ORIGINAL REPORT
RESPONSIVENESS OF THE INTERNATIONAL CLASSIFICATION OF FUNCTIONING, DISABILITY AND HEALTH (ICF) CLINICAL FUNCTIONING INFORMATION TOOL (ClinFIT) IN ROUTINE CLINICAL PRACTICE IN AN AUSTRALIAN INPATIENT REHABILITATION SETTING
Bhasker AMATYA, DMedSc, MPH, MD1,2,3*, Alaeldin ELMALIK, MBBS, FAFRM (RACP)1,2,3, Krystal SONG PHD, MBBS, FAFRM (RACP)1,2,3, Su Yi LEE, PHD, MBBS, FAFRM (RACP)1,2,3, Mary P. GALEA, PHD, BAPPSCI (PHYSIO), BA, GRAD DIP PHYSIO, Grad Dip NEUROSCI1,2,3 and Fary KHAN, MBBS, MD, FAFRM (RACP)1,2,3,4*
From the 1Department of Rehabilitation Medicine, Royal Melbourne Hospital, 2Department of Medicine (Royal Melbourne Hospital), University of Melbourne, Parkville, 3Australian Rehabilitation Research Centre, Royal Melbourne Hospital, Parkville and 4School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia
Objective: To examine the responsiveness of the International Classification of Functioning, Disability and Health (ICF) Clinical Functioning Information Tool (ClinFIT) in routine clinical practice in an Australian context.
Methods: A prospective observational study with consecutive recruitment of inpatients at a tertiary rehabilitation facility. The assessments were at admission (T0), discharge (T1) and 3-month post-discharge (T2), using the following questionnaires: ClinFIT, Functional Independence Measure (FIM) and European Quality of Life (EQ-5D-5L). Extension Indices (EI) were calculated for the ClinFIT set, and responsiveness measured as a change in scores over time. The association between FIM and ClinFIT scores was explored.
Results: Participants (n = 91, mean age 66.8±13.0 years, 52% male, 48% following stroke) reported ≥ 1 issue related to ClinFIT categories. ClinFIT total raw scores improved significantly across all health conditions compared with T0 (median (interquartile range): 196 (110, 228)) at both T1: 69 (37, 110); p < 0.001 and T2: 46.5 (20.8, 77); p < 0.001, with a medium effect size (r = 0.61 for both). There were significant changes in EI in the entire ClinFIT set from T0 to T1, and from T0 to T2 (p < 0.001 for both), with small to medium effect sizes. Analyses confirmed significant correlation in improvements between ClinFIT and FIM scores.
Conclusion: ClinFIT is useful in evaluating patient functioning and can detect changes in functioning over time and across different health conditions.
LAY ABSTRACT
Regular patient evaluation and clinical assessment is needed to maximize positive outcomes from rehabilitation intervention. This prospective study assessed the responsiveness of the International Classification of Functioning, Disability and Health (ICF) – Clinical Functioning Information Tool (ClinFIT) for the assessment of functioning in an inpatient rehabilitation facility in Australia. Overall, 91 patients with different health conditions (majority with stroke) were assessed at admission, discharge, and at 3 months after discharge, using the ClinFIT set, and compared with another routinely used instruments, the Functional Independence Measure (FIM). The findings showed that the ClinFIT set is useful in evaluating patients’ functioning over time across different health conditions.
Key words: International Classification of Functioning, Disability and Health; ICF; rehabilitation; function; disability; participation; impairment.
Citation: J Rehabil Med 2022; 54: jrm00159. DOI: http://dx.doi.org/10.2340/jrm.v54.159
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Feb 4, 2022; Epub ahead of print: Feb 17, 2022; Published: Apr 20, 2022
Correspondence address: Bhasker Amatya, Department of Rehabilitation Medicine, Royal Melbourne Hospital, 34–54 Poplar Road, Parkville, Victoria 3052, Australia. E-mail: bhasker.amatya@mh.org.au
Competing interests and funding: All authors are active members of the International Society of Physical and Rehabilitation Medicine (ISPRM) and Fary Khan is an Executive Member of the ISPRM ClinFIT Task Force. The authors have no other conflicts of interest to declare.
This study was supported by internal resources of the Rehabilitation Department, Royal Melbourne Hospital, Australia. No commercial party having a direct financial interest in the results of the research supporting this article have or will confer a benefit upon the authors or upon any organization with which the authors are associated.
Rehabilitation is considered a key health strategy for the 21st century to optimize “functioning” of persons with a health condition experiencing or likely to experience disability (1). Rehabilitation outcomes are dependent on a person’s level of disability, and are negatively influenced by various factors (disease duration, cognitive impairment, cerebellar dysfunction, sphincteric symptoms, disease-specific factors, environment and adaptive mechanisms) (2, 3). Regular patient evaluation and clinical assessment is needed to maximize positive outcomes, especially over time. The ongoing clinical improvement/deterioration process itself is of interest to clinicians, policy developers, government, and consumers. Rehabilitation is a complex process where assessment and treatment are case-sensitive and delivered in an iterative “learning” approach. Rehabilitation is thus difficult to standardize in terms of definitions, settings, content type, and intensity (4, 5). Hence, the evaluation of the efficacy and effectiveness of the rehabilitation process is challenging.
The aim of any rehabilitation intervention is to improve functioning and allow assessment of clinical outcomes along the patient journey (hospital to community), for strategic decision-making in clinical practice (6). This information allows for health service planning and management, resource allocation, and development of policies and programmes. A wide range of objective (physician-based) and subjective (patient-reported) outcomes measures are available to assess clinical and other outcomes in rehabilitation. In Australia and New Zealand, the Australasian Rehabilitation Outcomes Centre (AROC) holds a centralized registry, which gathers a standard set of information on both process and outcomes for every person admitted for inpatient rehabilitation (8). It uses the Functional Independence Measure (FIM) as the primary outcome measure (8–10), whilst the change in FIM scores from admission to discharge and “FIM efficiency” (FIM gain/length of stay) are surrogate markers for service efficiency and subsequent funding. Although this FIM-based classification model has the advantage of simplicity, and minimizes the burden of data collection, many argue that it is too simplistic an approach for adequate evaluation in the context of complex neurological rehabilitation, where a range of physical, cognitive and behaviour-related-impairments, disabilities and restrictions in participation also play a role (10, 11). Some FIM items are more predictive of care requirements than others, and this may vary across different clinical conditions, requiring item-level analysis to understand the impact of rehabilitation within these conditions (10, 11). Moreover, due to the floor and ceiling effects of FIM scores, “FIM efficiency” is a less sensitive indicator of cost-effectiveness (13, 14).
Despite the use of various outcome measures in rehabilitation settings, there is little consensus on how various health domains should be measured. Currently used outcome measures vary in scope and mode of measurement. The concepts of health and functional status, well-being, activity and participation, and quality of life (QoL), are often applied interchangeably in clinical practice and research, which makes it difficult to understand, interpret and compare study results (15). There is no standardized and/or generic information on relevance, coverage and clinical applicability of these measures in complex rehabilitation populations. Importantly, many proprietary instruments are not affordable for routine clinical practice, especially in low resource countries, where the need is greatest. A number of new measures are emerging and issues of what, when and how to measure patient outcomes in rehabilitation remain fundamental to the debate. Selecting the most appropriate and affordable outcome measure for use in routine clinical settings, and outcome research globally, is a priority.
