ORIGINAL REPORT
REFERRALS TO EARLY SPECIALIZED REHABILITATION AFTER TRAUMATIC BRAIN INJURY DURING THE COVID-19 PANDEMIC
Cathrine TVERDAL, MPhil1,2, Cathrine BRUNBORG, MSc3, Eirik HELSETH, MD, PhD1,2, Nada ANDELIC, MD, PhD4,5, Marte KOCH, BSN4, Cecilie RØE MD, PhD2,4,5, Mads AARHUS, MD, PhD1 and Torgeir HELLSTRØM, MD, PhD4,
From the 1Department of Neurosurgery, Oslo University Hospital, 2Faculty of Medicine, Institute of Clinical Medicine, University of Oslo, 3Oslo Centre for Biostatistics and Epidemiology, Research Support Services, Oslo University Hospital, 4Department of Physical Medicine and Rehabilitation, Oslo University Hospital and 5Research Centre for Habilitation and Rehabilitation Models and Services (CHARM), Faculty of Medicine, Institute of Health and Society, University of Oslo, Oslo, Norway
Objective: To quantify potential changes in direct referral to early specialized rehabilitation during the COVID-19 pandemic and the injury pattern of patients hospitalized with traumatic brain injury (TBI) at a level 1 trauma centre.
Methods: In this registry-based study, data were retrieved from the Oslo TBI Registry-Neurosurgery and included adult patients with injury-related intracranial findings admitted to Oslo University Hospital (OUH). The study focused on a period of time when OUH was in any level of preparedness because of the COVID-19 pandemic; March 2020 to August 2021. For comparison, the study used patients hospitalized for TBI in 2018 and 2019.
Results: A total of 1,310 hospitalized patients with TBI were divided into 2 groups; pre-pandemic and pandemic. Direct referral to early rehabilitation was maintained. Patient volume remained stable, and there were no differences between the groups regarding patient characteristics and acute management, although there was a significantly higher proportion of TBIs secondary to electric scooter accidents in the pandemic group. Results from univariable and multivariable logistic regression showed a multifaceted reality, but younger age, none or mild pre-injury comorbidity and severe disability due to TBI at discharge from acute care remained stable strong predictors of direct referral to rehabilitation.
Conclusion: For patients with moderate-severe TBI, the direct pathway to early specialized rehabilitation was maintained during 2020–21. However, the pandemic continued and the long-term impact for rehabilitation services is not yet known.
LAY ABSTRACT
After traumatic brain injury, early rehabilitation is important in order to achieve optimal functional outcome. The COVID-19 pandemic has put the health services under pressure, and hospitals in Norway were reorganized to be able to treat a large number of patients with COVID-19. Therefore, this study investigated to what extent the transfer to early rehabilitation has been affected and whether there have been changes in volume and characteristics of patients admitted to hospital with traumatic brain injury. In conclusion, the direct pathway to early rehabilitation was maintained during the pandemic years 2020 and 2021. Moreover, there was no change in the number of hospital admissions or patient characteristics, and acute management was maintained. However, the true long-term consequences for rehabilitation are not yet known.
Key words: traumatic brain injury; pathway; early specialized rehabilitation; COVID-19; trauma centre.
Citation: J Rehabil Med 2022; 54: jrm00334. DOI: https://dx.doi.org/10.2340/jrm.v54.2203
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Aug 14, 2022; Epub ahead of print: Sep 9, 2022; Published: Oct 03, 2022
Correspondence address: Torgeir Hellstrøm, Department of Physical Medicine and Rehabilitation, Oslo University Hospital, Oslo, Norway. E-mail: torgeir.hellstrom@ous-hf.no
Competing interests and funding: The authors have no conflicts of interest to declare.
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) incited a global public health response through government, commercial, and behaviour protocols aimed at reducing spread of the disease. The 2 first cases of COVID-19 in Oslo, the capital of Norway, were confirmed on 27 February 2020. On 28 February 2020, the City Government was on emergency alert, and on 13 March 2020, the Norwegian national authorities decided to implement strict national interventions. On 11 March 2020, Oslo University Hospital’s (OUH) preparedness response to COVID-19 was to postpone non-urgent operations, reorganize services and reallocate medical staff. This included converting the early rehabilitation ward to a COVID-19 ward and transferring the rehabilitation of patients with traumatic brain injury (TBI) to the neurosurgery ward.
