ORIGINAL REPORT
IMPAIRMENTS OF THE ARM AND HAND ARE HIGHLY CORRELATED DURING SUBACUTE STROKE
Lydia N. REID, BSc1, Sean P. DUKELOW, MD, PhD2,3 and Stephen H. SCOTT, PhD1,4,5
From the 1Centre for Neuroscience Studies, Queen’s University, Kingston, Ontario, 2Hotchkiss Brain Institute, University of Calgary, Calgary, AB, 3Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary, AB, 4Department of Biomedical and Molecular Science, Queen’s University, Kingston, Ontario and 5Providence Care Hospital, Kingston, Ontario, Canada
Background: The classical description of post-stroke upper limb impairment follows a proximal-to-distal impairment gradient. Previous studies are equivocal on whether the hand is more impaired than the arm.
Objective: To compare impairment of the arm and hand during subacute stroke.
Method: A total of 73 individuals were evaluated for impairment of the upper limb within 30 days (early subacute) and within 90–150 days (late subacute) of stroke. Impairments were quantified using the Chedoke-McMaster Stroke Assessment (CMSA) for the arm and hand, Purdue Pegboard task, and a robotic Visually Guided Reaching task.
Results: In the early phase 42% of participants in the early phase and 59% in the late phase received the same CMSA score for the ar and hand, with 88% and 95% of participants in the early and late phases, respectively, receiving a 1-point difference. Strong correlations exist between the CMSA arm and hand scores (early r = 0.79, late r = 0.75), and moderate – strong correlations exist between CMSA arm and hand scores and Purdue Pegboard and Visually Guided Reaching performances (r = 0.66–0.81). No systematic differences were found between the arm and hand.
Conclusion: Impairments in the arm and hand during subacute stroke are highly correlated and do not support the presence of a proximal-to-distal gradient.
LAY ABSTRACT
Motor impairments are a common occurrence after stroke, and are classically believed to present in a gradient from more impairment in the hand to less impairment in the arm. In this study, participants who had recently had a stroke underwent assessment with the Chedoke-McMaster Stroke Assessment, the Purdue Pegboard task, and a Visually Guided Reaching task to quantify impairment and performance of the arm and hand. Levels of impairment in the arm and hand, as measured with the Chedoke-McMaster Stroke Assessment, were found to be highly correlated. The study also showed strong correlations between quantitative measures of performance for both the arm and hand. Overall, our results do not support the presence of a proximal-to-distal gradient of impairment during subacute stroke.
Key words: stroke; upper extremity; exoskeleton device; ataxia.
Citation: J Rehabil Med 2023; 55: jrm2174. DOI: https://dx.doi.org/10.2340/jrm.v55.2174.
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Mar 27, 2023; Published: May 23, 2023
Correspondence address: Lydia N. Reid, Centre for Neuroscience Studies, Room 237, Botterell Hall, Queen’s University, Kingston K7L 3N6, Ontario, Canada
Competing interests and funding: SHS is the co-founder and chief scientific officer of Kinarm, the company that commercializes the robotic technology used in the current study. The authors LR and SPD have no competing interests to declare.
A common occurrence following stroke is impairment in upper limb motor function, impacting an individual’s ability to perform daily activities, such as pouring a drink or buttoning clothing (1). Such tasks require the hand to grasp, and the arm to move and stabilize the hand in space. Thus, both hand and arm function are essential for full recovery and are key objectives for rehabilitation (2, 3).
Early recovery research by T. E. Twitchell observed that there was a general pattern of recovery following stroke through which each patient progressed (4). Among these observations was the finding that grip strength tended to recover last, and only if, the arm was fully recovered. This contributed to the classical perception that motor recovery of the upper limb followed a proximal-to-distal gradient, with more severe impairments associated with the hand (5–7). Historically, support for this idea came from the anatomical structure of the motor system: the motor cortex has a larger representation of the hand compared with the arm (8, 9), and thus, a greater proportion of descending projections from the contralateral hemisphere targeting proximal vs distal muscles (10). Also, more corticospinal tract neurones synapse directly onto hand motoneurones compared with arm motoneurones (11, 12), and there are more alternative descending motor pathways that influence the arm (13). Taken together, this could suggest that disruption to the corticospinal tract could disproportionately affect motor function of the hand compared with the arm.
In contrast, studies comparing active range of motion, joint individuation (14), and normalized strength for upper limb segments reported minimal differences between proximal and distal portions of the upper limb (15, 16) and found no evidence of an impairment gradient following stroke. The primary objective of this study was to re-visit this issue by comparing measures of arm and hand impairment. Comparing impairments of the arm and hand can be challenging, given that the actions of each are unique; for example, reaching with the arm vs grasping with the hand. Therefore, the primary comparison of this study used the Chedoke-McMaster Stroke Assessment (CMSA) (17). CMSA provides a criteria-based, 7-point ordinal scale that categorizes arm- and hand-specific impairments individually into stages of recovery, thereby allowing for comparison of arm and hand impairments. In addition, the combined arm and hand CMSA is highly correlated (r = 0.95) with the Fugl-Meyer Assessment-Upper Extremity (17).