ICF Clinical Functioning Information Tool (ClinFIT)
In 2001, the World Health Organization (WHO) endorsed a universal reference framework, the International Classification of Functioning, Disability and Health (ICF), to describe “functioning” in relation to the health condition of an individual (16). This framework considers disability as the umbrella term for impairments, activity limitation and participation restriction and views disability and functioning as an outcome of the interaction between health conditions (diseases, disorders and injuries) and contextual factors. The ICF classification system comprises a taxonomy of life areas of possible relevance to people with health problems, coded into over 1,400 hierarchically ordered categories. The ICF is organized into 2 parts “Functioning and Disability”, with the components “Body functions and structures (b and s)” and “Activities and participation (d)”; and “Contextual factors” with the components ‘Environmental factors’ (e) and Personal factors (not yet classified). Initially, the WHO prioritized the development of the ICF Core Sets, which represent the selection of ICF categories for either comprehensive assessment (Comprehensive ICF Core Set) or a minimal standard of clinical reporting (Brief ICF Core Set) for a given health condition in clinical settings (17). These sets describe specific biopsychosocial issues relevant to a specific health condition and thus provide a stable reference point for defining “what should be measured” (17, 18). ICF Core Sets for various health conditions were developed through a comprehensive multi-phase consensus process (17), were validated globally.
A number of studies evaluated the reliability of activity and participation category scores of the ICF Rehabilitation Set, and compared with the reliability of the FIM item scores (19). It demonstrated, for example, that inter-rater and intra-rater reliability of ICF-based activity and participation items were comparable or better than for corresponding FIM items, and the use of ICF categories as components of rehabilitation outcome measures was recommended (19). In 2014, an ICF Generic Set (with 7 ICF categories) was developed comprising a minimal set of “functioning” domains that capture experiences of individuals and populations across clinical cohorts, contexts and settings (3, 20, 21). An extended version of this set, the ICF Rehabilitation Set (with 30 ICF categories), was also established, representing a minimal set of ICF categories relevant to rehabilitation. Measurement approaches based on this ICF Rehabilitation Set have gone through robust validity and psychometric analyses (including Rasch analysis) (3, 22–26). Recently, the WHO, in collaboration with the International Society of Physical and Rehabilitation Medicine (ISPRM) endorsed a measurement tool based on the ICF Rehabilitation Set, called the “Clinical Functioning Information Tool (ClinFIT)”. The ClinFIT aims to facilitate the assessment of “functioning” in clinical, research and other health-related settings. ClinFIT provides a common metric of “functioning” to ensure comparability of data across studies and populations in rehabilitation clinical practice across the continuum of care (1, 27).
This study examined the responsiveness of the ClinFIT in routine clinical practice in an Australian context using routinely collected outcome measures such as the FIM as an external comparator.
METHODS
Study design
A prospective observational study was conducted following the STROBE guideline (28) and in accordance with the principles of the Declaration of Helsinki. This study was approved by the Institutional Research and Ethics Committee of the Royal Melbourne Hospital (HREC: 2019.118).
Setting and study participants
This pilot study was part of a quality improvement initiative, at the 40-bed medically supervised inpatient rehabilitation unit of Royal Melbourne Hospital, a tertiary referral centre in Victoria, Australia. As a routine practice, all patients admitted to the unit are systematically assessed by a rehabilitation physician, and, based on this assessment, provided with a goal-directed individualized interdisciplinary rehabilitation programme. Allied health teams provided specialized input using individual and group therapies, such as progressive physical training programmes to improve mobility and strength, task re-acquisition and adaptive techniques to improve function including activities of daily living (ADL) and cognitive training, and behaviour management programmes.
All patients consecutively admitted (between July 2019 and April 2021) to the rehabilitation ward who met the inclusion criteria were eligible to participate in the study. The patient subgroups comprised those with neurological, orthopaedic musculoskeletal, and oncological health conditions. The criteria for inclusion were: age 18 years and above, ability and willingness to give informed consent. Those with severe cognitive issues or other psychiatric disorders that prevented the patient from being able to understand or provide responses to questionnaires were excluded. All eligible patients were invited to participate in this study by an independent admitting physician/nurse, who explained the purpose, benefit and risk of participation.
Procedure
All clinicians received formal training by the authors (AE, KS, FK), comprising introduction to the ICF model, the ClinFIT Set, and the study protocol. Consistent with routine clinical practice, every patient admitted to the rehabilitation ward was assessed with comprehensive clinical and function-oriented outcome measures at admission and discharge by the interdisciplinary team. Information collected included demographics (age, sex, marital status, education, employment), disease-related information (diagnosis, spasticity and other symptoms), medications and co-morbidities. A face-to-face structured interview was used to conduct assessments within 24 h of admission to the service (T0), using standardized instruments (see measures section below). These assessments took approximately 20 min. Staff did not prompt patients, but assisted those who had difficulty with completing the questionnaires. All participants received individualized interdisciplinary rehabilitation based on clinical need. Further assessments were conducted at discharge from the ward (T1) and at 3-months post-discharge (T2) (telephone follow-up) using the same set of standardized instruments. Any adverse events during rehabilitation (e.g. falls, injury during treatment) and patient concerns or comments were also captured.
Measures
The Functional Independence Measure (FIM) (29) considers 18 categories, of which 13 items are related to motor function. FIM assesses the level of function (29) along 4 subscales: Self-care, Transfers, Locomotion and Sphincter control; and 5 items related to the cognition along subscales: communication, psychological and cognition. Participants are rated on each item on a scale ranging from 1 to 7 (1 = total assistance, 2 = maximal assistance, 3 = moderate assistance, 4 = requires physical assistance, 5 = needs supervision, 6 = modified independence, 7 = independent) by trained staff. The FIM total sum score ranges from 18 to126, with motor subscale total ranging from 13 to 91 and cognitive subscale total ranging from 5 to 35. The score reflects dependency in each area measured, with a lower score indicating the lowest level of functioning .
ICF Clinical Functioning Information Tool (ClinFIT). ClinFIT consists of 30 categories that define a minimum set of information on “functioning” and disability that should be collected across health conditions along the continuum of rehabilitation care (20). Nine items are in the “Body Functions” domain: and 21 items in the “Activities and Participation” domain (Appendix 1) (24, 30, 31). These categories, accompanied by clinically meaningful descriptions, were used by clinicians to assess functioning problems patients experience as reflected in each ClinFIT item on an 11-point numerical rating scale, ranging from 0 = no problem to 10 = complete problem (Appendix 1).
Euro-Quality of Life (EQ-5D-5L). EQ-5D-5L (32) measures health problems along 5 dimensions: mobility, self-care, daily activity, pain/discomfort, and anxiety/depression. Items for these 5 dimensions are rated across 5 ordinal levels: no problems, slight problems, moderate problems, severe problems, and extreme problems. The sixth item within the scale assessed participant’s current overall health using a visual analogue scale (VAS) from 0 (the worst health state they can imagine) to 100 (the best health state on that day they can imagine). EQ-5D-5L index score was generated using 5-level responses to each item, based on a published crosswalk algorithm, which provides index-based scores ranging from –0.594 to 1.0 in the UK population, with lower values signifying worse health.
Statistical methods
All data on patient demographics and disease characteristics are presented descriptively. A series of both parametric and non-parametric tests were used to determine the differences in items rating between T0–T1 and T0–T2. To detect changes in ClinFIT raw total scores between admission (T0) and different assessment time-points (T1 and T2), the count-based “Extension Index” (EI) was calculated for the total ClinFIT set, and its “body function (b)” and “activity and participation (d)” domains. The EI is calculated as [(the count of categories with rating score 1–10/the total number of categories) ×100] (23, 33). The transformed value ranges from 0 to 100, with the lowest value representing no issues with body function, and no limitation/restriction in activity and participation (23, 33, 34). The paired sample t-test was used to compare the EI of the ClinFIT set between T0–T1 and T0–T2 periods. Spearman’s rank correlation coefficients (ρ) were used to assess the correlation between EI for changes between T0 and T1 and T0 and T2 in the entire ClinFIT set and the activity and participation domains and change in total FIM score (34). A series of Wilcoxon signed ranked tests were used to compare change scores from admission to discharge and 3-month follow-up for the FIM and EQ-5D-5L scores. In addition, stratified analyses (Mann–Whitney U tests) by health condition groups (stroke vs other health conditions) compared change in scores in the different outcome measures over time. Effect size statistics were calculated and assessed against Cohen’s criteria (0.2 as small, 0.5 as medium and 0.8 as large effect) (35, 36). A p-value < 0.05 was considered statistically significant. All analyses used the IBM SPSS Statistics Package Version 27 (Chicago, IL, USA).