Comparing TBI referrals during lockdown with previous years, many studies showed a decrease in the incidence of referrals and admissions during lockdown periods (1–6). However, the relief of lockdown measures was accompanied by increases in TBIs (2, 7). The observed changes may be explained by lockdown measures resulting in people staying at home, which led to global reductions in road traffic accidents (RTAs) (1, 8–10). Neurotrauma was more likely to take place indoors at private residences, accompanied by increases in falls, which were seen among > 65-year-olds (6). Regarding management of TBI, Manivannan et al. (9) found a significant decrease in surgical management of TBI, with a comparable increase in conservative management. This may be a reflection of a change towards milder TBI during the pandemic. However, Goyal et al. (8) similarly experienced a significant increase in the proportion of conservatively managed TBI, despite significant increases in severity of TBI. There is a considerable variation between countries (11), and the eventual impact on unmet service needs for the patients with TBI during the pandemic is largely unexplored.
Depending on its severity, TBIs can result in cognitive, social, emotional and motor deficits requiring extensive rehabilitative therapy. Early initiation of a continuous care and rehabilitation pathway can improve functional outcomes and reduce hospitalization costs for patients with severe TBI (12, 13). Hence, a continuous care pathway including specialized rehabilitation has been a goal for the services over the last years (14). However, during the COVID-19 pandemic, both the non-urgent neurosurgical and the rehabilitation services were downscaled. Combining trauma referral and TBI severity with referral to specialized rehabilitation provides the opportunity to explore the pandemic’s impact on the patients’ needs. Therefore, the aim of this study was to quantify potential changes in direct referral to early specialized rehabilitation during the COVID-19 pandemic. The study also aimed to evaluate changes in injury pattern and severity of patients with TBI and neurosurgical treatment at OUH during the pandemic period.
METHODS
Study setting and participants
Oslo University Hospital (OUH) is the only level 1 trauma centre with neurosurgical services in the south-east region of Norway and the primary trauma referral hospital for Oslo residents. Thus, all neurosurgery in the region is performed solely at OUH. Patients with TBI are transported directly to OUH if they are in need of emergency neurosurgical procedures or neurosurgical evaluation. After treatment at the acute care units (intensive care unit (ICU) and/or neurosurgical ward), the patient can be discharged to specialized rehabilitation, to their local hospital or home.
The patient cohort in this study was retrieved from the Oslo TBI Registry-Neurosurgery, a quality control registry maintained by the neurosurgical department at OUH. The registration is prospective, and data are derived manually from electronic medical records and stored in a Medinsight database (15). Inclusion criteria for Oslo TBI Registry – Neurosurgery are: (i) TBI; (ii) cerebral-computed tomography (CT)/CT angiography (CTA), or cerebral-magnetic resonance imaging (MRI)/ magnetic resonance angiography (MRA) with findings of acute trauma (haemorrhage, fracture, traumatic axonal injury (TAI) vascular injury); (iii) admitted to OUH within 7 days post-injury; and (iv) Norwegian social security number.
This study included patients admitted to OUH in the period when the hospital was in a level of preparedness because of the COVID-19 pandemic; 11 March 2020 was the start-date for change in preparedness level at OUH and 17 August 2021 was the end-date. Subgroup analyses were performed on patients hospitalized and discharged between 27 March 2020 and 4 May 2020, and 29 March 2021 and 26 April 2021 because of further increased preparedness level, and the rehabilitation ward in these periods was converted to a COVID-19 ward. Patients admitted between 1 January 2018 and 31 December 2019 were used for comparison. This study included adult patients (age ≥ 18 years), alive at discharge and resident in the south-east region of Norway.
Endpoint
The study endpoint was the discharge destination from acute care units at OUH. Discharge destinations are categorized as home, specialized rehabilitation, local hospital, general rehabilitation, nursing home and other. The endpoint variable is binary; either direct transfer to specialized rehabilitation or not. Patients discharged to specialized inpatient rehabilitation are categorized as “yes”. All other discharge places are considered as “no”. The specialized inpatient rehabilitation programme focusses on reducing the extent of the brain injury, preventing complications, promoting functional recovery and reducing impairment, as well as minimizing the distress of the patient and caregivers (12). The treatment includes medical assessment and treatment of post-traumatic amnesia, physiotherapy, cognitive assessment and training, occupational therapy, speech therapy, nutrition, dietary services and psycho-social support.