While the CMSA is useful to compare levels of impairment between arm and hand, it is limited in its ability to objectively quantify behaviour. Clinical ordinal scales are useful for informing treatment, but often lack the sensitivity to detect small but meaningful functional changes. Continuous measures that quantify behaviour can provide an objective measure of motor impairment, and potentially a finer resolution of recovery. Therefore, the second objective of this study was to compare CMSA arm and hand scores with quantitative measures. The Purdue Pegboard (PPB) test was used to quantify coordination and dexterity of both the arm and hand (18). A robotic 2-D visually guided reaching task was used to quantify arm impairment, since continuous and quantifiable movement data is recommended by the recent Stroke Recovery and Rehabilitation Roundtable (SRRR) (19, 20).
METHODS
Participants
The participant pool for this study was selected from a database that includes individuals with stroke who have completed clinician-reported assessments as well as a timed task performance test and robotic-based performance test to quantify upper arm sensory, motor, and/or cognitive impairment. These individuals were recruited at 2 Kingston, Ontario locations (St Mary’s on the Lake, and Providence Care Hospitals) and 2 Calgary, Alberta locations (Foothills Medical Centre and the Dr Vernon Fanning Care Centre). Inclusion criteria for participants with stroke were: first time clinically evident, unilateral stroke and able to follow simple instructions in English. Participants with pre-existing neurological disorders or ongoing orthopaedic injury to the arm were excluded. All participants were evaluated within 30 days of the stroke event and again within 90–150 days of stroke, which aligns with Stroke Recovery and Rehabilitation Roundtable recommendations for collection of data over a recovery period (20). This study was approved by the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board (#ANAT042-05) and the University of Calgary’s Conjoint Health Research Ethics Board (#22123).
Chedoke-McMaster Stroke Assessment
Participants completed a battery of traditional clinical assessments. Motor impairments of the arm and hand were quantified using the CMSA impairment inventory, which is based on Twitchell’s stages of recovery (0 = flaccid appendage, 7 = normal movement) (4, 17). The CMSA assesses the arm and hand with separate inventories. Each inventory involves completing tasks of either arm- or hand-specific movements, which is guided by a skilled assessor and scored accordingly based on their performance (17). A description of the level of impairment in each stage is provided in Table I (17).
Purdue Pegboard
This study also examined the dexterity of the limb using the PPB test (18), which involves 2 trials of 30s each, in which the participant must place as many pegs into holes on the board as possible, with the mean of the 2 trials used as the score. This task involves both manual dexterity as well as gross movement and requires use of both the arm and the hand to perform the necessary motor actions to complete the task.
Visually Guided Reaching: a robotic horizontal target-reaching task
A standard battery of tasks was performed using the Kinarm Exoskeleton laboratory (Kinarm, Kingston, Ontario, Canada) (21). Participants were seated and the exoskeletons were adjusted to align with the participant’s shoulder and elbow joints, providing weight support and maintaining the movement of the arms in the horizontal workspace. A virtual reality system aligned with the horizontal workspace provided visual feedback of spatial targets and hand position. A trained operator gave direction to the participants and monitored the performance in real time to ensure that the task was completed appropriately.
Arm motor impairment was assessed using 1 of 2 versions of a Visually Guided Reaching (VGR) task (19, 22). The first variant of the VGR task included 8 peripheral targets presented on a screen, spaced 45° apart and 10 cm from the initial central target. Each trial began with the patient moving their index finger (represented by a 0.4-cm radius white circle) to the central target (a 1.0-cm radius red circle). After a random time period, a peripheral target (1.0-cm radius red circle) was illuminated, and participants were given 3s to complete the reach. The peripheral targets were presented in a pseudorandom order and each target was presented 6 or 8 times. A second variant of the task was similar, but with only 4 peripheral targets. After reaching and maintaining the hand at the peripheral target for a brief period of time, the central target was re-illuminated, and participants made a reaching movement back to the centre target. The 4-target reaching task has been shown in previous studies to provide a nearly identical performance in terms of classification sensitivity, specificity, and accuracy compared with the 8-target task (23).
