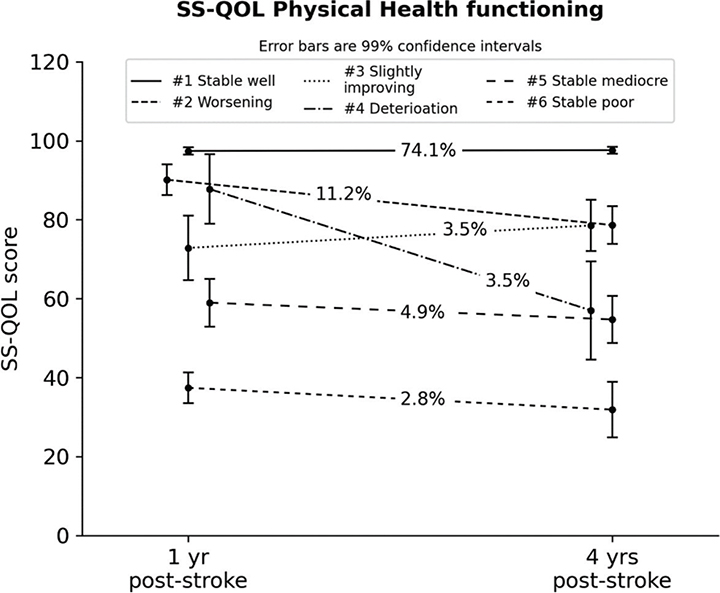
Fig. 4. Latent trajectories for the Stroke-Specific Quality of Life (SS-QOL) Physical Health Component (with 99% confidence intervals). #Trajectory number. yr: year.
Visual and language trajectories
The 5-class solution was preferred since the extraction of further trajectories was not feasible due to a local maxima (i.e. the model was not replicable most probable due to insufficient data heterogeneity). The stable and best-functioning trajectory (#1) represented almost 4-fifths of the participants with a very precise estimation as all individual data clustered very closely around the trajectory mean, hence prohibiting any further meaningful breakup of this class. The remaining trajectories thus represented few participant cases that also prohibited any further breakup. The trajectory means are shown in Fig. 5. The second-best trajectory (#2, 9%) had a lower initial score compared with the majority trajectory, but evidenced a minor but significant improvement at follow-up (Mchange = 5.3, p = 0.008). Participants in the 2 next trajectories evidenced mean scores indicative of improvement (#3, ~6%; Mchange = 9.8, p = 0.01) vs deterioration (#4, ~4%; Mchange = –26.3, p < 0.001), whereas the trajectory with the lowest SS-QOL scores (#5) had a stable level of functioning.
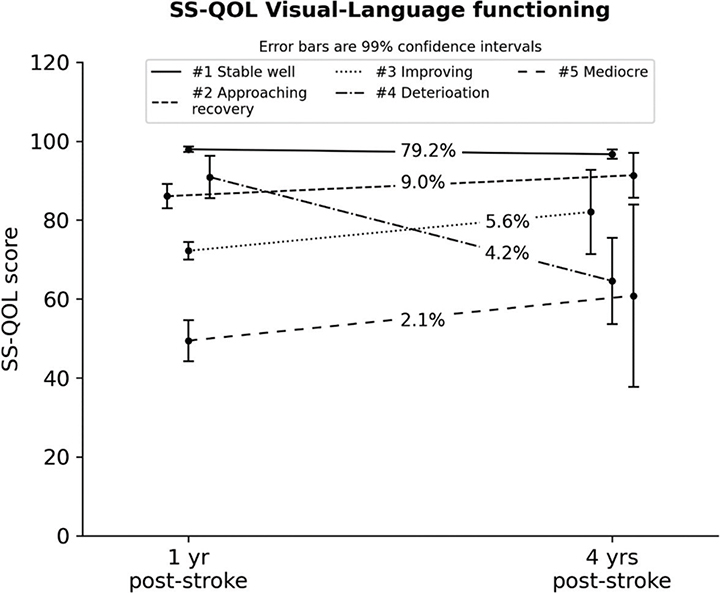
Fig. 5. Latent trajectories for the Stroke-Specific Quality of Life (SS-QOL) Visual-Language Component (with 99% confidence intervals). #Trajectory number.