RESULTS
A total of 91 patients consecutively admitted to the rehabilitation ward who met the inclusion criteria and provided written consent were recruited to the study. During the study, 3 participants were lost to follow-up at the 12-week (T2) follow-up assessment, as 2 could not be contacted and 1 was deceased. The majority of participants (n = 81, 89%) were discharged to their primary residence.
Sociodemographic and clinical characteristics
Descriptive statistics for patient demographics and disease characteristics are shown in Table I.
Participants were predominantly male (n = 47, 52%), and Caucasian (n = 81, 89%), with mean age of 66.8±13.0 (range = 23.8–92.1) years. The majority had a main diagnosis of stroke (n = 44, 48%) followed by musculoskeletal disorders (n = 20, 22%) and brain tumours (n = 4, 4.4%). Overall, 93% (n = 85) of participants reported 1 or more comorbidities, hypertension being the most common (n = 68, 84%), followed by depression (n = 25, 28%) and diabetes (n = 9, 10%). More than half of the participants were taking > 3 medications (polypharmacy) and common impairments reported included: lower limb (67%) and/or upper limb impairments (54%), cognitive dysfunction (43%), sensory issues (34%) and vision deficits: 45 (49%). More than two-thirds of participants reported some degree of fatigue (87%) and pain (78%) (Table I).
| Scales | T0 AdmissionMd (IQR) n = 91 | T1 DischargeMd (IQR) n = 91 | T2 3-monthMd (IQR) n = 88 | Z values** | Effect size# | ||
| T0–T1 | T0–T2 | T0–T1 | T0–T2 | ||||
| ClinFIT (Total raw score) (0-300) | 196 (110, 228) | 69 (37, 110) | 46.5 (20.8, 77) | –8.28* | –8.10* | 0.61 | 0.61 |
| FIM motor Total (13–91) | 62 (50, 69) | 79 (74, 82) | 80 (72.5, 85) | –7.65* | –7.47* | 0.57 | 0.56 |
| Self care (6–42) | 29 (24, 34) | 38 (35.1, 40) | 38 (34, 41) | –7.56* | –6.78* | 0.56 | 0.51 |
| Sphincter control (2-14) | 12 (10, 13) | 13 (13, 14) | 14 (12, 14) | –7.06* | –5.96* | 0.52 | 0.45 |
| Mobility (3–21) | 15 (12, 15) | 18 (16, 18) | 18 (18, 19.6) | –7.51* | –7.14* | 0.56 | 0.53 |
| Locomotion (2–14) | 6 (3, 6) | 10 (8.1, 11.9) | 11 (10, 12.6) | –7.43* | –7.44* | 0.55 | 0.56 |
| FIM cognition Total (5–35) | 29 (26, 32) | 32 (31, 35) | 33 (32, 35) | –6.92* | –6.40* | 0.51 | 0.48 |
| Communication (2–14) | 13 (12, 14) | 14 (14, 14) | 14 (14, 14) | –5.40* | –5.09* | 0.40 | 0.38 |
| Psycho-social (1–7) | 5 (5, 6) | 6 (6, 7) | 6 (6, 7) | –5.93* | –5.39* | 0.44 | 0.40 |
| Cognition (2–14) | 10 (10, 12) | 12 (11, 14) | 13 (12, 14) | –6.50* | –6.10* | 0.48 | 0.46 |
| EQ-5D | |||||||
| Mobility (1–5) | 4 (3, 4) | 2 (2, 3) | 2 (1, 3) | –8.181* | –7.481* | 0.61 | 0.56 |
| Self-care (1–5) | 4 (3, 4) | 2 (1, 2) | 2 (1, 2) | –7.862* | –7.175* | 0.58 | 0.54 |
| Daily activity (1–5) | 4 (3, 4) | 2 (2, 2) | 2 (1, 3) | –7.702* | –7.059* | 0.57 | 0.53 |
| Pain/discomfort (1–5) | 3 (2, 4) | 2 (1, 2) | 2 (1, 2) | –7.071* | –5.205* | 0.52 | 0.39 |
| Anxiety/depression (1–5) | 3 (2, 3) | 1 (1, 2) | 1.5 (1, 2) | –6.694* | –4.219* | 0.50 | 0.32 |
| Index value (–0.59–1.0)*** | 0.31 (0.04, 0.55) | 0.68 (0.59, 0.80) | 0.64 (0.55, 0.83) | -8.278* | -7.371* | 0.61 | 0.55 |
| Overall health (0–100) | 40 (30, 60) | 75 (65, 85) | 70 (60, 80) | -7.891* | -6.500* | 0.58 | 0.49 |
| *. Correlation significant at the 0.001 level (2-tailed). **. Wilcoxon signed-ranks test. ***. EQ-5D index-based summary score (UK). #. Effect size statistics (r) Cohen’s criteria: (0.2 = small, 0.5 = medium, 0.8 = large effect). ClinFIT: Clinical Functioning Information Tool; EQ-5D: Euro-Quality of life scale; FIM: Functional Independence Measure; IQR: interquartile range; Md: median; n=total number of participants. |
|||||||
Change scores over time in FIM and quality of life measures
Summary data for all outcome measures at different assessment points are provided in Table II. At discharge (T1), a significant improvement in participants’ functional and cognitive outcomes was noted in FIM motor “total” and all subscales: “self-care”, “sphincter”, “locomotion”, “mobility”, “communication” (p < 0.001 for all), with medium effect size (r = 0.51 to 0.61), and FIM cognition “total”, and “communication”, “psychosocial” and “cognition” subscales (p < 0.001 with small to medium sizes (r = 0.40–0.51). The QoL and overall health of participants improved significantly (EQ-5D-5L and overall health scores) (p < 0.01 for all), with medium effect sizes (r = 0.50–0.61).
Most participants were discharged home (n = 80, 88%), and the majority maintained their functional and cognitive improvement (FIM scores) at 3-month follow-up (T2). Significant functional improvements were maintained by most participants, as measured by FIM motor “total” and each subscale (p < 0.001 for all) with a medium effect size (r = 0.52–0.61). The improvements were also maintained for the FIM cognition “total” and each cognition subscales (p < 0.001 for all); however, the magnitude of the effect was reduced to small (r = 0.38–0.48). The QoL and overall health of participants remained statistically significant (p < 0.01 for all) with small to medium effect sizes (r = 0.32–0.56) (Table II).
