ORIGINAL REPORT
FEASIBILITY AND POTENTIAL EFFECTS OF ROBOT-ASSISTED PASSIVE RANGE OF MOTION TRAINING IN COMBINATION WITH CONVENTIONAL REHABILITATION ON HAND FUNCTION IN PATIENTS WITH CHRONIC STROKE
Chia-Yu HSU1,2, MD, PhD, Chu-Ming WU1, MD, Chieh-Cheng HUANG3, MD, Hung-Hai SHIE1, MSc and Yuh-Show TSAI2, PhD
From the 1Department of Rehabilitation Medicine, Ten-Chan General Hospital, 2Department of Biomedical Engineering, Chung Yuan Christian University and 3Department of Neurology, Ten-Chan General Hospital, Taoyuan City, Taiwan
Objective: To assess the effects of exoskeleton robot-assisted passive range of motion for induction training in combination with conventional hand rehabilitation in patients with chronic stroke.
Design: Single-cohort feasibility study.
Subjects: Chronic stroke with severe upper extremity hemiparesis.
Methods: Thirty sessions of therapy over a period of 10 weeks. Each session started with 30 min robot-assisted passive range of motion for the hand, followed by 30 min conventional hand rehabilitation. The Fugl-Meyer Assessment for upper extremity, arm subscore of Motricity Index, Functional Independence Measure and Fugl-Meyer assessment for sensation (Fugl-Meyer assessment-sensory) were conducted at pre-intervention (pre) and after the 16th (16-post) and 30th (30-post) sessions of interventions.
Results: Twelve patients with chronic stroke were recruited. The Fugl-Meyer assessment for upper extremity (16-post vs 30-post, p = 0.011), arm subscore of Motricity Index (pre vs 30-post, p = 0.012) and Functional Independence Measure (pre vs 30-post, p = 0.007; 16-post vs 30-post, p = 0.016) improved significantly after the therapy. However, FMA-sensory did not change significantly.
Conclusion: Exoskeleton robot-assisted passive range of motion of the hand using an exoskeleton can be considered as an induction therapy before starting conventional therapy for hand rehabilitation in patients with chronic stroke. Further randomized control trials are needed to verify the therapeutic benefits.
LAY ABSTRACT
Motor recovery of hand dexterity is challenging during the chronic phase of stroke. Patients achieve different levels of hand function during the acute or subacute phase of stroke. Those receiving conventional physical therapy during the chronic phase of stroke usually experience difficulty in hand dexterity improvement after achieving motor recovery plateau. This pilot study investigated the effects of robot-assisted passive range of motion training in combination with conventional rehabilitation on hand function in a cohort of patients with chronic stroke who underwent follow-up at an outpatient rehabilitation clinic. The affected upper extremity function, strength and general function improved significantly after the therapy for the 12 patients recruited to this study. Using robot-assisted passive range of motion training as an induction therapy in combination with conventional rehabilitation may be beneficial for patients with chronic stoke who have impairment of hand function.
Key words: robot; rehabilitation; hand; stroke; wearable exoskeleton.
Citation: J Rehabil Med 2022; 54: jrm00323. DOI: https://dx.doi.org/10.2340/jrm.v54.1407
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jul 5, 2022; Epub ahead of print: Aug 4, 2022; Published: Aug 26, 2022
Correspondence address: Yuh-Show Tsai, Department of Biomedical Engineering, Chung Yuan Christian University, No. 200, Zhongbei Road, Zhongli District, Taoyuan City 320, Taiwan (R.O.C.). E-mail: yuhshow@cycu.edu.tw
Competing interests and funding: The authors have no conflicts of interest to declare.
The numbers of incident strokes, prevalent stroke survivors and disability-adjusted life-years lost due to stroke are large and have increased since 1990 (by 68%, 84% and 12%, respectively) (1). In Netherlands, patients at 6 months after ischemic stroke, 67% of patients are left with impairments of upper extremity (UE) movement and only 11.6% reach complete functional recovery in hand dexterity (2); these impairments typically present as hand weakness and abnormal contractions of the UE. Studies in Netherlands and UK have reported hemiparetic patients who have not regained hand function 6 months after a stroke (2–5). Motor recovery of hand dexterity is slow and challenging, leading to limited hand activities and occupational disability. Therefore, facilitating post-stroke motor recovery of hand dexterity is crucial for stroke rehabilitation.