Cognitive-social-mental trajectories
For the CSM component, model fit stopped improving after specifying 7 classes. The largest trajectory (#1), characterizing stable and very high-functioning participants (~49%), had a lower prevalence compared with the best PH trajectory. Among the stable trajectories, 2 participant classes represented mediocre (#5, ~13%) and lower (#6, ~4%) functional levels. Two other trajectories represented improvement classes, 1 labelled as a recovery class (#2, ~13%; Mchange = 14.8, p < 0.001) by regaining approximately the same functional level as the majority class, while another trajectory evidenced substantial improvements from a lower level (#3, ~4%; Mchange = 36.4, p < 0.001). Two other trajectories represented participants showing worsening (#4, ~14%; Mchange=–24.5, p < 0.001) and marked deterioration in functioning (#7, ~3%; Mchange = –73.3, p < 0.001) (Fig. 6).
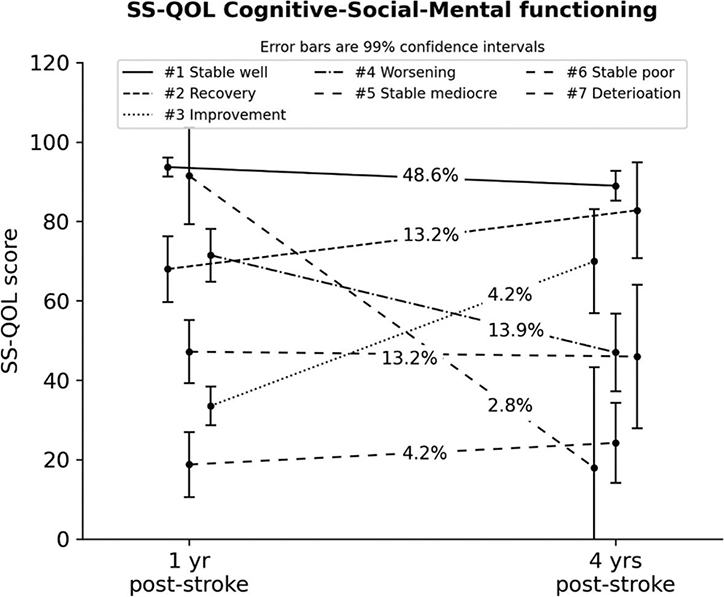
Fig. 6. Latent trajectories for the Stroke-Specific Quality of Life (SS-QOL) Cognitive-Social-Mental Component (with 99% confidence intervals). #Trajectory number. yr: year.
Fatigue as a distal outcome (including adjustment and class predictions)
Fatigue was added as a “distal outcome” together with covariables for adjusting the fatigue score as well as predicting trajectory memberships. In all 3 outcome analyses, the first (and best functioning) trajectory represented participants with the lowest fatigue score compared with all other trajectories (Table II). For the SS-QOL PH component, the first trajectory had significantly lower fatigue scores (p < 0.01) than all other trajectories except trajectory #5 in this component. For the SS-QOL VL component, differences were less pronounced, with only trajectory #4 being significantly higher on fatigue scores (p < 0.01) than all other trajectories. For the SS-QOL CSM component, the first trajectory (#1) was significantly better than trajectories #3–6, whereas the second trajectory (#2) in the CSM component was better than trajectory #3–5. The low number of participants in the poorer trajectories is probably the number one reason for the lack of significance despite worse fatigue scores.