Sensitivity to change in ClinFIT scores
Table III lists the patient-reported problems due to their health conditions using ClinFIT in “Body Functions (b)” and “Activities and Participation (d)” domains. The majority of participants reported at least 1 issue related to one of the ClinFIT categories. The most affected ClinFIT category at admission (T0) was “d450 Walking” (97.8%), followed by “b455 Exercise tolerance functions” (96.7%), “d230 Carrying out daily routine” (96.7%) and “d455 Moving around” (96.7%) (Fig. 1).
| ClinFIT items | Admission (T0)n = 91 | Discharge (T1)n = 91 | 3-months (T2)n = 88 | Z value^ | Effect size# | |||||
| N§ (%) | Md~ (IQR) | N§ (%) | Md~ (IQR) | N§ (%) | Md~ (IQR) | T0–T1 | T0–T2 | T0–T1 | T0–T2 | |
| b130 - Energy & drive functions | 86 (94.5) | 7 (5, 9) | 81 (89.0) | 3 (1, 5) | 62 (70.5) | 2 (0, 3) | –7.85* | –7.69* | 0.58 | 0.57 |
| b134 - Sleep functions | 82 (90.1) | 7 (4, 9) | 72 (79.1) | 3 (1, 5) | 55 (62.5) | 1 (0, 3) | –7.67* | –7.50* | 0.57 | 0.56 |
| b152 - Emotional functions | 84 (92.3) | 7 (3, 8) | 72 (79.1) | 2 (1, 4) | 53 (60.2) | 1 (0, 2) | –7.58* | –7.41* | 0.56 | 0.55 |
| b280 - Sensation of pain | 74 (81.3) | 6 (3, 8) | 64 (70.3) | 2 (0, 3) | 46 (52.3) | 1 (0, 2) | –7.34* | –7.10* | 0.54 | 0.53 |
| b455 - Exercise tolerance functions | 88 (96.7) | 8 (4, 9) | 77 (84.6) | 3 (1, 4) | 53 (60.2) | 1 (0, 2) | –7.69* | –7.71* | 0.57 | 0.58 |
| b620 - Urination functions | 53 (58.2) | 1 (0, 6) | 32 (35.2) | 0 (0, 1) | 28 (31.8) | 0 (0, 2) | –5.48* | –3.90* | 0.41 | 0.29 |
| b640 - Sexual functions | 50 (54.9) | 1 (0, 7) | 37 (40.7) | 0 (0, 3) | 28 (31.8) | 0 (0, 2) | –4.47* | –3.08** | 0.33 | 0.23 |
| b710 - Mobility of joint functions | 85 (93.4) | 8 (4, 9) | 77 (84.6) | 3 (1, 5) | 66 (75.0) | 1.5 (0.3, 3.8) | –7.81* | –7.31* | 0.58 | 0.55 |
| b730 - Muscle power functions | 85 (93.4) | 8 (5, 9) | 81 (89.0) | 3 (1, 5) | 66 (75.0) | 1 (0.3, 3) | –7.80* | –7.58* | 0.58 | 0.57 |
| d230 - Carrying out daily routine | 88 (96.7) | 8 (5, 9) | 80 (87.9) | 3 (1, 5) | 64 (72.7) | 2 (0, 3) | –8.08* | –7.70* | 0.60 | 0.58 |
| d240 - Handling stress & other psychological demands | 83 (91.2) | 7 (3, 8) | 73 (80.2) | 2 (1, 4) | 52 (59.1) | 1 (0, 3) | –7.37* | –7.34* | 0.55 | 0.55 |
| d410 - Changing basic body position | 78 (85.7) | 8 (4, 9) | 65 (71.4) | 2 (0, 4) | 54 (61.4) | 1 (0, 3) | –7.52* | –7.40* | 0.56 | 0.55 |
| d415 - Maintaining a body position | 76 (83.5) | 7 (2, 8) | 61 (67.0) | 2 (0, 4) | 41 (46.6) | 0 (0, 2) | –7.37* | –7.17* | 0.55 | 0.54 |
| d420 - Transferring oneself | 82 (90.1) | 8 (4, 8) | 66 (72.5) | 2 (0, 4) | 53 (60.2) | 1 (0, 2) | –7.76* | –7.71* | 0.58 | 0.58 |
| d450 - Walking | 89 (97.8) | 8 (6, 10) | 82 (90.1) | 3 (1, 5) | 59 (67.0) | 2 (0, 3) | –7.79* | –7.80* | 0.58 | 0.58 |
| d455 - Moving around | 88 (96.7) | 8 (5, 10) | 83 (91.2) | 3 (2, 5) | 71 (80.7) | 3 (1, 6.8) | –7.73* | –6.00* | 0.57 | 0.45 |
| d465 - Moving around using equipment | 85 (93.4) | 6 (3, 8) | 70 (76.9) | 2 (1, 4) | 61 (69.3) | 2 (0, 4) | –7.16* | –5.94* | 0.53 | 0.44 |
| d470 - Using transportation | 83 (91.2) | 8 (5, 9) | 74 (81.3) | 3 (1, 5) | 49 (55.7) | 1 (0, 3) | –7.23* | –7.72* | 0.54 | 0.58 |
| d510 - Washing oneself | 82 (90.1) | 8 (4, 9) | 68 (74.7) | 2 (0, 4) | 44 (50.0) | 0.5 (0, 2.8) | –7.68* | –7.55* | 0.57 | 0.56 |
| d520 - Caring for body parts | 79 (86.8) | 7 (3, 9) | 55 (60.4) | 1 (0, 3) | 33 (37.5) | 0 (0, 1.8) | –7.55* | –7.40* | 0.56 | 0.55 |
| d530 - Toileting | 77 (84.6) | 6 (3, 8) | 47 (51.6) | 1 (0, 2) | 23 (26.1) | 0 (0, 1) | –7.53* | –7.45* | 0.56 | 0.56 |
| d540 - Dressing | 83 (91.2) | 7 (3, 8) | 69 (75.8) | 2 (1, 3) | 44 (50.0) | 0.5 (0, 3) | –7.48* | –7.27* | 0.55 | 0.54 |
| d550 - Eating | 55 (60.4) | 1 (0, 3) | 27 (29.7) | 0 (0, 1) | 10 (11.4) | 0 (0, 0) | –5.43* | –5.31* | 0.40 | 0.40 |
| d570 - Looking after one’s health | 83 (91.2) | 7 (3, 8) | 70 (76.9) | 2 (1, 3) | 43 (48.9) | 0 (0, 2) | –7.78* | –7.63* | 0.58 | 0.57 |
| d640 - Doing housework | 84 (92.3) | 8 (6, 10) | 80 (87.9) | 3 (1, 5) | 68 (77.3) | 2 (1, 5) | –7.58* | –7.24* | 0.56 | 0.54 |
| d660 - Assisting others | 82 (90.1) | 8 (5, 9) | 78 (85.7) | 3 (1, 5) | 57 (64.8) | 1 (0, 2.8) | –7.21* | –7.11* | 0.53 | 0.53 |
| d710 - Basic interpersonal interactions | 61 (67.0) | 4 (0, 8) | 51 (56.0) | 1 (0, 4) | 33 (37.5) | 0 (0, 1.8) | –6.44* | –6.48* | 0.48 | 0.48 |
| d770 - Intimate relationship | 58 (63.7) | 5 (0, 8) | 55 (60.4) | 1 (0, 4) | 40 (45.5) | 0 (0, 3) | –5.93* | –4.31* | 0.44 | 0.32 |
| d850 - Remunerative employment | 69 (75.8) | 8 (1, 10) | 68 (74.7) | 5 (0, 10) | 56 (63.6) | 2.5 (0, 10) | –3.61* | –3.67* | 0.27 | 0.27 |
| d920 - Recreation and leisure | 86 (94.5) | 8 (5, 10) | 83 (91.2) | 4 (2, 7) | 69 (78.4) | 3 (1, 5) | –7.22* | –6.58* | 0.54 | 0.49 |
| ^. Wilcoxon signed-ranks test. *. Correlation significant at the 0.001 level & **0.01 level (2-tailed) ~. Md = Median score for each ClinFITitem; §. N = number of participants scoring ≥ 1 in ClinFIT rating scores. #. Effect size statistics (r) Cohen’s criteria: (0.2 = small, 0.5 = medium, 0.8 = large effect). ClinFIT: Clinical Functioning Information Tool; IQR: interquartile range; n=total number of participants. |
||||||||||

Fig. 1. Top 10 most commonly affected Clinical Functioning Information Tool (ClinFIT) categories at admission (T0).