Demographics, pre-injury comorbidity, injury characteristics and functional outcome
Age was stratified into 18–29 years, 30–49 years, 50–64 years, 65–79 years, 80+ years and sex into male/female. Living status was recorded as: independently at home, home with assistance, institution or other, and further dichotomized into living independently at home or not. Trauma mechanism was divided into falls, RTA or other. High-energy trauma was defined as falls from a height ≥3 m, RTAs or other high-energy accidents. The Glasgow Coma Scale score (GCS) was defined as the lowest score documented prior to intubation or admission to OUH. TBI severity was measured by Head Injury Severity Score (HISS) (minimal: GCS 15 and no loss of consciousness or amnesia, mild: GCS 14 or 15 plus amnesia, or brief loss of consciousness (< 5 min), or impaired alertness or memory, moderate: GCS 9–13 or loss of consciousness ≥ 5 min or focal neurological deficit, or severe: GCS ≤ 8) (16). Pre-injury comorbidity was classified by the American Society of Anaesthesiologists Physical Status Classification System (ASA-PS) (17). Assigning a Physical Status classification level is a clinical decision based on multiple factors. Here we report on classification from 1 = normal healthy, 2 = mild systemic, 3 = severe systemic, 4 = life-threatening. The Glasgow Outcome Score (GOS) on the day of discharge was based on information from multidisciplinary medical records. The GOS is divided into 5 categories: GOS 1 dead (D), GOS 2 vegetative state (VS), GOS 3 severe disability (SD), GOS 4 moderate disability (MD) or GOS 5 good recovery (GR) (18), and only 2 through 5 were applicable in the current study population. The reasons for reduced GOS were grouped into: (i) TBI, (ii) TBI in combination with extracranial injury and/or comorbidity, and (iii) other.
Hospital management
The hospital’s management strategy was divided into conservative management (no surgery required), insertion of intracranial pressure (ICP) sensor, or intracranial procedures. Intracranial procedures included evacuation of mass lesion (haematoma/haemorrhage), cerebrospinal fluid drainage, decompressive hemicraniectomy, repair of dura or fractured skull (duraplasty/cranioplasty), and vascular surgery. Calculation of length of stay (LOS) is based on dates and each date counted as a full day.
Statistical analysis
Patient characteristics are reported as frequency (percentage), mean (standard deviation; SD), or median (interquartile range; IQR) depending on distribution. For comparison between groups, we used Student's test or Mann–Whitney U test for continuous variables, and χ2 tests or Fisher’s exact test for categorical variables, as appropriate. All tests were 2-sided and set 5% significant level. The statistical procedure and selection of variables for the multivariable logistic regression models was built on a previous study from OUH (14). In brief, univariable and multivariable logistic regressions were performed to examine possible risk factors of differentiating between patients discharged to specialized rehabilitation and patients discharged elsewhere. The possible risk factors were selected based on previous literature and clinical relevance, and possible multicollinearity examined using Pearson correlation analyses, correlating variables were not included (r upper limit 0.7). The model was assessed by calibration and discrimination, and calibration evaluated by the Hosmer and Lemeshow goodness-of-fit test. Discrimination was evaluated by analysis of the area under the receiver operating characteristic curve (AUC ROC). Results are presented with odds ratio (OR), 95% confidence interval (95% CI) and p-value, and data were analysed with IBM SPSS Statistics, Version 26.0. Armonk, NY, USA: IBM Corp.
Ethics
OUH data protection officer (DPO) has approved Medinsight database (approval number 2016/17569), this study was applied to OUH DPO and approved on 5 October 2021.
RESULTS
Patient cohort
A total of 1,310 patients with TBI fulfilling the inclusion criteria were divided in 2 groups based on admission dates. The pre-pandemic group covered a time-period of 24 months and included 758 patients. The pandemic group covered a period of 18 months and included 552 patients. A comparison of patient and injury characteristics in pre-pandemic and pandemic period is shown in Table I. There were no significant differences in the characteristics between the groups with respect to mean age (58 years in both groups (SD 20)), and sex (65–68% male). The majority were healthy, with ASA-PS score of 1–2. However, there was a difference in pre-injury ASA-PS score; the pandemic group had a higher proportion of patients with ASA-PS ≥ 3 (43–33%). The majority lived independently at home in both groups (84–85%). The trauma mechanisms were similar with falls as a dominating cause (58–56% of the cases). The major difference between the groups was the proportion of injured electric scooter riders (1–8%). The distribution of TBI severity was similar across the periods.