| SS-QOL Physical Health | #1 (106) Stable, well-functioning | #2 (16) Worsening | #3 (5) Slightly improving | #4 (5) Deterioration | #5 (7) Stable mediocre | #6 (4) Stable poor | ||
| Fatigue M SE | 3.59 0.16 | 5.25 0.31 | 5.35 0.23 | 5.51 0.46 | 4.14 0.69 | 5.95 0.63 | ||
| diff | a | b | b | b | a, b | b | ||
| Fatigue adj | Trajectory predictors (OR 95% CI.) | |||||||
| Covariates | beta se | #1 | #2 | #3–6 | ||||
| Male | –0.99*** 0.29 | ref | 0.64 0.22–1.89 | 0.36* 0.14–0.96 | ||||
| Severe stroke | –0.14 0.28 | ref | 3.69* 1.21–11.24 | 1.88 0.67–4.75 | ||||
| SS-QOL Visual-Language |
#1 (114) Stable, well-functioning |
#2 (13) Approaching recovery |
#3 (8) Improving |
#4 (6) Deterioration |
#5 (3) Mediocre |
|||
| Fatigue M SE | 3.74 0.16 | 4.54 0.36 | 4.57 0.48 | 6.37 0.56 | 4.87 0.80 | |||
| diff | a | a | a | b | a, b | |||
| Fatigue adj | Trajectory predictors (OR.95% CI) | |||||||
| Covariates | beta se | #1 | #2 | #3–5 | ||||
| Male | –0.78** 0.31 | ref | 0.34 0.09–1.26 | 0.42 0.14–1.29 | ||||
| Age 18–55 years | 1.01** 0.41 | ref | 1.51 0.24–9.38 | 0.53 0.10–2.69 | ||||
| Age >75 years | 0.67* 0.31 | ref | 1.39 0.28–6.89 | 0.78 0.17–3.69 | ||||
| Age 56–75 years | ref | |||||||
| Living alone | 0.64* 0.31 | ref | 0.83 0.17–4.17 | 0.48 0.10–2.31 | ||||
| Working | –0.73* 0.36 | ref | 3.73 0.64–21.88 | 1.38 0.27–6.98 | ||||
| Severe stroke | 0.16 0.28 | ref | 0.28 0.05–1.66 | 2.97* 1.05–8.45 | ||||
| Haemorrhage | –0.07 0.42 | ref | 5.43* 1.06–27.85 | 2.26 0.59–8.62 | ||||
| SS-QOL Cognitive-Social-Mental | #1 (70) Stable, well-functioning |
#2 (19) Recovery |
#3 (6) Improving |
#4 (20) Worsening |
#5 (19) Stable mediocre |
#6 (6) Stable poor |
#7 (4) Deterioration |
|
| Fatigue M SE | 3.04 0.19 | 3.67 0.34 | 5.18 0.60 | 5.37 0.20 | 5.54 0.34 | 5.02 0.67 | 4.51 0.73 | |
| diff | a | a, b | b, c | c | c | b, c | a, b, c | |
| Fatigue adj | Trajectory predictors (OR.95% CI) | |||||||
| Covariates | beta se | #1 | #2–3 | #4 and 7 | #5–6 | |||
| Male | –0.56* 0.26 | ref | 0.68 0.22–2.13 | 0.32* 0.12–0.88 | 0.22** 0.08–0.64 | |||
| Living alone | 0.76** 0.28 | ref | 2.43 0.80–7.37 | 1.16 0.38–3.57 | 0.59 0.16–2.24 | |||
| Notes. beta se: beta coefficient and standard error (in subscript) with asterisk indicating significance; * p<0.05; ** p<0.01; *** p<0.001; Fatigue M SE: Trajectory specific mean fatigue score with standard error (in subscript); diff: Mean fatigue scores between latent trajectories with different subscript letters are significantly different at p < .01; Fatigue adj: Overall mean fatigue score adjusted for covariables; Trajectory predictors: Multinomial regression coefficients for the covariate prediction of trajectory membership. | ||||||||
The covariate effects (irrespective of class membership) showed significantly lower fatigue scores among males across all 3 SS-QOL components compared with females, but with more uncertainty regarding the CSM component (p < 0.05) than for the PH and VL components (p < 0.001 and p < 0.01, respectively). Living alone implied more fatigue in the CSM component compared with cohabitation (p < 0.01), whereas younger participants (55 years or below, p < 0.01) and older participants (above age 75 years, p < 0.05) reported more fatigue in the VL component compared with middle old age (56–75 years age span).
The multinomial logistic regression analyses with class membership as a nominal dependent variable had to combine participants belonging to the poorer functioning classes due to the low number of participants in these classes (see Table II). Overall, few predictors contributed significantly to the prediction of the trajectories. Sex was, however, a significant predictor (p < 0.01) of class membership within the CSM component, indicating lower odds for males of belonging to poorer functioning trajectories compared with females. In addition, severe stroke, and haemorrhage predicted membership in the poorer trajectories of the SS-QOL PH and VL components, but as the uncertainty of these effects was higher (p < 0.05), we refer to Table II without interpreting these effects further.
DISCUSSION
The current study contributes new knowledge regarding stroke-specific HRQoL trajectories from 1 to 4 years post-stroke and the presence of fatigue in the functional trajectories. The study included persons with predominantly mild or moderate stroke. Changes in functional status were measured with the SSQoL scale, which could be effectively reduced to 3 overall functional component scores according to a principal component analysis: physical health, visual-language, and the cognitive-social-mental functional components.