Most participants, irrespective of their health condition, showed improvement from admission to discharge and at 3 months (Fig. 2). Compared with the admission (T0) (median (Md), interquartile range (IQR); 196 (110, 228)), there was a significant improvement in participants across all health conditions in ClinFIT total raw score at both study assessment points T1: 69 (37, 110); p < 0.001 and T2: 46.5 (20.8, 77); p < 0.001, with medium effect size (r = 0.61 for both) (Table II). Summary data for all 30 ClinFIT categories at different assessment periods are shown in Table III. Similarly, compared with admission (T0), there was significant improvement across all 30 ClinFIT categories at discharge (T1) and 3-month follow-up (p < 0.01 for all), with small to medium effect sizes (r = 0.3– 0.6). (Table III).

Fig. 2. Composite Radar Chart* illustrating the median scores for each Clinical Functioning Information Tool (ClinFIT) item at admission (T0), discharge (T1) and 3 months (T2) (n=9.1). *The composite radar chart gives a graphical representation of the functional profile from the ClinFIT data. The 30-scale items are arranged as spokes of a wheel (codes out the circumference), with International Classification of Functioning, Disability and Health (ICF) rating score from 0=no problem to 10=complete problem, running from the centre outwards. The blue-shaded area represents the median admission (T0) scores, yellow-shaded area the median discharge (T1) scores and green-shaded area the median scores at 3-month follow-up (T2).
Table IV shows the mean change in the Extension Index (EI) of the total ClinFIT set over time at different assessment time-points. There were significant changes in EI in the entire ClinFIT set from admission (T0) to discharge (T1) (z –6.96, p < 0.001) and from T0 to T2 (p < 0.001), with large effect sizes (0.81 and 1.11, respectively). The changes in the EI were significant for both ClinFIT “b” and “d” domains at both assessment time-points, indicating participants have fewer issues with their body function and less restriction in their everyday activities (Table IV).
| ClinFIT components | EI# (Mean ± SD) | t, p value^ | Effect size (95% CI)* | ||||
| T0 | T1 | T2 | T0–T1 | T0–T2 | T0–T1 | T0–T2 | |
| BF (b) | 83.9 ± 19.0 | 80.2 ± 24.3 | 58.0 ± 31.3 | 6.43, p < 0.001 | 8.81, p < 0.001 | 0.68 (0.45, 0.90) | 0.94 (0.69, 1.19) |
| A&P (d) | 86.4 ± 19.1 | 73.5 ± 26.2 | 56.5 ± 29.9 | 7.17, p <0.001 | 10.3, p < 0.001 | 0.75 (0.52, 0.98) | 0.94 (0.83, 1.36) |
| Total (b+d) | 85.6 ± 18.1 | 73.2 ± 24.2 | 57.0 ± 29.5 | 7.74, p < 0.001 | 10.4, p < 0.001 | 0.81 (0.57, 1.04) | 1.11 (0.84, 1.37) |
| #. EI = extension index was calculated as: (Number of problem categories/total number of categories) × 100 ^. Paired sample t-test. *. Effect size statistics (d) Cohen’s d criteria: 0.2 = small, 0.5 = medium, 0.8 = large effect A&P: Activity and participation; BF: Body function; ClinFIT: Clinical Functioning Information Tool; SD: standard deviation. |
|||||||
Correlation between change scores in FIM and ClinFIT sets
A series of correlation analyses were used to describe the strength and direction of the linear relationship between change scores at different assessment time-points in FIM and ClinFIT sets. There were significant small correlations between total FIM changes and the ClinFIT total EI changes at from admission to T1 (ρ = 0.27, p = 0.009) and at T2 (ρ = 0.29, p = 0.006). Furthermore, excluding the ClinFIT “body structure (b)” categories from the analysis (as FIM items are specifically based on “activity and participation”), there was a small to moderate correlation between change scores in total FIM and the EI for “activity and participation (b)” domain at T1 (ρ = 0.28, p = 0.008) and T2 (ρ = 0.34, p = 0.002).
Subgroup analysis based on the diagnosis
Almost half the participants (n = 44, 48.4%) had stroke. The most reported ClinFIT categories at admission were comparable between participants with stroke and other health conditions (multiple sclerosis (MS), musculoskeletal disorders, brain tumours and others) (Fig. 3). The most commonly affected categories in both groups were in “activities and participation (d)” domains, specifically in performing ADLs, mobility, emotional function and participation. The most commonly reported categories by stroke survivors (> 95%) included: “Emotional functions (b152)”, “Carrying out daily routine (d230)”, “Walking (d450)”, “Moving around (d455)” and “Assisting others (d660)”. Participants with other health conditions reported (> 97%): “Walking (d450)”, “Exercise tolerance (b455)”, “Carrying out daily routine (d230)”, “Moving around (d455)”, “Energy and drive functions (b130)”, and “Moving around using equipment (d465)”. The least concerning categories reported by all participants ( < 66%) included: “Intimate relationships (d770)” and “Sexual function (b640)”.
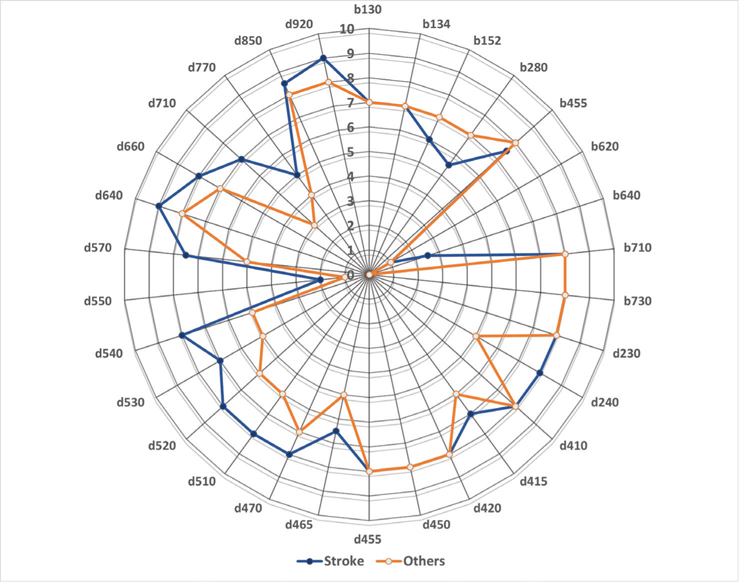
Fig. 3. Composite Radar Chart* illustrating the median scores for each Clinical Functioning Information Tool (ClinFIT) item at admission (T0), between participants with stroke (n=44) vs other health conditions (n=47). *The composite radar chart gives a graphical representation of the functional profile from the ClinFIT data (median admission scores). The 30-scale items are arranged as spokes of a wheel (codes out the circumference), with ICF rating score from 0=no problem to 10=complete problem, running from the centre outwards.