Performance on VGR was quantified using 14 parameters reflecting spatial and temporal features of hand motion (Kinarm, KST Summary, https://kinarm.com/kinarm-products/kinarm-standard-tests/). These parameters are described in Table II. Raw parameter scores were converted to normalized Z-scores using statistical models of performance previously calculated from a large group of healthy controls. Briefly, the data is transformed using Box-Cox power transforms (24) and multiple linear regression to account for age, sex, and handedness. The Task Score is a composite score of the parameter Z-scores and was calculated using root-sum-squares. For the VGR task, the weight of each parameter is calculated based on the number of successful trials, excluding parameters that are highly correlated (r < 0.9) with another parameter. The Task Score is a positive value, with zero denoting the best performance and increasing scores denoting poorer performance (25). By calculating Z-scores and Task Scores using models developed from healthy controls, it is then possible to identify if participants with stroke are impaired relative to the expected performance of an individual of similar age, sex, and handedness. Performance was considered impaired if the Task Score exceeded 1.96, which corresponds to the bottom fifth percentile of performance for healthy participants (26).
Clinical data (i.e. CMSA and PPB) was not always collected on the same day as robotic assessment to reduce fatigue, but the upper limit for the number of days between clinical and robotic assessments was 4 (mean number of days between assessments: 0.93).
Statistical analysis
Non-parametric tests were utilized throughout this paper because CMSA and PPB measures are quantified by ordinal scales. Differences between the medians of CMSA arm and hand scores were determined using the Wilcoxon signed-rank test and a χ2 test of independence was performed to test for association between CMSA arm and hand scores. Correlations between clinical scores, PPB, and VGR were determined using Spearman’s correlation coefficient for ranked data. A Bonferroni correction was used to adjust the level of significance for Spearman’s correlations, there were 10 correlations and therefore the p-value was adjusted to 0.005. Difference between correlations was determined by comparing bootstrap confidence intervals set to 95% (27). Correlations below 0.50 were defined as weak, between 0.50–0.75 were defined as moderate, between 0.75–0.90 were defined as strong, and above 0.90 was defined as very strong (28). Statistical calculations were performed with Matlab Statistics and Machine Learning Toolbox (MATLAB version: 9.13.0 (R2022b), Natick, Massachusetts: The MathWorks Inc).
RESULTS
Participant characteristics
The study examined 73 individuals with first-time, hemiparetic stroke. Subjects included 20 females, mean age 62 years, age range 33–86 years. Demographic information on the participants is shown in Table III.
Comparison of CMSA arm and hand during early subacute phase
The CMSA was used to assess the level of impairment in the arm and hand. The distribution of CMSA scores for both the arm and hand were skewed towards higher values, indicating that, overall, the population tended towards milder impairment of the upper limb (29). A score of 5 or higher on the CMSA arm and hand scales was achieved by 62% and 73% of participants, respectively (Fig. 1A and B). Of those, there were 22 participants (30% of total) who received a score of 7 on the CMSA arm score and 16 (22% of total) who received a 7 on the CMSA hand score. Notably, this indicates a ceiling effect with the CMSA assessment in this population.
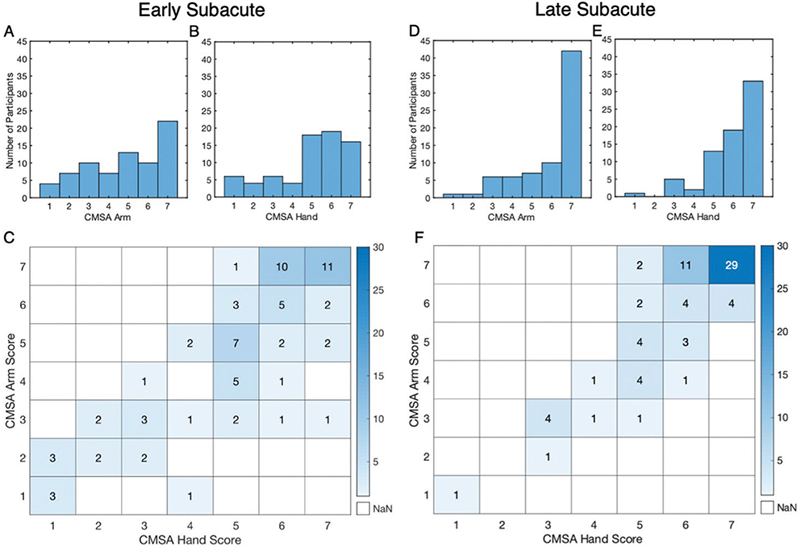
Fig. 1. Comparison of Chedoke-McMaster Stroke Assessment (CMSA) Scores for the Arm and Hand. (A–B) Distribution of participant CMSA scores during early subacute phase. (C) Cross-tabulation matrix of CMSA Hand vs Arm scores for each participant. χ2(6,73), p = 0.02. (D–E) Distribution of participant CMSA scores during late subacute phase. (F) Cross-tabulation matrix of CMSA Hand vs Arm scores, χ2(6,73), p = 0.