Repeated exercises of the affected hand have proven beneficial for hand function recovery in patients with stroke (6). Continuous passive motion (CPM) exercise, which is a repetitive movement, has been applied for patients with contractures (7). It is commonly implemented for patients with stroke with spasticity before occupational hand therapy. Studies have also demonstrated that passive movement alters the inhibitory state of the central nervous system and further affects motor responses (8, 9), which may facilitate activity-dependent plasticity (10). However, clinical evidence favouring the use of passive motion for motor recovery in patients after stroke is not sufficient.
The high intensity of sensorimotor end-effector robot-aided training targeting the affected shoulder and elbow has resulted in improved UE function in patients with chronic stroke (11–16). Most of these groups studying robot-aided training showed larger improvements in the proximal UE, which was compatible with the principle of training specificity (17). However, there have been few studies of the use of a wearable exoskeleton robotic hand device for dexterity training of the affected hand (18). Studies using robot-assisted CPM report it as beneficial for arm- and hand-function improvement in patients after stroke (19, 20); the provision of a training programme for the distal part of UE warrants investigation.
The aim of this study is to evaluate the feasibility and efficacy of robot-assisted hand rehabilitation for improving functional abilities of the affected hand in patients with chronic hemiplegic stroke. A powered exoskeleton hand was used to provide automatic passive range of motion (PRoM) exercise in patients who were more than 6 months since stroke onset. The study hypothesis is that adding robot-assisted hand exercise as an induction therapy to conventional rehabilitation therapy could improve the function of the paretic hand.
METHODS
Patients
A total of 12 patients with stroke were recruited at the Ten-Chan General Hospital, Taoyuan City, Taiwan, from September 2017 to June 2019. Inclusion criteria were: age > 20 years; diagnosis of haemorrhagic or ischaemic stroke with severe UE hemiparesis (Brunnstrom recovery stage I–III). Exclusion criteria were: severe pain and instability in the wrist of the affected arm; severe cognitive impairment, aphasia, hemispatial neglect and apraxia; and joint contractures > 20° in the affected hand. The experimental protocol was approved by the institutional review board of the hospital. Written informed consent was obtained from all patients.
Robotic hand device
The wearable exoskeleton robotic hand device (HS 001, Rehabotics Medical Technology Corporation, Hsinchu County, Taiwan) was used for this study. The device provides 3 finger movement models, which are single-finger, 5-fingers and mirror-guided models. Through the exoskeletal hand, the patient’s affected hand finger could be moved by the device to perform flexion/extension movements. The single- and 5-finger modes (the movement speeds of finger extension and flexion are approximately 3 s, respectively) were used to conduct PRoM exercises for patients (Fig. 1); each mode could achieve a maximum of 15 repetitions per min.
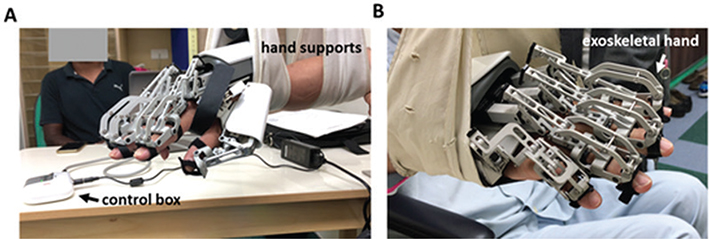
Fig. 1. Wearable exoskeleton robotic hand device. (A) Left-side view, (B) right-side view.
Training programme
The patients were provided with 30 sessions of therapy by a well-trained occupational therapist over a period of 10 weeks, with each session starting with 30 min of passive robotic hand therapy followed by 30 min of conventional hand rehabilitation. In the robotic hand therapy, each finger would receive 3 min single-finger mode and 15 min 5-finger mode with a maximum of 270 repetitions in total. In the conventional hand training, the therapist conducted one-on-one individualized programmes focused on arm and hand function. Treatment included function-oriented specific tasks, such as reach, grasp, transport and release of various objects between different targets. All patients underwent basic rehabilitation following the guidelines according to the Bobath concept (21).