Functional trajectories
In concordance with previous research on HRQoL trajectories post-stroke, the latent class analyses based on the SS-QOL components showed a heterogeneous pattern of trajectories describing stability, deterioration, and improvement across time (12, 14). The analyses revealed that the majority displayed a stable trajectory characterized by a high level of function during the entire follow-up period. Furthermore, most participants shared a quite homogeneous pattern of stable high functioning in the VL and the PH components. The finding of a predominantly high functioning and stable class should be highlighted as positive, giving the long-term follow-up of 4 years in this study. Although the initial stroke severity was predominantly mild or moderate, the population was also ageing, which might cause physical or cognitive deterioration (35). Moreover, 63% of the participants had comorbidity, which previously showed significant positive associations with physical dependence and cognitive impairment (36). Hence, comorbidity or multimorbidity could be a negative determinant for deteriorating trajectories, but this was not clearly evident in the current analyses.
In the current study ~74% had a stable and high level of physical function. Similar results (65% with stable high physical functioning) were found in a study assessing trajectories up to 1-year post-stroke (14). However, this study excluded subjects that had comorbidity that could interfere with study outcomes, whereas the current study did not apply these exclusion criteria. Nevertheless, the current study had a 10% higher rate of stable high physical function among the participants. Although the literature is scarce concerning person-oriented analysis beyond 1 year, others have investigated changes in physical factors based on mean scores. For example, a disease-overarching study (37) found a general improvement in function 3 years after rehabilitation. The change in functioning was measured with the generic Medical Outcome Study Short Form (SF-36). When dividing the disease groups, the neurological group had a decrease in the physical component scores after 1 year, but an increase after 3 years. This underscores the importance of investigating the long-term consequences of stroke, as measures of functions may differ according to the time of measurement. As suggested by Berget et al., persons with neurological conditions may need more time to achieve stable improvement (37). Another study by van de Port et al. (38) showed a gradual deterioration in mobility status during the follow-up period of 3 years after stroke, with deterioration in mobility in 21% of the participants and an improvement in 7%. The above-mentioned studies did not, however, employ latent class growth analysis/cluster-based techniques.
The CSM component, addressing cognitive, social, and mental functioning, also had a large trajectory characterizing stable and very high-functioning individuals’ post-stroke (~49%). However, the largest CSM trajectory had a lower prevalence compared with both the PH and the VL component’s best trajectories, which indicates more heterogeneity in the CSM change processes across time. The current study finding is comparable to another study (14) that found 45% at a stable high psychosocial HRQoL trajectory in the SS-QOL-12 at 1-year post-stroke. In the current study, the CSM component had the highest variation of different trajectories and was also the only model that showed a group with a near full-recovery trajectory (~13%), as well as a smaller improving trajectory (~4%). This finding contradicts arguments that functional recovery and HRQoL reach a plateau within 3–6 months after stroke onset (14). On the contrary, the results of long-term recovery and improving trajectories indicate possibilities for further adjustments and positive changes beyond 6 months. As discussed by Mierlo et al., psychosocial adaptation is a continuous process that may stretch over a longer period, and individuals may stabilize on adjusted expectations to recovery (14).
Fatigue and covariables
Not unexpectedly, the highest functioning trajectories in all 3 components of the SS-QOL represented participants with the lowest fatigue score compared with all other trajectories. PSF has previously been associated with both physical and mental functional impairments, which may contribute to differences in post-stroke rehabilitation needs, as the group is heterogeneous both in sample composition and the management of their functional impairments (17, 22, 23). PSF and HRQoL have, in several studies, shown a negative correlation (11, 17), and psychosocial and behavioural factors might be important for both triggering and maintaining fatigue (23). In addition, self-perceived fatigue might be affected by homeostatic factors, such as impaired central regulation of physical activity, dependent on energetic and neural feedback (22). It is challenging to compare the current study findings with other studies, as we have not found previous studies investigating fatigue as an outcome predicted by both functional HRQoL trajectories and covariables. However, a study identifying trajectories of PSF over 18 months (23) identified 3 trajectories, characterized by different levels of fatigue: low (FSS <3), moderate (FSS 3–4), and high (FSS ≥ 5). In the current study, a total of 9 trajectories within the PH, VL and CSM components had mean scores of fatigue above 5; however, the functional trajectories varied in terms of deterioration [4], improvement [2] or low stability [3] scores in the SS-QOL. These findings indicate that severe fatigue (FSS ≥ 5) may be present even if aspects of HRQoL are improving, and the relationship between HRQoL and PSF is therefore not essentially streamlined. As discussed by Kjeverud et al., the symptoms of fatigue might be due to specific physical or cognitive impairments, psychological stress, inadequate coping styles, or combinations of these (23).