CMSA scores for the arm and hand had a rank correlation of 0.79 (95% CI 0.64–0.87, p << 0.005), indicating a strong relationship (Fig. 1C). The χ2 test also yielded significant results, indicating that the CMSA scores for the arm and hand are associated. The results show that 42% of participants received the same score for the arm and hand and 88% of individuals were within 1 point. A similar proportion of individuals scored higher on the arm as individuals who scored higher on the hand, with 30% having a higher arm score and 27% having a higher hand score. Only 3 participants (4%) within the sample displayed a difference of 3 points or more between the arm and hand scores; notably, all with higher hand scores than arm scores. Across all participants, the median for the arm score was 5 (Q1:3, Q3:7) and the median for the hand score was 5 (Q1:4, Q3:6), and these were not statistically different (p = 0.47).
Comparison of CMSA arm and hand during late subacute phase
Differences in the distribution of scores during the late subacute phase were skewed towards the highest values, with median CMSA scores of 7 (Q1:5, Q3:7) for the arm and 6 (Q1:5, Q3:7) for the hand, which are 1–2 points higher compared with the early subacute phase. Very few individuals have scores of 3 or below, whereas 59 participants (81%) received a 5 or above for the arm, and 65 participants (89%) received a 5 or above for the hand. Forty-two participants (58%) received a 7 for the arm and 33 participants (45%) received a 7 for the hand (Fig. 1D and E), indicating a ceiling effect that is present in both phases.
The relationship between CMSA arm and hand scores remained strong in the late subacute phase, with a rank correlation of 0.75 (95% CI 0.58–0.85, p << 0.005) (Fig. 1F) and a significant test of association (χ2(6,73) p = 0). The proportion of individuals that received the same arm and hand score also remained high, at 58%, with 21% scoring higher in the arm and 21% scoring higher on the hand. Only 4 participants received a hand score that was 2 points different from their arm score, and none received 3 or more points different in the late subacute phase.
Comparison of arm and hand with PPB
In the current cohort there was a significant floor effect with PPB during the early subacute phase, with 27% of participants receiving 0, the lowest possible score, indicating that the participant was unable to place a single peg in the board during either of the 30-s trials (Fig. 2A).
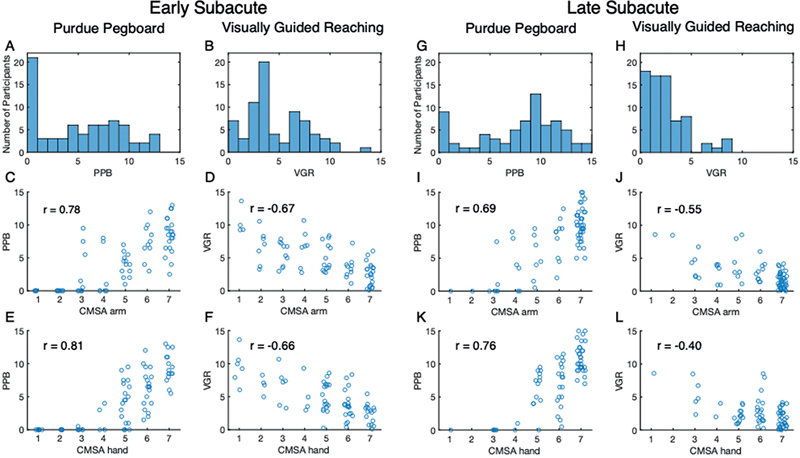
Fig. 2. Comparison of Chedoke-McMaster Stroke Assessment (CMSA) scores with Purdue Pegboard (PPB) and Visually Guided Reaching (VGR) task. (A–F) Early subacute phase (A) Distribution of scores attained on the PPB task with each bin containing the half-point above (e.g. bin of 0 contains scores of 0 and 0.5; bin of 1 contains scores of 1 and 1.5). (B) Distribution of VGR Task Scores with each ordinal bin containing Task Scores including and above each bin (e.g. bin 0 contains 0.00–0.99; bin 1 contains 1.00–1.99). (C–F) Scatter plots of PPB and VGR Task Scores vs CMSA scores with Spearman’s r. Jitter was applied according to x = x0.02z, where z is a randomly generated number within the interval (0,1). (C) PPB vs CMSA Arm score, r = 0.78, p < 0.005. (D) VGR Task Score vs CMSA Arm score, r = –0.67, p < 0.005. (E) PPB vs CMSA Hand score, r = 0.81, p < 0.005. (F) VGR Task Score vs CMSA Hand score, r = –0.66, p < 0.005. (G–L) Late subacute phase (G) Distribution of PPB scores. (H) Distribution of VGR Task Scores during late subacute phase. (I) PPB vs CMSA Arm score, r=0.69, p < 0.005. (J) VGR Task Score vs CMSA Arm score, r = –0.55, p < 0.005. (K) PPB vs CMSA Hand score, r = 0.76, p<0.005. (L) PPB vs CMSA Hand Score, r = –0.40, p < 0.005.