Outcome assessments
Hand motor function was assessed before (pre), after the 16th training session (16-post) and after the 30th training session (30-post). The primary outcome was the change in the Fugl-Meyer assessment (FMA), which was used to evaluate sensorimotor recovery in patients with particular attention to the hand and wrist section (maximum score 66 in motor function and 24 in sensory function) to assess the functional capacity of the affected hand (22).
For other assessments, the arm subscore of Motricity Index (MI) scale was used to measure strength in UE after stroke. The weighted score based on the ordinal 6-point scale of the Medical Research Council was used to measure the maximum isometric muscle strength and the motor recovery rate of the patients (100% = maximum MI) (23). Functional Independence Measure scale (FIM) was used to assess the degree of independence and need-of-assistance in basic activities of daily living at the time of enrolment and at the end of the study. FIM is an 18-item ordinal scale, rated from 1 (total dependence) to 7 (total independence) per item; furthermore, 13 items of this scale and the subscale motor-FIM were used to evaluate motor disability (24). All the assessments were performed by an independent occupational therapist.
Finally, the visual analogue scale (VAS) was used to access the level of operator difficulty for the occupational therapist in managing the device (0 (extremely simple)–10 (extremely difficult)) (25). The therapist was required to report any adverse events occurring during the study with respect to the use of the robotic hand.
Statistical analysis
Friedman’s test and multiple Wilcoxon signed-ranks tests (IBM, SPSS Inc. Version 12.0. Chicago, Illinois, USA) were performed to investigate hand functional changes in pre, 16-post and 30-post interventions. All data are represented as median (interquartile range). The significance levels in Wilcoxon signed rank tests were set to 0.0167 with Bonferroni’s adjustment.
RESULTS
The demographic and clinical characteristics of the patients before intervention are shown in Table I. Twelve patients with chronic stroke were recruited for the study; 6 with cerebral haemorrhage, 4 cerebral infarction, 1 atherothrombotic cerebral embolism, and 1 cardiogenic cerebral embolism stroke. The median age of patients was 55.5 years (15.5 years), and median time from stroke onset was 12.0 months (15.0 months).
All patients completed the training programme. Data for FMA-UE, MI, FIM and FMA-sensory at pre, 16-post and 30-post assessments are shown in Table II, and the mean of clinical assessment scores are shown in Table III. Motor function of the UE in all patients was severely impaired (FMA-UE = 13.5 (12.0)) at pre-assessment. At 30-post with robot-assisted PRoM exercises for the hand and finger followed by conventional occupational hand function training, the FMA-UE had improved significantly (16-post: 13.5 (17.0), 30-post: 16.5 (15.0), n = 12, p = 0.011).
| Assessment | Pre. | 16-post | 30-post | Friedman’s test, p | Wilcoxon signed-rank test, p |
| FMA-UE-motor | 13.5 (12.0) | 13.5 (17.0) | 16.5 (15.0) | 0.004 | 0.048a 0.04b 0.011c |
| MI-UE | 26.5 (29.0) | 36.5 (28.5) | 37.0 (22.5) | 0.001 | 0.068 0.012 0.027 |
| FIM | 82.5 (57.5) | 85.5 (36.5) | 85.5 (35.5) | < 0.001 | 0.028 0.007 0.016 |
| FMA-UE sensation | 22.0 (10.5) | 23.5 (12.0) | 23.5 (12.0) | 0.002 | 0.027 0.017 0.157 |
| Friedman’s test with α=0.05; a: pre vs. 16-post; b: pre vs 30-post; c: 16-post vs 30-post. | |||||
| Data are presented as median (interquartile range), FMA: Fugl–Meyer Assessment; MI: Motricity Index; FIM: Functional Independence Measure scale, UE: upper extremity. | |||||
UE strength measured using arm subscore of MI at pre, 16-post and 30-post interventions improved significantly after the training programme (pre: 26.5 (29.0), 30-post: 37.0 (22.5), n = 12, p = 0.012). Functional independence was evaluated using FIM assessment; function significantly improved during the training (pre: 82.5 (57.5), 16-post: 85.5 (36.5), 30-post: 85.5 (35.5); pre vs 30-post, p = 0.007; 16-post vs 30-post, p = 0.016). However, sensory function did not change significantly (FMA-sensory scores at pre: 22.0 (10.5), 30-post: 23.5 (12.0), n = 12, p = 0.017).