Similar results of higher fatigue among females than males have been reported (39), but not consistently (40). The multinomial logistic regression analyses in the current study found sex as a significant predictor of the trajectories in the CSM component, indicating better CSM function for males than for women. Comparisons with other studies are not available, but 1 study reported an association between female sex and markedly improving QoL trajectories in general (12). Living alone also implied more fatigue in the CSM component, which might be an expression of less social support as well as more individual demands in practical daily tasks. Previous studies both support and dispute that PSF is more common among those living alone (40). Within the VL components’ trajectories, participants with younger and older ages reported more fatigue compared with middle-aged subjects. Visual problems are associated with more self-reported fatigue (11). Younger participants may experience more demands (e.g. work and family life), and may have higher expectations in their own overall everyday capacities compared with older individuals. Previous research is inconclusive regarding the association between age and fatigue, and a commonly debated question regarding this discrepancy is that the oldest individuals with stroke often are non-responders or not included in studies (40).
Study strengths and limitations
To our knowledge, the current study is one of very few to identify distinct long-term functional HRQoL changes and the presence of fatigue in the diverse trajectories, hence representing new knowledge that is of clinical importance for understanding post-stroke prospects. Questionnaires with a high completeness rate, combined with high-quality data from the national Norwegian Stroke Register, represent a strength of this study as the risk of information bias is minimized.
Although the current study is decent in sample size for conventional regression analyses, it may be considered smaller for a latent class analysis as several of the identified classes were of minor size. This means that findings related to the small classes need to be interpreted with caution, and need replication, whereas the findings related to the majority classes may be considered sturdier.
A limitation of the current study is the possibility of a non-response bias as those who had more severe strokes were more likely to respond in the primary study that this study recruited from. Interpretations of results are therefore relevant for individuals with mild and moderate stroke, but extend less well to stroke survivors with more severe strokes.
Conclusion
This study identified a variation of stable, deteriorating and improving trajectories of function between 1 year and 4 years post-stroke. Most of the participants belonged to a stable high-functioning trajectory, indicating that most individuals with predominantly mild or moderate strokes are doing well in terms of functioning 4 years post-stroke. Fatigue was more present in the deteriorating, mediocre, slightly improving, or stable low functional trajectories, and men or those living with a significant other had lower odds of belonging to these trajectories. The results indicate that a noteworthy portion of individuals vary in function beyond the first year post-stroke. Furthermore, functional recovery and improved HRQoL are possible even long-term post-stroke, which may reduce the burden of fatigue.
ACKNOWLEDGEMENTS
This study has been made possible by the Dam Foundation. The publication charges for this article have been funded by a grant from the publication fund of UIT The Arctic University of Norway.
Contributors: Angela Brady LeGrice, University of York St John, UK.
Ethical clearance: The Regional Committee for Research Ethics in Medicine and Health Sciences in North Norway approved the study (Institutional Protocol Number 2017/1966).