At discharge, participants with stroke improved significantly in cognitive function and some activity compared with those with other diagnoses (FIM total, p = 0.014). At discharge, the estimated difference in scores between stroke and other health condition groups was significantly in favour of the stroke group in FIM cognition total (p < 0.001) and subscales: “communication” (p < 0.001), “cognition” (p = 0.002); and FIM “locomotion” (p = 0.002). There was no significant difference in the QoL and overall health of participants (EQ-5D-5L subscales and overall health scores) (Table V).
| Scales (range) | Discharge (T1) – Admission (T0)^ | 3-Month (T2) – Admission (T0)^ | ||||||
| Stroke (n=44) | Others** (n=47) | Z value# | p-value* | Stroke (n=42) | Others** (n=46) | Z value# | p-value* | |
| FIM Total (18–126) | 22.5 (15, 35) | 16 (10, 23) | –2.452 | 0.014 | 26.5 (14.8, 40) | 20.5 (14, 28.3) | –1.903 | 0.057 |
| Motor Total (13–91) | 16 (12, 28.3) | 14.5 (9.8, 22) | –1.681 | 0.093 | 19.5 (11.5, 31.3) | 18 (10.8, 27.5) | –0.710 | 0.477 |
| Self-care (6–42) | 8.5 (4, 12) | 6 (3, 10) | –1.488 | 0.137 | 9 (4.5, 16) | 6.5 (4, 12) | –1.343 | 0.179 |
| Sphincter (2–14) | 1 (0.3, 2) | 1 (1, 3) | –0.694 | 0.488 | 1 (0, 3) | 1 (0, 3) | –0.400 | 0.689 |
| Mobility (3–21) | 3 (3, 6) | 3 (2, 6) | –1.381 | 0.167 | 4 (3, 9) | 3 (3, 7) | –0.672 | 0.501 |
| Locomotion (2–14) | 5 (3, 7) | 3 (1, 7) | –2.001 | 0.045 | 5.5 (4, 8) | 6 (1.8, 7.3) | –0.713 | 0.465 |
| Cognition Total (5–35) | 5 (1.8, 6.3) | 1 (0, 4) | –3.695 | <0.001 | 6 (3, 9.3) | 1 (0, 4) | –4.498 | <0.001 |
| Communication (2–14) | 1.5 (0, 3) | 0 (0, 0) | –3.485 | <0.001 | 2 (0, 4) | 0 (0, 1) | –4.015 | <0.001 |
| Psychosocial (1–7) | 1 (0, 1.8) | 0 (0, 1) | –1.926 | 0.054 | 1 (0.8, 2) | 0 (0, 1) | –3.715 | <0.001 |
| Cognition (2–14) | 2 (1, 3) | 1 (0, 2) | –3.111 | 0.002 | 2 (1, 5) | 0 (0, 2) | –3.493 | <0.001 |
| EQ-5D Mobility (1–5) | –2 (–2, –1) | –2 (–2, –1) | –0.394 | 0.694 | –2 (–2.3, –1) | –2 (–2, –1) | –0.398 | 0.690 |
| Self-care (1–5) | –2 (–2, –1) | –2 (–2, –1) | –0.724 | 0.469 | –2 (–2.3, –1) | –2 (–2, –1) | –0.679 | 0.497 |
| Daily activity (1–5) | –1.5 (–2, –1) | –2 (–2, –1) | –0.315 | 0.753 | –1.5 (–2, –1) | –1 (–2, 0) | –0.994 | 0.320 |
| Pain/discomfort (1–5) | –1 (–2, 0) | –1 (–2, 0) | –0.221 | 0.825 | –1 (–2, 0) | –1 (–2, –0.3) | –0.730 | 0.465 |
| Anxiety/depression (1–5) | –1 (–2, –1) | –1 (–2, 0) | –1.620 | 0.105 | –1 (–2, 0) | 0 (–2, 0) | –1.860 | 0.063 |
| Overall Health (0–100) | 30 (20, 40) | 25 (10, 40) | –1.225 | 0.220 | 30 (19.8, 41.3) | 25 (7.5, 40) | –2.140 | 0.032 |
| ClinFIT | ||||||||
| Total raw score (0 – 300) | 89.5 (46.8, 140) | 95 (40.5, 128.8) | –0.318 | 0.751 | 151.5 (68.5, 179) | 113.5 (54.5, 156.8) | –1.734 | 0.083 |
| Extension Index§ (0–100) | ||||||||
| Body function (b) | 11.1 (0, 22.2) | 0.0 (0, 22.0) | –0.100 | 0.920 | 22.2 (11.1, 47.2) | 22.2 (0, 44.4) | –1.290 | 0.197 |
| Activity & participation (d) | 9.5 (0, 22.6) | 4.8 (0, 14.3) | –0.454 | 0.650 | 35.7 (14.3, 57.1) | 19.0 (7.1, 44.1) | –1.478 | 0.123 |
| Total (b+d) | 10.0 (0, 23.4) | 6.7 (0, 16.7) | –0.406 | 0.685 | 31.7 (15.9, 57.5) | 20.0 (5.9, 46.7) | –1.631 | 0.103 |
| ^. Values provided as median (interquartile range). *. Correlation significant at all levels <0.05 level (2-tailed) are shown in bold. #. Mann–Whitney U test. §. Extension index was calculated as: (number of problem categories/total number of categories) × 100. **. Participants with other health conditions, such as musculoskeletal, other neurological conditions, cancer, etc.). ClinFIT: Clinical Functioning Information Tool; SD: standard deviation, EQ-5D: Euro-Quality of Life scale, FIM: Functional Independence Measure, n: total number. |
||||||||
At 3-month follow-up, compared with those with other diagnoses, participants with stroke showed improvement in their overall health (EQ-5D “overall health” (p = 0.032), but no difference was noted in other EQ-5D-5L items. The participants with stroke also showed significant improvement in FIM cognition total and its subscales (p < 0.001 for all), but not in FIM motor subscales. Although participants with other diagnoses, compared with the stroke group, reported some improvement in ClinFIT total raw score at T2, this was not statistically significant (p = 0.08). No difference between groups was noted for the ClinFIT EI (Table V).
DISCUSSION
This study was designed to support the mandate of the ISPRM ClinFIT Task Force to continue the development and global implementation of ClinFIT in clinical practice, quality management, and research (1). Furthermore, the study was conducted in response to a clinical need for comprehensive evaluation of functional outcomes in rehabilitation settings by treating clinicians, for effective timely and appropriate care delivery. This study demonstrates that ClinFIT is useful for the assessment of functioning in rehabilitation settings across a range of health conditions. The ClinFIT scores were responsive and showed improvements in patient functioning, i.e. body functions, as well as activities and participation after an inpatient rehabilitation programme, with medium effect sizes, which were maintained at 3-months follow-up. These improvements, specifically in cognitive function (as measured by FIM), were more pronounced in participants with stroke at discharge compared with participants with other diagnoses. The changes in the ClinFIT total raw score and EI and QoL scores were comparable between these 2 groups; however, at 3-month follow-up the participants with stroke indicated greater improvement in their overall health and cognitive functioning. Furthermore, there was a significant low to moderate correlation between the changes in the ClinFIT EI and changes in FIM scores at discharge and 3-month follow-up.
Despite multiple studies evaluating the psychometric properties, reliability and validity of the ICF Rehabilitation-30 Set (3, 22, 24–26, 37), only a few studies have examined its application for assessment of clinical outcomes in inpatient rehabilitation programmes (23, 34). Consistent with the findings of this study, Kinoshita et al. showed marked improvement in the EI of the ICF Rehabilitation Set at discharge after a structured inpatient multidisciplinary rehabilitation programme in stroke patients (n = 108), with medium to large effect sizes (34) and that the improvement in the ICF Rehabilitation Set correlated significantly with FIM scores (34). Another study conducted earlier by the same group of authors (n = 117), evaluated the “activity and participation (d)” component of the ICF rehabilitation set in stroke inpatient rehabilitation wards (38). The authors divided the “d” component of the set into 3 sub-components: cognition-related activity, motor-related activity, and participation, and compared these with the FIM scores. The authors reported significant and strong correlations between the values of the entire “d” component and sub-components (cognition-related activity and motor-related activity) of the ICF Rehabilitation Set and FIM score, but a weak correlation between FIM and the participation sub-component (38). One recent study (n = 104 participants) evaluated the clinical effectiveness of the multidisciplinary rehabilitation approach using the ICF Rehabilitation Set to evaluate patients’ ADL status in a convalescent rehabilitation ward (23). The authors reported a significant improvement in EI in participants who received 2-weekly serial assessment/discussion using the ICF Rehabilitation Set compared with participants receiving assessment using the FIM (31.6±18.5 vs 17.3±18.4, respectively; p < 0.001) (23). Many of these results are consistent with the findings of this study demonstrating significant improvement in EI for both “body function” and “activity and participation” domains of the ClinFIT. In addition to above-mentioned studies, the current study also assessed the participants at 3 months post-discharge and demonstrated that improvement was maintained, with small to medium effect sizes.