Finally, the study evaluated the feasibility of the use of the robot device in patients. VAS results indicated that the occupational therapist could use and understand the robotic device easily (evaluated at pre: 3.0 (2.0), 16-post: 2.5 (2.75), 30-post: 2.0 (2.75); pre vs 30-post, p = 0.016) (Fig. 2). No adverse events were reported.
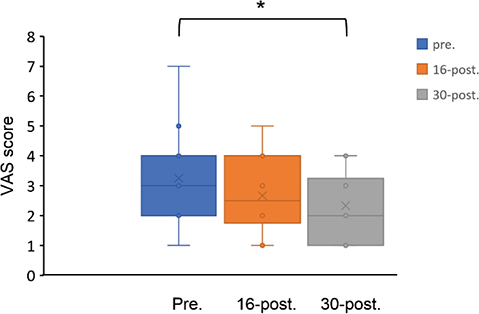
Fig. 2. Box and whisker plot of visual analogue scale (VAS) was rated by the occupational therapist who assessed the difficulty in managing the robot device for the participants (0 (extremely simple)–10 (extremely difficult)). *p<0.016, Wilcoxon signed-rank test, VAS: visual analogue scale, pre: pre-intervention, 16-post: after the 16th session of intervention, 30-post: after the 30th session of intervention.
DISCUSSION
This study combined robot-assisted PRoM exercises and conventional hand training for patients with stroke onset longer than 6 months prior to an outpatient rehabilitation setting. The findings demonstrate that the combinational therapy significantly improved affected hand function and strength, as well as overall function, as indicated by FMA-UE, MI and FIM. The results also showed high feasibility of use of the robotic device for patients, as indicated by the VAS. A benefit of the robot-assisted PRoM exercise is that, once the therapist has set up the robotic device, the patient can be left alone with the device, and this reduces the need for one-on-one attention while performing repetitive motions of a single PRoM exercise of the hand. Finally, no adverse effects were reported in this study.
Robotic systems can provide standardized, repetitive, reproducible and interactive forms of hand training. The advantages of using robots in neurorehabilitation includes favouring attention, boosting motivation and adherence to treatment (26); they are beneficial for multi-sensory and sensorimotor integration (27). Studies showed that robot-assisted therapy is safe and well tolerated and has a positive impact on muscle strength and function of the paretic arm (28–30). Furthermore, robot-assisted exercises can optimize labour efficiency by allowing for unsupervised practise of highly repetitive exercises that would otherwise require direct supervision; for example, flexion/extension exercise of warm-up before functional training. The positive results of the current study with the use of the exoskeleton robotic hand training were in agreement with those of previous studies using a soft robotic hand (31), Gloreha device (32) and Amadeo device (33) for patients with chronic stroke. The current study found that adding robotic hand training before conventional rehabilitation could significantly improve UE function and muscle strength, which were in accordance with other studies using electromyography-driven exoskeleton hand robot devices (34, 35). For FMA-UE motor scale, although the median improvement from baseline to follow-up was 3 points on the group level, which is below what is considered of minimal clinical importance, patients 1, 4, and 9 had better improvement, up to 6 points. This suggests that the robotic-assisted hand training may have potential therapeutic effects is this subgroup of stroke patients who were relatively younger (< 60 years old), onset less than 2 years and intracerebral haemorrhage. A simple training programme using repetitive passive motions was provided as an induction therapy before conventional occupational hand therapy according to the Bobath concept (36). First, it helps the paretic hand complete a hand movement and stretches hand muscles and soft tissue to reduce spasticity (37) and prevent contracture (38). Secondly, the patient could achieve the training task more easily in the following rehabilitation exercise while potentially increasing the number of repetitions, and hence the intensity of practice post-stroke (39). The enhanced somatosensory input may help motor planning and result in better hand movement with faster motor recovery and expedited motor learning, which are considered related to neuroplasticity in the lesioned brain areas (6). Purely passive movement could activate some cortical areas similarly to voluntary movements (40). Thirdly, neurophysiological studies demonstrated that PRoM exercises may decrease the inhibition effect in the affected brain areas (9), facilitating the neural functional compensation to the damaged brain areas. The use of an exoskeleton robotic hand with structure design of individual finger modules and metacarpophalangeal, proximal and distal interphalangeal joints can provide individual finger movements. The video-guided passive motion hand exercise has also demonstrated motor improvement in patients with stroke (17). The mechanism using passive motion as an introduction to the following convention hand training warrants further investigation.