REFERENCES
- Larsen LP, Johnsen SP, Andersen G, Hjollund NH. Determinants of self-rated health three months after stroke. J Stroke Cerebrovasc Diseases 2016; 25: 1027–1034. DOI: 10.1016/j.jstrokecerebrovasdis.2015.12.014
- Mavaddat N, Van der Linde R, Savva GM, Brayne C, Mant J. What determines the self-rated health of older individuals with stroke compared to other older individuals? A cross-sectional analysis of the Medical Research Council Cognitive Function and Aging Study. BMC Geriatr 2013; 13: 85. DOI: 10.1186/1471-2318-13-85
- Fini NA, Holland AE, Keating J, Simek J, Bernhardt J. How physically active are people following stroke? systematic review and quantitative synthesis. Phys Ther 2017; 97: 707–717. DOI: 10.1093/ptj/pzx038
- Al-Qazzaz NK, Ali SH, Ahmad SA, Islam S, Mohamad K. Cognitive impairment and memory dysfunction after a stroke diagnosis: a post-stroke memory assessment. Neuropsychiatr Dis Treat 2014; 10: 1677–1691. DOI: 10.2147/ndt.S67184
- Kim JS. Post-stroke mood and emotional disturbances: pharmacological therapy based on mechanisms. J Stroke 2016; 18: 244–255. DOI: 10.5853/jos.2016.01144
- Boosman H, Schepers VP, Post MW, Visser-Meily JM. Social activity contributes independently to life satisfaction three years post stroke. Clin Rehabil 2011; 25: 460–467. DOI: 10.1177/0269215510388314
- Pedersen SG, Friborg O, Heiberg GA, Arntzen C, Stabel HH, Thrane G, et al. Stroke-Specific Quality of Life one-year post-stroke in two Scandinavian country-regions with different organisation of rehabilitation services: a prospective study. Disabil Rehabil 2021; 43: 3810–3820. DOI: 10.1080/09638288.2020.1753830
- Heiberg G, Friborg O, Pedersen SG, Thrane G, Stabel HH, Feldbaek Nielsen J, et al. Post-stroke health-related quality of life at 3 and 12 months and predictors of change in a Danish and Arctic Norwegian Region. J Rehabil Med 2020; 52: jrm00096. DOI: 10.2340/16501977-2716
- Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke 1999; 30: 1362–1369. DOI:10.1161/01.STR.30.7.1362
- Pedersen SG, Heiberg GA, Nielsen JF, Friborg O, Stabel HH, Anke A, et al. Validity, reliability and Norwegian adaptation of the Stroke-Specific Quality of Life (SS-QOL) scale. SAGE Open Med 2018; 6: 1–10. DOI: 10.1177/2050312117752031
- Pedersen SG, Lokholm M, Friborg O, Halvorsen MB, Kirkevold M, Heiberg G, et al. Visual problems are associated with long-term fatigue after stroke. J Rehabil Med 2023; 55: jrm00374. DOI: 10.2340/jrm.v55.4813
- Pucciarelli G, Lee CS, Lyons KS, Simeone S, Alvaro R, Vellone E. Quality of life trajectories among stroke survivors and the related changes in caregiver outcomes: a growth mixture study. Arch Phys Med Rehabil 2019; 100: 433–440. DOI: 10.1016/j.apmr.2018.07.428
- Pan JH, Song XY, Lee SY, Kwok T. Longitudinal analysis of quality of life for stroke survivors using latent curve models. Stroke 2008; 39: 2795–2802. DOI: 10.1161/STROKEAHA.108.515460
- van Mierlo M, van Heugten C, Post MWM, Hoekstra T, Visser-Meily A. Trajectories of health-related quality of life after stroke: results from a one-year prospective cohort study. Disabil Rehabil 2018; 40: 997–1006. DOI: 10.1080/09638288.2017.1292320
- Winter Y, Klotsche J, Ringel F, Spottke A, Klockgether T, Urbach H, et al. Characterizing the individual course of health-related quality of life after subarachnoid haemorrhage: latent growth mixture modelling. J Stroke Cerebrovasc Dis 2023; 32: 106913. DOI: 10.1016/j.jstrokecerebrovasdis.2022.106913
- Forslund MV, Perrin PB, Sigurdardottir S, Howe EI, van Walsem MR, Arango-Lasprilla JC, et al. Health-related quality of life trajectories across 10 years after moderate to severe traumatic brain injury in Norway. J Clin Med 2021; 10. DOI: 10.3390/jcm10010157
- Elf M, Eriksson G, Johansson S, von Koch L, Ytterberg C. Self-reported fatigue and associated factors six years after stroke. PloS One 2016; 11: 1–9. DOI: 10.1371/journal.pone.0161942
- Sand KM, Midelfart A, Thomassen L, Melms A, Wilhelm H, Hoff JM. Visual impairment in stroke patients – a review. Acta Neurol Scand Suppl 2013; Suppl 127: 52–56. DOI: 10.1111/ane.12050