The ISPRM ClinFIT task force envisages that ClinFIT is used as a universal clinical data collection tool, tailored to the needs of the clinicians in different rehabilitation settings and across different patient populations along the continuum of care (1). The ClinFIT copyright is owned by the WHO and the ISPRM, therefore it is freely available and can be used by any country worldwide (1). Despite their differences, ClinFIT includes more exhaustive categories, specifically in the “activity and participation” domains that are more relevant from the rehabilitation perspective, and can be used to evaluate broader aspects of patients’ functioning, compared with other commonly used measures, such as FIM . It is feasible to use in busy clinical settings and suitable to describe patients’ functional status comprehensively in inpatient rehabilitation settings. Furthermore, this study reports the responsiveness of the ClinFIT scores over time, generating interval score (EI) over time (from admission to discharge and 3-months post-discharge) providing information on various aspects of functional deficits and disability. These scores, consistent with scores obtained on the regularly collected FIM tool, demonstrate the beneficial impact of structured interdisciplinary rehabilitation programmes. This can be used to enhance goal setting and structured rehabilitation plans to suit patient needs for better clinical outcomes. However, experts in the field have yet to recommend the use of various ICF Core Sets and ClinFIT, as outcome assessment measures in routine clinical practice. This remains open for further discussion; more reliability studies are needed in routine clinical practice applying the ClinFIT set concurrently with clinical observations and evaluation, specifically with control groups and longer-term follow up.
The findings of this study add to the existing evidence in this area and demonstrate the usefulness of ICF-based functioning assessment in routine rehabilitation clinical practice setting. Further studies should also explore whether the ClinFIT set can also be used for the standardized reporting of functioning, which may enable comparisons within and between health systems. Previously, Kinoshita et al. demonstrated the validity of the “activity and participation (d)” component of the ICF Rehabilitation Set in stroke patients by categorizing sub-components: cognition-related activity, motor-related activity and participation (38). Further research is needed to examine whether meaningful concepts and psychometrically sound subscales/domains within the tool can be created to enhance the interpretation of the outcome data across the care continuum.
Study limitations
This study has several limitations. First, the study was conducted in a single tertiary hospital rehabilitation unit and most participants had neurological conditions (mainly stroke) and musculoskeletal disorders, which may limit the generalizability of the findings. The study cohort, however, covered a wide geographical population, with different diagnoses, and complexity in terms of disease severity, symptoms and comorbidities, which may portray the true picture of the sub-acute patient population in an Australian context. Secondly, this is a longitudinal observational study without a control group, which limits the ability to draw causal relationships between the rehabilitation interventions and improvements in patient outcomes. However, this was not the intention of the current study, and further studies with a control group will be needed to evaluate responsiveness. Thirdly, assessment of a wide range of function and activities may differ between inpatient and community (home) environments. As follow-up data (T2) were self-reported from the participants’ homes and communities, it is dependent on participant interpretation, and the assessor can thus only extrapolate from their inpatient capabilities. This study was conducted during the COVID-19 pandemic (with over 18 months of multiple lockdowns and restrictions in Melbourne), which impacted participant recruitments and attrition. Many rehabilitation services ceased and/or were interrupted during the lockdown, and “on-line” services were used. The correlational analysis between the ClinFIT and FIM sets should be interpreted cautiously due to differences in characteristics of these tools. For example, the ClinFIT only comprises 13 FIM items directly linked to its categories, and 5 FIM items: “bowel management”, “comprehension”, “expression”, “problem-solving”, and “memory” cannot be directly linked to the ClinFIT categories. However, as ClinFIT categories are the second-level item of the complex ICF hierarchical structure and taxonomy, it automatically includes many of these missed items as third-level items within the defined ICF categories (39). Furthermore, ClinFIT, as other ICF Core Sets, were developed through a rigorous international multilevel consensus process (17) and it is noted that ClinFIT was not based on nor intended to replicate the FIM or any other measure. It was beyond the scope of this study to further evaluate the content of the ClinFIT set. Further, we acknowledge that the EI was not calculated for each item but for the entire ClinFIT set and its domains, and the magnitude of change score may not represent the accurate value for each item. Finally, the current study had a short 3-month follow-up. Future studies are required to evaluate ClinFIT in community settings with longer follow-up periods.
CONCLUSION
This study demonstrates the usefulness of the ClinFIT set, which represents the minimal information that should be collected, regardless of health conditions in rehabilitation settings. The ClinFIT scores of functioning were responsive to change over time in clinical practice and across a range of health conditions. Implementation of such a structured universal tool in routine clinical rehabilitation practice will potentially allow for comprehensive evaluation of health outcomes and monitoring quality of care. The information can provide a foundation for understanding the level of functioning and its impact on daily life, which is vital for goal setting and delivery of the targeted intervention. This pilot study was designed to promote and facilitate broader acceptance of the ICF-based model in rehabilitation settings at large, particularly in low-resource countries in which proprietary instruments, such as FIM, may not be affordable. Furthermore, collaboration across rehabilitation sectors is required for the implementation of ClinFIT as a universal tool to assess patient function in daily clinical practice across various rehabilitation settings globally.
ACKNOWLEDGEMENTS
The authors are grateful to all participants in this study and thank the multidisciplinary team at the rehabilitation ward of the Royal Park Campus, Royal Melbourne Hospital. We thank Loren Oscari for patient assessments and data entry. The views expressed in this article are exclusive of the authors unless referenced.
REFERENCES
- Frontera W, Gimigliano F, Melvin J, Li J, Li L, Lains J, et al. ClinFIT: ISPRM’s Universal Functioning Information Tool based on the WHO’s ICF. J Int Soc Phys Rehabil Med 2019; 2: 19–21.
- Grasso MG, Troisi E, Rizzi F, Morelli D, Paolucci S. Prognostic factors in multidisciplinary rehabilitation treatment in multiple sclerosis: an outcome study. Mult Scler 2005; 11: 719–724.
- Prodinger B, Reinhardt JD, Selb M, Stucki G, Yan T, Zhang X, et al. Towards system-wide implementation of the International Classification of Functioning, Disability and Health (ICF) in routine practice: developing simple, intuitive descriptions of ICF categories in the ICF Generic and Rehabilitation Set. J Rehabil Med 2016; 48: 508–514.
- Wade DT. Research into the black box of rehabilitation: the risks of a Type III error. Clin Rehabil 2001; 15: 1–4.
- Whyte J, Hart T. It’s more than a black box; it’s a Russian doll: defining rehabilitation treatments. Am J Phys Med Rehabil 2003; 82: 639–652.
- Khan F, Pallant JF. Use of the International Classification of Functioning, Disability and Health (ICF) to identify preliminary comprehensive and brief core sets for multiple sclerosis. Disabil Rehabil 2007; 29: 205–213.
- Khan F, Amatya B. Use of the International Classification of Functioning, Disability and Health (ICF) to describe patient-reported disability in primary brain tumour in an Australian community cohort. J Rehabil Med 2013; 45: 434–445.