Although the results showed significant changes in outcomes, this study has some limitations. First, there was no control group. The single-cohort study design did not allow any distinction between the relative contribution of robotic and conventional training. Validation for examining the treatment effect is required. Secondly, the sample size was small; a larger sample size is needed to confirm the results and long-term benefits. Thirdly, it was difficult to control the attention of the patient. Further larger, high-quality, randomized controlled studies are needed to verify the therapeutic benefits of this technique.
CONCLUSION
Exoskeleton robot-assisted PRoM hand training may be a good induction therapy before conventional therapy for hand rehabilitation of patients with chronic stroke. Further randomized control trials are needed to verify the therapeutic benefits of this technique.
ACKNOWLEDGEMENTS
The authors thank all patients who participated in the study. This study was supported by grant number 106005 of Ten-Chan General Hospital.
REFERENCES
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. The Lancet 2014; 383: 245–255.
- Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003; 34: 2181–2186.
- Heller A, Wade DT, Wood VA, Sunderland A, Hewer RL, Ward E. Arm function after stroke: measurement and recovery over the first three months. J Neurol Neurosurg Psychiatr 1987; 50: 714–719.
- Wade D, Langton-Hewer R, Wood VA, Skilbeck C, Ismail H. The hemiplegic arm after stroke: measurement and recovery. J Neurol Neurosurg Psychiatr 1983; 46: 521–524.
- Sunderland A, Tinson D, Bradley L, Hewer RL. Arm function after stroke. An evaluation of grip strength as a measure of recovery and a prognostic indicator. J Neurol Neurosurg Psychiatr 1989; 52: 1267–1272.
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA 2006; 296: 2095–2104.
- Liebesman J, Cafarelli E. Physiology of range of motion in human joints: a critical review. Crit Rev Phys Rehabil Med 1994; 6: 131.
- Lewis GN, Byblow WD. Modulations in corticomotor excitability during passive upper-limb movement: is there a cortical influence? Brain Res 2002; 943: 263–275.
- Lindberg PG, Schmitz C, Engardt M, Forssberg H, Borg J. Use-dependent up- and down-regulation of sensorimotor brain circuits in stroke patients. Neurorehabil Neural Repair 2007; 21: 315–326.
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain 2001; 124: 1171–1181.
- Ferraro M, Palazzolo J, Krol J, Krebs H, Hogan N, Volpe B. Robot-aided sensorimotor arm training improves outcome in patients with chronic stroke. Neurology 2003; 61: 1604–1607.
- Lambercy O, Dovat L, Yun H, Wee SK, Kuah CW, Chua KS, et al. Effects of a robot-assisted training of grasp and pronation/supination in chronic stroke: a pilot study. J Neuroeng Rehabil 2011; 8: 1–12.
- Cho KH, Song W-K. Robot-assisted reach training with an active assistant protocol for long-term upper extremity impairment poststroke: a randomized controlled trial. Arch Phys Med Rehabil 2019; 100: 213–219.
- Lum PS, Burgar CG, Shor PC, Majmundar M, Van der Loos M. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Arch Phys Med Rehabil 2002; 83: 952–959.
- Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med 2010; 362: 1772–1783.
- Fazekas G, Horvath M, Troznai T, Toth A. Robot-mediated upper limb physiotherapy for patients with spastic hemiparesis: a preliminary study. J Rehabil Med 2007; 39: 580–582.
- Daly JJ, Hogan N, Perepezko EM, Krebs HI, Rogers JM, Goyal KS, et al. Response to upper-limb robotics and functional neuromuscular stimulation following stroke. J Rehabil Res Dev 2005; 42: 723–736.
- Ockenfeld C, Tong RK, Susanto EA, Ho S-K, Hu X-l. Fine finger motor skill training with exoskeleton robotic hand in chronic stroke: Stroke rehabilitation. 2013 IEEE 13th International Conference on Rehabilitation Robotics (ICORR); Seattle, WA, USA: IEEE, 2013; p. 1–4.
- Volpe BT, Ferraro M, Lynch D, Christos P, Krol J, Trudell C, et al. Robotics and other devices in the treatment of patients recovering from stroke. Curr Neurol Neurosci Rep 2005; 5: 465–470.