- Radman N, Staub F, Aboulafia-Brakha T, Berney A, Bogousslavsky J, Annoni JM. Poststroke fatigue following minor infarcts: a prospective study. Neurology 2012; 79: 1422–1427. DOI: 10.1212/WNL.0b013e31826d5f3a
- Ferguson SL, G. Moore EW, Hull DM. Finding latent groups in observed data: A primer on latent profile analysis in Mplus for applied researchers. Int J Behav Dev 2020; 44: 458–468. DOI: 10.1177/0165025419881721
- Lerdal A, Bakken LN, Kouwenhoven SE, Pedersen G, Kirkevold M, Finset A, et al. Poststroke fatigue – a review. J Pain Symptom Manage 2009; 38: 928–949. DOI: 10.1016/j.jpainsymman.2009.04.028
- Penner IK, Paul F. Fatigue as a symptom or comorbidity of neurological diseases. Nat Rev Neurol 2017; 13: 662–675. DOI: 10.1038/nrneurol.2017.117
- Kjeverud A, Østlie K, Schanke AK, Gay C, Thoresen M, Lerdal A. Trajectories of fatigue among stroke patients from the acute phase to 18 months post-injury: a latent class analysis. PloS one 2020; 15: e0231709. DOI: 10.1371/journal.pone.0231709
- Lerdal A, Wahl A, Rustoen T, Hanestad BR, Moum T. Fatigue in the general population: a translation and test of the psychometric properties of the Norwegian version of the fatigue severity scale. Scand J Public Health 2005; 33: 123–130. DOI: 10.1080/14034940410028406
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46: 1121–1123. DOI: 10.1001/archneur.1989.00520460115022
- Cumming TB, Packer M, Kramer SF, English C. The prevalence of fatigue after stroke: a systematic review and meta-analysis. Int J Stroke 2016; 11: 968–977. DOI: 10.1177/1747493016669861
- Ozyemisci-Taskiran O, Batur EB, Yuksel S, Cengiz M, Karatas GK. Validity and reliability of fatigue severity scale in stroke. Top Stroke Rehabil 2019; 26: 122–127. DOI: 10.1080/10749357.2018.1550957
- Boosman H, Passier PE, Visser-Meily JM, Rinkel GJ, Post MW. Validation of the Stroke Specific Quality of Life scale in patients with aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 2010; 81: 485–489. DOI: 10.1136/jnnp.2009.184960
- Muus I, Williams LS, Ringsberg KC. Validation of the Stroke Specific Quality of Life Scale (SS-QOL): test of reliability and validity of the Danish version (SS-QOL-DK). Clin Rehabil 2007; 21: 620–627. DOI: 10.1177/0269215507075504
- Muthén LK, Muthén B. Mplus User’s Guide. (1998–2017) 2017 [cited 2023 Jan 16]; Version 8. Available from: https://www.statmodel.com/download/usersguide/MplusUserGuideVer_8.pdf
- Nylund-Gibson K, Choi AY. Ten frequently asked questions about latent class analysis. Transl Issues Psychol Sci 2018; 4: 440–461. DOI: 10.1037/tps0000176
- Schwarz G. Estimating the Dimension of a model. Ann Stat 1978; 6: 461–464.
- McLachlan G, Peel D. Finite Mixture Models. 1. Aufl. ed. Hoboken: Hoboken: Wiley-Interscience; 2004. DOI: 10.1002/0471721182
- Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Modeling 2007; 14: 535–569. DOI: 10.1080/10705510701575396
- Aamodt EB, Alnaes D, de Lange AG, Aam S, Schellhorn T, Saltvedt I, et al. Longitudinal brain age prediction and cognitive function after stroke. Neurobiol Aging 2023; 122: 55–64. DOI: 10.1016/j.neurobiolaging.2022.10.007
- She R, Yan Z, Hao Y, Zhang Z, Du Y, Liang Y, et al. Comorbidity in patients with first-ever ischemic stroke: Disease patterns and their associations with cognitive and physical function. Front Aging Neurosci 2022; 14: 887032. DOI: 10.3389/fnagi.2022.887032
- Berget AM, Moen VP, Hustoft M, Eide GE, Skouen JS, Strand LI, et al. Long-term change and predictors of change in physical and mental function after rehabilitation: a multi-centre study. J Rehabil Med 2023; 55: jrm00358. DOI: 10.2340/jrm.v55.2809
- van de Port IG, Kwakkel G, van Wijk I, Lindeman E. Susceptibility to deterioration of mobility long-term after stroke: a prospective cohort study. Stroke 2006; 37: 167–171. DOI: 10.1161/01.STR.0000195180.69904.f2
- Lerdal A, Bakken LN, Rasmussen EF, Beiermann C, Ryen S, Pynten S, et al. Physical impairment, depressive symptoms and pre-stroke fatigue are related to fatigue in the acute phase after stroke. Disabil Rehabil 2011; 33: 334–342. DOI: 10.3109/09638288.2010.490867
- Choi-Kwon S, Kim JS. Poststroke fatigue: an emerging, critical issue in stroke medicine. Int J Stroke 2011; 6: 328–336