The PPB task showed a strong correlation with both the CMSA arm and hand score, with correlations of 0.78 (95% CI 0.62–0.87, p << 0.005) and 0.81 (95% CI 0.70–0.88, p << 0.005), respectively (Fig. 2C and E). However, these high correlations mask important features of the underlying relationships; most individuals that scored below 5 on the CMSA arm or hand scores received 0 on the PPB, as noted above. Subjects who scored 5 or higher on either CMSA score showed a wide range of PPB scores. With a CMSA score of 7, participants may have scored anywhere from 3 to 13 on the PPB.
In the late subacute phase, the number of participants that receive 0 reduces to only 11% (Fig. 2G). The correlations between PPB and the CMSA arm and hand scores decrease in the late phase, but remain moderate to strong and significant (0.69 95% CI 0.53–0.80, p < 0.005, and 0.78 95% CI 0.64–0.84, p < 0.005, respectively) (Fig. 2I and K). Although the proportion of overlapping confidence intervals for these correlations also decreases slightly, from 59% in the early phase to 52% in the late phase, they still have considerable overlap, denoting no significant difference in the relationship between the PPB and CMSA arm and hand scores. Floor effects that were observed in the early phase are also seen, where individuals who score below 5 on the CMSA are likely to score 0 on the PPB. There are several exceptions to this in the CMSA arm vs PPB relationship (Fig. 2I), where individuals that received 3 or 4 on the CMSA arm score could still place 4–9 pegs in the board.
Comparison of arm and hand with Visually Guided Reaching
VGR Task Scores ranged from 0 to 14 in the early subacute phase of stroke, with 64 participants (88%) receiving a Task Score above 1.96, which denotes performance worse than 95% of healthy controls and is considered impaired. The distribution of Task Scores (Fig. 2B) displays a skew towards lower values, which is the end of lesser impairment and is akin to the skew seen in the early subacute CMSA scores (Fig. 1A and B).
The relationships between VGR and CMSA arm and hand scores are moderate, with a correlation of –0.67 (95% CI –0.51 to –0.78, p < 0.005) between the VGR Task Score and CMSA arm score and –0.66 (95% CI –0.50 to –0.77. p << 0.005) between the Task Score and CMSA hand score (Fig. 2D and F). These correlations also show considerable overlap in the confidence intervals. Of the 22 participants (30%) who received a 7 on the CMSA arm score, which denotes normal movement of the limb, only 9 received a Task Score of less than 1.96, denoting healthy performance. Likewise, of the 16 participants (22%) who received a 7 on the CMSA hand score, only 5 received a task score below 1.96. At a given CMSA score, there is considerable variability in their reaching Task Scores. For example, Task Scores ranged from approximately 3 to 8 for individuals with a 5 for CMSA of the hand or arm. In contrast, of the 9 participants with a Task Score below 1.96 (unimpaired), only 1 participant had a CMSA arm score below 7 and 4 participants had CMSA hand scores below 7.
VGR Task Scores tend to be shifted to lower values in the late subacute phase, with 34 participants (47%) scoring below 1.96, indicating an unimpaired performance, and 92% of participants scoring below 5 (Fig. 2H).
Correlations between VGR and CMSA in the late subacute phase decrease to a weak to moderate strength, with correlations of –0.55 (95% CI –0.36 to –0.70, p < 0.005) between VGR Task Score and CMSA arm score and –0.40 (95% CI –0.17 to –0.58, p < 0.005) between VGR Task Score and CMSA hand score (Fig. 2J and L). The overlap of confidence intervals between the VGR Task Score and the arm and hand also decreased in the late phase; whereas the confidence intervals during the early phase overlapped by approximately 93%, the late phase confidence intervals overlap by only 42%.
DISCUSSION
This study compared the levels of impairment of the arm and hand in a cohort of individuals during the early and late subacute phases of stroke. Strong correlations were found between the CMSA arm and hand scores, and levels of impairment for the hand and arm were similar with this tool. Both CMSA scores also correlated with the PPB and VGR Task Score. While the correlations decreased from the early to the late subacute phase, correlations were still fair to moderate, and levels of impairment were similar. The results of this study indicate that impairments of the arm and hand are highly correlated and that this relationship is preserved throughout the subacute phase of stroke recovery.