- Villafañe JH, Taveggia G, Galeri S, Bissolotti L, Mullè C, Imperio G, et al. Efficacy of short-term robot-assisted rehabilitation in patients with hand paralysis after stroke: a randomized clinical trial. Hand 2018; 13: 95–102.
- Lennon S, Ashburn A. The Bobath concept in stroke rehabilitation: a focus group study of the experienced physiotherapists’ perspective. Disabil Rehabil 2000; 22: 665–674.
- Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 1975; 7: 13–31.
- Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. Eur Neurol 1980; 19: 382–389.
- Granger CV, Cotter AC, Hamilton BB, Fiedler RC. Functional assessment scales: a study of persons after stroke. Arch Phys Med Rehabil 1993; 74: 133–138.
- Downie W, Leatham P, Rhind V, Wright V, Branco J, Anderson J. Studies with pain rating scales. Ann Rheumat Dis 1978; 37: 378–381.
- Colombo R, Pisano F, Mazzone A, Delconte C, Micera S, Carrozza MC, et al. Design strategies to improve patient motivation during robot-aided rehabilitation. J Neuroengin Rehabil 2007; 4: 1–12.
- Nef T, Mihelj M, Riener R. ARMin: a robot for patient-cooperative arm therapy. Med Biol Eng Comput 2007; 45: 887–900.
- Masiero S, Poli P, Rosati G, Zanotto D, Iosa M, Paolucci S, et al. The value of robotic systems in stroke rehabilitation. Expert Rev Med Dev 2014; 11: 187–198.
- Orihuela-Espina F, Roldán GF, Sánchez-Villavicencio I, Palafox L, Leder R, Sucar LE, et al. Robot training for hand motor recovery in subacute stroke patients: a randomized controlled trial. J Hand Therapy 2016; 29: 51–57.
- Vanoglio F, Bernocchi P, Mulè C, Garofali F, Mora C, Taveggia G, et al. Feasibility and efficacy of a robotic device for hand rehabilitation in hemiplegic stroke patients: a randomized pilot controlled study. Clin Rehabil 2017; 31: 351–360.
- Shi XQ, Heung HL, Tang ZQ, Li Z, Tong KY. Effects of a soft robotic hand for hand rehabilitation in chronic stroke survivors. J Stroke Cerebrovasc Dis 2021; 30: 105812.
- Lee H-C, Kuo F-L, Lin Y-N, Liou T-H, Lin J-C, Huang S-W. Effects of robot-assisted rehabilitation on hand function of people with stroke: a randomized, crossover-controlled, assessor-blinded study. Am J Occupat Ther 2021; 75: 1–11.
- Calabrò RS, Accorinti M, Porcari B, Carioti L, Ciatto L, Billeri L, et al. Does hand robotic rehabilitation improve motor function by rebalancing interhemispheric connectivity after chronic stroke? Encouraging data from a randomised-clinical-trial. Clin Neurophysiol 2019; 130: 767–780.
- Hu X, Tong K, Wei X, Rong W, Susanto E, Ho S. The effects of post-stroke upper-limb training with an electromyography (EMG)-driven hand robot. Journal of Electromyog Kinesiol 2013; 23: 1065–1074.
- Singh N, Saini M, Kumar N, Srivastava MP, Mehndiratta A. Evidence of neuroplasticity with robotic hand exoskeleton for post-stroke rehabilitation: a randomized controlled trial. J Neuroeng Rehabil 2021; 18: 1–15.
- Yue Z, Zhang X, Wang J. Hand rehabilitation robotics on poststroke motor recovery. Behav Neurol 2017; 2017: 3908135.
- Carey JR. Manual stretch: effect on finger movement control and force control in stroke subjects with spastic extrinsic finger flexor muscles. Arch Phys Med Rehabil 1990; 71: 888–894.
- Williams P. Use of intermittent stretch in the prevention of serial sarcomere loss in immobilised muscle. Ann Rheumat Dis 1990; 49: 316–317.
- Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PloS One 2014; 9: e87987.
- Carel C, Loubinoux I, Boulanouar K, Manelfe C, Rascol O, Celsis P, et al. Neural substrate for the effects of passive training on sensorimotor cortical representation: a study with functional magnetic resonance imaging in healthy subjects. J Cereb Blood Flow Metab 2000; 20: 478–484.