- Eagar K. The Australian National Sub-Acute and Non-Acute Patient casemix classification. Aust Health Rev 1999; 22: 180–196.
- Lowthian P, Disler P, Ma S, Eagar K, Green J, de Graaff S. The Australian National Sub-acute and Non-acute Patient Casemix Classification (AN-SNAP): its application and value in a stroke rehabilitation programme. Clin Rehabil 2000; 14: 532–537.
- Turner-Stokes L, Vanderstay R, Stevermuer T, Simmonds F, Khan F, Eagar K. Comparison of rehabilitation outcomes for long term neurological conditions: a cohort analysis of the Australian Rehabilitation Outcomes Centre dataset for adults of working age. PLoS One 2015; 10: e0132275.
- Turner-Stokes L, Sutch S, Dredge R, Eagar K. International casemix and funding models: lessons for rehabilitation. Clin Rehabil 2012; 26: 195–208.
- Heinemann AW, Linacre JM, Wright BD, Hamilton BB, Granger C. Relationships between impairment and physical disability as measured by the functional independence measure. Arch Phys Med Rehabil 1993; 74: 566–573.
- Hall KM, Mann N, High WMJ, Wright J, JS K, Wood D. Functional measures after traumatic brain injury: Ceiling effects of FIM, FIM+FAM, DRS, and CIQ. J Head Trauma Rehabil 1996; 11: 27–39.
- Turner-Stokes L, Williams H, Siegert RJ. The Rehabilitation Complexity Scale version 2: a clinimetric evaluation in patients with severe complex neurodisability. J Neurol Neurosurg Psychiatry 2010; 81: 146–153.
- Cieza A, Stucki G. Content comparison of health-related quality of life (HRQOL) instruments based on the international classification of functioning, disability and health (ICF). Qual Life Res 2005; 14: 1225–1237.
- World Health Organization (WHO). International classification of functioning, disability and health (ICF). 2001; Geneva: WHO; 2001.
- Stucki G, Pollock A, Engkasan JP, Selb M. How to use the International Classification of Functioning, Disability and Health as a reference system for comparative evaluation and standardized reporting of rehabilitation interventions. Eur J Phys Rehabil Med 2019; 55: 384–394.
- Cieza A, Hilfiker R, Chatterji S, Kostanjsek N, Ustun BT, Stucki G. The International Classification of Functioning, Disability, and Health could be used to measure functioning. J Clin Epidemiol 2009; 62: 899–911.
- Kohler F, Connolly C, Sakaria A, Stendara K, Buhagiar M, Mojaddidi M. Can the ICF be used as a rehabilitation outcome measure? A study looking at the inter- and intra-rater reliability of ICF categories derived from an ADL assessment tool. J Rehabil Med 2013; 45: 881–887.
- Cieza A, Oberhauser C, Bickenbach J, Chatterji S, Stucki G. Towards a minimal generic set of domains of functioning and health. BMC Public Health 2014; 14: 218.
- Li J, Prodinger B, Reinhardt JD, Stucki G. Towards the system-wide implementation of the International Classification of Functioning, Disability and Health in routine practice: Lessons from a pilot study in China. J Rehabil Med 2016; 48: 502–507.
- Gao Y, Yan T, You L, Li K, Zhang L, Zhang M. Psychometric properties of the International Classification of Functioning, Disability and Health Rehabilitation Set: a Rasch analysis. Int J Rehabil Res 2021; 44: 144–151.
- Kinoshita S, Abo M, Okamoto T. Effectiveness of ICF-based multidisciplinary rehabilitation approach with serial assessment and discussion using the ICF rehabilitation set in a convalescent rehabilitation ward. Int J Rehabil Res 2020; 43: 255–260.
- Selb M, Gimigliano F, Prodinger B, Stucki G, Pestelli G, Iocco M, et al. Toward an International Classification of Functioning, Disability and Health clinical data collection tool: the Italian experience of developing simple, intuitive descriptions of the Rehabilitation Set categories. Eur J Phys Rehabil Med 2017; 53: 290–298.
- Senju Y, Mukaino M, Prodinger B, Selb M, Okouchi Y, Mizutani K, et al. Development of a clinical tool for rating the body function categories of the ICF generic-30/rehabilitation set in Japanese rehabilitation practice and examination of its interrater reliability. BMC Med Res Methodol 2021; 21: 121.
- Mukaino M, Prodinger B, Yamada S, Senju Y, Izumi SI, Sonoda S, et al. Supporting the clinical use of the ICF in Japan - development of the Japanese version of the simple, intuitive descriptions for the ICF Generic-30 set, its operationalization through a rating reference guide, and interrater reliability study. BMC Health Serv Res 2020; 20: 66.
- Gimigliano F, De Sire A, Gastaldo M, Maghini I, Paoletta M, Pasquini A, et al. Use of the International Classification of Functioning, Disability and Health Generic-30 Set for the characterization of outpatients: Italian Society of Physical and Rehabilitative Medicine Residents Section Project. Eur J Phys Rehabil Med 2019; 55: 258–264.
- Knottnerus A, Tugwell P. STROBE--a checklist to Strengthen the Reporting of Observational Studies in Epidemiology. J Clin Epidemiol 2008; 61: 323.
- Ottenbacher KJ, Hsu Y, Granger CV, Fiedler RC. The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil 1996; 77: 1226–1232.
- Frontera W. The organizations of physical and rehabilitation medicine in the world: The International Society of Physical and Rehabilitation Medicine. J Int Soc Phys Rehabil Med 2019; 2: S130–133.
- Prodinger B, Cieza A, Oberhauser C, Bickenbach J, Ustun TB, Chatterji S, et al. Toward the International Classification of Functioning, Disability and Health (ICF) Rehabilitation Set: A Minimal Generic Set of Domains for Rehabilitation as a Health Strategy. Arch Phys Med Rehabil 2016; 97: 875–884.
- EuroQoL Group. EQ-5D 2017 [cited 2020 April 14]; Available from: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/
- Raggi A, Leonardi M, Ajovalasit D, D’Amico D, Bussone G. Disability and functional profiles of patients with migraine measured with ICF classification. Int J Rehabil Res 2010; 33: 225–231.
- Kinoshita S, Abo M, Okamoto T, Kakuda W, Miyamura K, Kimura I. Responsiveness of the functioning and disability parts of the International Classification of Functioning, Disability, and Health core sets in postacute stroke patients. Int J Rehabil Res 2017; 40: 246–253.
- Cohen J. Statistical power analysis for the behavioural sciences. 2nd edn. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988.
- Pallant J. SPSS survival manual: a step by step guide to data analysis using IBM SPSS. 5th ed. New York: McGraw-Hill, 2013.
- Zhang M, Zhang Y, Xiang Y, Lin Z, Shen W, Wang Y, et al. A team approach to applying the International Classification of Functioning, Disability and Health Rehabilitation set in clinical evaluation. J Rehabil Med 2021; 53: jrm00147.
- Kinoshita S, Abo M, Miyamura K, Okamoto T, Kakuda W, Kimura I, et al. Validation of the “Activity and participation” component of ICF Core Sets for stroke patients in Japanese rehabilitation wards. J Rehabil Med 2016; 48: 764–768.
- Kohler F, Connolly C, Sakaria A, Stendara K, Buhagiar M, Mojaddidi M. Response to letter to the Editor by Gunnar Grimby and Asa Lundgren-Nilsson, on ‘comments on the article “can the ICF be used as a rehabilitation outcome measure? A study looking at the inter- and intra-rater reliability of the ICF categories derived from an ADL assessment tool.”’. J Rehabil Med 2013; 45: 931.