Previous studies examining this issue have focused on measures of strength and range of motion to assess the levels of impairment in the upper extremity (4, 6, 14–16). Some studies found weakness was greater for proximal compared with distal muscles (4, 6), whereas others found no difference in strength or functional range for the upper limb segments. Notably, studies that compared post-stroke impairment with intact control participants found evidence of a proximal-to-distal gradient (6), whereas studies that used the ipsilesional arm as a matched control for the contralesional arm found no difference in impairment between the segments of the limb (15, 16). Several studies have shown that impairments are present in the ipsilesional arm post-stroke (30, 31). Thus, there may be a general reduction in strength in both limbs that preferentially impacts the hand compared with the arm.
This comparison of CMSA scores with other motor tasks provides further support that impairment of the hand and arm are tightly coupled. It may not be surprising that PPB is significantly correlated with both CMSA scores, given that the task requires both arm and hand function to complete. The VGR task, however, only quantifies arm motor function, but was almost equally correlated with CMSA scores for the arm and the hand.
The findings of this study challenge the results of early clinical research that led to the view that the hand is commonly more impaired than the arm following stroke, an assumption that can impact the decisions made by clinicians regarding recovery and rehabilitation. The current findings suggest that, when informing rehabilitation strategies, it should not be assumed that the upper limb will follow the traditional proximal-to-distal gradient of impairment during recovery. Furthermore, the tight coupling between the arm and hand suggests that the level of impairment of one may be inferred from assessment of the other. This may be advantageous in situations where assessment time is limited due to time constraints or patient fatigue, or when assessment technology is limited to measuring just the arm or hand.
As noted above, previous studies focused on strength and range of motion to compare proximal and distal components of the limb. The current approach was to use an assessment tool that quantified the ability to perform motor actions. This is somewhat challenging, as motor actions for the arm and hand are unique (e.g. reaching vs grasping), therefore comparing impairment of the two may not be possible. However, the CMSA score for the arm and hand is based on Twitchell’s original observations on the return of function following hemiplegia and Brunnstrom’s stages of recovery (4, 17, 32). Critically, each level in the scales reflects characteristics that are indicative of a certain level of impairment; from flaccid paralysis, through different levels of synergistic motor actions, to full function. Both the arm and hand will pass through these levels of impairment, but they are assessed according to tasks that are appropriate for the respective structure (e.g. when moving out of flaccid paralysis, touching the hand to the chin is a task in the arm scale, whereas touching the index finger to the thumb is a task in the hand scale) (17). While the arm and hand clearly have different motor actions, this use of stages of impairment to assess performance means that a given level of impairment reflects the ability to control different muscle groups to generate effector-appropriate actions. From this perspective, there does not appear to be a general difference in the impairments observed for the hand or arm, at least at the group level when measured with the CMSA. Most individuals received CMSA scores that were the same or were within 1 point for the arm and hand. Notably, of the 9 individuals with hand and arm scores that were different by 2 points or more, 8 received lower scores for the arm compared with the hand. Thus, substantive asymmetry in performance using this scale was more likely to reflect greater proximal impairment than distal impairment.
The general severity of post-stroke deficits has been tied to the degree of damage to the corticospinal tract (CST) (33–35). It has been thought that the proximal-to-distal gradient of impairments is a result of disruption to the CST. The proportion of the CST descending from the damaged hemisphere has more projections to motoneurones of distal muscles. Furthermore, the proportion of the CST that descends uncrossed from the undamaged hemisphere has more projections to proximal motoneurones (8, 12); however, the functional relevance of these connections may be limited for recovery (13). A gradient in the upper extremity may more likely be driven by the compensatory control of other motor pathways (e.g. the reticulospinal or the rubrospinal tract) that provide compensatory control over the proximal muscles of the limb (10, 36). It has been speculated that these alternative pathways allow for volitional contraction and strength to return to the proximal muscles, but that the CST is required for dexterity, inter-joint coordination, and overall recovery of the upper limb (13, 37). This is, however, taken with the caveat that recent evidence suggests the reticulospinal tract does contribute to hand function (38), but further work is needed to understand how alternate descending pathways may facilitate recovery of the arm and hand. The results of this study support the finding that impairment is a result of disruption to the CST, and that alternative pathways may provide some level of compensatory control. This is evidenced by the presence of some motor control in participants with low CMSA scores, as captured by the VGR Task Score, but the lack of dexterity required to place even a single peg in the board during the PPB task.
Limitations to this study include notable instances of floor and ceiling effects with the PPB and CMSA, respectively. These tools were chosen based on their use as a reliable tool for the assessment of impairment and to gain an understanding of how impairment would be classified in the clinical field. Despite this, a floor effect was seen in the PPB during the early subacute phase, where 27% of participants received a score of 0 (Fig. 2A). Impairments can be quite severe during the early subacute phase of stroke, including challenges with both strength and dexterity. Thus, it is perhaps not surprising that a task that only captures the number of successful trials will lead to a floor effect. The CMSA provided some stratification of individuals during the early phase by separating those with flaccid paralysis from those with some ability to generate limited gross motor actions. However, in the late subacute phase, the CMSA scores displayed a ceiling effect, as 58% and 45% of participants scored a 7 for the arm and hand, respectively. The VGR task provided further stratification of individuals at each stage of the CMSA, which is one reason why many support the use of kinematic-based scales that measure the quality of movement (39–42). Such approaches are less impacted by floor and ceiling effects and provide a continuous scale to assess performance. This removes ceiling effects, as performance can be assessed even through the entire healthy range. Such resolution may not be necessary for present clinical applications but is essential for exploring potential benefits of therapeutic interventions. Finally, the CMSA mixes spasticity and motor function, partly because the tool was created based on the Brunnstrom stages of recovery and the disappearance of spasticity over various stages of recovery. While the other tools, such as Fugl-Meyer Assessment (FMA) (43), do not explicitly measure spasticity, a patient with spasticity will still have issues with the FMA tasks and this impacts the individual’s score.
In conclusion, this study found strong correlations between CMSA scores of the arm and hand over both the early and late subacute phases of stroke recovery. The study also found moderate to strong correlations between clinical scores and measures of performance, such as the PPB and the VGR task. The results of this study show that impairments of the arm and hand are highly correlated during the subacute phase of stroke and do not support the presence of a proximal-to-distal gradient.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the assistance of Kim Moore, Simone Appaqaq, Helen Bretzke, Ethan Heming, Mary Jo Demers, Megan Metzler, Janie Yajure, and Mark Piitz in the collection and management of the experimental data. We would also like to thank Dr Catherine Lowrey for help in editing the manuscript. Queen’s University is situated on traditional Haudenosaunee and Anishinaabek territory.
Financial support was provided by an Ontario Research Fund and the Canadian Institute of Health Research.
REFERENCES
- Nakayama H, Jørgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil 1994; 75: 394–398.
- Hatem SM, Saussez G, Della Faille M, Prist V, Zhang X, Dispa D, et al. Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci 2016; 10: 442.
- Kapandji I. The upper limb as logistical support for the hand. The Hand 1981; 1: 94–106.
- Twitchell T. The restoration of motor function following hemiplegia in man. Brain 1951; 74: 443–480.
- Muellbacher W, Richards C, Ziemann U, Wittenberg G, Weltz D, Boroojerdi B, et al. Improving hand function in chronic stroke. Arch Neurol 2002; 59: 1278–1282.
- Colebatch J, Gandevia S. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain 1989; 112: 749–763.
- Saladin L, Fredericks C. Cerebrovascular disease: stroke. In: Saladin, LK, Fredericks, CM, editors. Pathophysiology of the motor systems: principles and clinical presentations. Philadelphia, PA: F.A. Davis company; 1996, 486–512.
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. J Physiol 1992; 448: 397–412.
- Sanes JN, Donoghue JP, Thangaraj V, Edelman RR, Warach S. Shared neural substrates controlling hand movements in human motor cortex. Science 1995; 268: 1775–1777.
- Isa T, Mitsuhashi M, Yamaguchi R. Alternative routes for recovery of hand functions after corticospinal tract injury in primates and rodents. Current Opin Neurol 2019; 32: 836–843.
- Porter R, Lemon R. Corticospinal function and voluntary movement. New York, NY: Oxford University Press; 1993.
- Rathelot J-A, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Nat Acad Sci 2009; 106: 918–923.
- Schambra HM, Xu J, Branscheidt M, Lindquist M, Uddin J, Steiner L, et al. Differential poststroke motor recovery in an arm versus hand muscle in the absence of motor evoked potentials. Neurorehabil Neural Repair 2019; 33: 568–580.
- Lang CE, Beebe JA. Relating movement control at 9 upper extremity segments to loss of hand function in people with chronic hemiparesis. Neurorehabil Neural Repair 2007; 21: 279–291.
- Andrews AW, Bohannon RW. Distribution of muscle strength impairments following stroke. Clin Rehabil 2000; 14: 79–87.
- Beebe JA, Lang CE. Absence of a proximal to distal gradient of motor deficits in the upper extremity early after stroke. Clin Neurophysiol 2008; 119: 2074–2085.
- Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Hullenaar SV, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke 1993; 24: 58–63.
- Tiffin J, Asher EJ. The Purdue pegboard; norms and studies of reliability and validity. J Appl Psychol 1948; 32: 234–247.
- Coderre AM, Abou Zeid A, Dukelow SP, Demmer MJ, Moore KD, Demers MJ, et al. Assessment of upper-limb sensorimotor function of subacute stroke patients using visually guided reaching. Neurorehabil Neural Repair 2010; 24: 528–541.
- Eng JJ, Bird M-L, Godecke E, Hoffmann TC, Laurin C, Olaoye OA, et al. Moving stroke rehabilitation research evidence into clinical practice: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2019; 14: 766–773.
- Mostafavi SM, Glasgow JI, Dukelow SP, Scott SH, Mousavi P. Prediction of stroke-related diagnostic and prognostic measures using robot-based evaluation. 2013 IEEE 13th International Conference on Rehabilitation Robotics (ICORR) 2013; 1–6.
- Semrau JA, Herter TM, Scott SH, Dukelow SP. Examining differences in patterns of sensory and motor recovery after stroke with robotics. Stroke 2015; 46: 3459–3469.
- Mostafavi SM, Dukelow SP, Glasgow JI, Scott SH, Mousavi P. Reduction of stroke assessment time for visually guided reaching task on KINARM exoskeleton robot. 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2014; 5296–5299.
- Box GE, Cox DR. An analysis of transformations. J Roy Statist Soc 1964; 26: 211–243.
- Simmatis L, Krett J, Scott SH, Jin AY. Robotic exoskeleton assessment of transient ischemic attack. PLOS One 2017; 12: e0188786.
- Kinarm. Dexterit-E 3.9 user guide. 2021; Kingston, ON, Canada 2021.
- Bishara AJ, Hittner JB. Confidence intervals for correlations when data are not normal. Behav Res Meth 2017; 49: 294–309.
- Koo TK, Li MY. A Guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016; 15: 155–163.
- Barreca SR, Stratford PW, Lambert CL, Masters LM, Streiner DL. Test-retest reliability, validity, and sensitivity of the chedoke arm and hand activity inventory: a new measure of upper-limb function for survivors of stroke. Arch Phys Med Rehabil 2005; 86: 1616–1622.
- Chestnut C, Haaland KY. Functional significance of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehabil 2008; 89: 62–68.
- Semrau JA, Herter TM, Kenzie JM, Findlater SE, Scott SH, Dukelow SP. Robotic characterization of ipsilesional motor function in subacute stroke. Neurorehabil Neural Repair 2017; 31: 571–582.
- Brunnstrom S. Movement therapy in hemiplegia. A neurophysiological approach. Open Journal of Nursing 1970: 113–122.
- Birchenall J, Térémetz M, Roca P, Lamy J-C, Oppenheim C, Maier MA, et al. Individual recovery profiles of manual dexterity, and relation to corticospinal lesion load and excitability after stroke –a longitudinal pilot study. Neurophysiol Cliniq 2019; 49: 149–164.
- Maraka S, Jiang Q, Jafari-Khouzani K, Li L, Malik S, Hamidian H, et al. Degree of corticospinal tract damage correlates with motor function after stroke. Ann Clin Transl Neurol 2014; 1: 891–899.
- Pineiro R, Pendlebury S, Smith S, Flitney D, Blamire A, Styles P, et al. Relating MRI changes to motor deficit after ischemic stroke by segmentation of functional motor pathways. Stroke 2000; 31: 672–679.
- Li S, Chen Y-T, Francisco GE, Zhou P, Rymer WZ. A unifying pathophysiological account for post-stroke spasticity and disordered motor control. Front Neurol 2019; 10: 468.
- Hammerbeck U, Tyson SF, Samraj P, Hollands K, Krakauer JW, Rothwell J. The strength of the corticospinal tract not the reticulospinal tract determines upper-limb impairment level and capacity for skill-acquisition in the sub-acute post-stroke period. Neurorehabil Neural Repair 2021; 35: 812–822.
- Maitland S, Baker SN. Ipsilateral motor evoked potentials as a measure of the reticulospinal tract in age-related strength changes. Front Aging Neurosci 2021; 13: 612352.
- Balasubramanian S, Colombo R, Sterpi I, Sanguineti V, Burdet E. Robotic assessment of upper limb motor function after stroke. Am J Phys Med Rehabil 2012; 91: S255–S269.
- Hidler J, Nichols D, Pelliccio M, Brady K. Advances in the understanding and treatment of stroke impairment using robotic devices. Top Stroke Rehabil 2005; 12: 22–35.
- Otaka E, Otaka Y, Kasuga S, Nishimoto A, Yamazaki K, Kawakami M, et al. Clinical usefulness and validity of robotic measures of reaching movement in hemiparetic stroke patients. J Neuroeng Rehabil 2015; 12: 66.
- Scott SH, Dukelow SP. Potential of robots as next-generation technology for clinical assessment of neurological disorders and upper-limb therapy. J Rehabil Res Dev 2011; 48: 335–353.
- Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 1975; 7: 13–31.