| Pre-pandemica n (%) 758 (100) | Pandemicb n (%) 552 (100) | Differences p-value | |
| Age In years, mean (SD) Age strata, years 18–29 30–49 50–64 65–79 80+ |
58 (20) 91 (12.0) 148 (19.5) 195 (25.7) 197 (26.0) 127 (16.8) |
58 (20) 61 (11.1) 116 (21.0) 146 (26.4) 152 (27.5) 77 (13.9) |
0.61 0.63 |
| Male | 516 (68.1) | 360 (65.2) | 0.28 |
| ASA-PS 1 Normal healthy 2 Mild systemic 3 Severe systemic 4 Life threatening |
272 (35.9) 239 (31.5) 238 (31.4) 9 (1.2) |
177 (32.1) 140 (25.4) 217 (39.3) 18 (3.3) |
> .001 |
| Living status Home independent |
634 (83.6) |
471 (85.3) |
0.41 |
| Substance dependence | 129 (17) | 110 (19.9) | 0.18 |
| Trauma mechanism Fall Motor vehicle Pedestrian Bicycle Electric scooter Sports Violence Self-harm Other/unknown |
438 (57.8) 67 (8.8) 20 (2.6) 71 (9.4) 5 (0.7) 34 (4.5) 48 (6.3) 23 (3.0) 53 (6.9) |
310 (56.2) 34 (6.2) 11 (2.0) 62 (11.2) 42 (7.6) 12 (2.2) 29 (5.3) 6 (1.1) 46 (8.3) |
> .001 |
| Alcohol at time of injury | 224 (29.6) | 167 (30.3) | 0.78 |
| Head Injury Severity Scale Minimal Mild Moderate Severe |
44 (5.8) 333 (43.9) 251 (33.1) 130 (17.2) |
43 (7.8) 228 (41.3) 188 (34.1) 93 (16.8) |
0.47 |
| High-Energy trauma (yes) | 250 (33) | 196 (35.5) | 0.34 |
| Isolated TBI | 401 (52.9) | 293 (53.1) | 0.95 |
| aAdmitted to OUH from 1 January 2018 to 31 December 2019 bAdmitted to OUH from 11 March 2020 to 17 August 2021 ASA-PS: American Society of Anaesthesiologists Physical Status Classification System, SD: standard deviation, TBI: traumatic brain injury. |
|||
Referral patterns
By comparing TBI referrals, acute treatment, and discharge destination, no difference was found between the pre-pandemic and pandemic groups (Table II). The monthly number of hospital admitted patients with TBI to OUH remained stable with natural fluctuation during both periods (Fig. 1). Of the 117 discharged directly to specialized rehabilitation in the pandemic group, 16% (19 patients) was admitted with mild TBI, 39% with moderate TBI (46 patients) and 44% with severe TBI (52 patients). A similar proportion was found in the pre-pandemic group; mild TBI 14%, moderate TBI 42% and severe TBI 44%. The proportion of direct discharge to specialized rehabilitation was similar when comparing the 2 groups (19–21%, Table II). When selecting patients with moderate-severe TBI (upon admission), the proportion was similar (32–35%). The monthly proportion of patients with moderate-severe TBI discharged to specialized rehabilitation is shown in Fig. 2. In a subgroup analysis, we found a decrease in the number of patients getting specialized rehabilitation during the second period in which the rehabilitation ward was converted to a COVID-19 ward (April 2021) (Fig. 2) compared with the first period (April 2020). However, due to the relatively short time and a low number of patients with moderate or severe TBI in both periods (n = 21), this was not investigated further. An overall summary of the patient cohort from both subgroups is shown in Table SI.
| Pre-pandemica | Pandemicb | p-value | |
| n = 758 (100%) | n = 552 (100%) | ||
| Referral Primary Secondary Emergency department/other |
265 (35.0) 253 (33.4) 240 (31.7) |
178 (32.2) 201 (36.4) 173 (31.1) |
0.46 |
| Intensive care unit admission (yes) | 456 (60.2) | 349 (63.2) | 0.26 |
| Length of stay at intensive care unit Days (median, interquartile range) |
3 (2-7.75) |
3 (2-9) |
0.07 |
| Treatment TBI Conservative Any neurosurgical procedure Intracranial pressure sensor Evacuation of mass lesion Intracranial surgeryc |
577 (76.1) 181 (23.9) 128 (16.9) 89 (11.7) 132 (17.4) |
426 (77.2) 126 (22.8) 94 (17.0) 65 (11.8) 94 (17.0) |
0.66 0.66 0.95 0.99 0.86 |
| Extracranial surgery (yes) | 147 (19.4) | 104 (18.8) | 0.80 |
| Discharge destination Home Local hospital Specialized rehabilitation Nursing home Other |
259 (34.2) 296 (39.1) 143 (18.9) 46 (6.1) 14 (1.8) |
174 (31.5) 222 (40.2) 117 (21.1) 32 (5.8) 7 (1.3) |
0.67 |
| GCS 15 at discharge | 510 (67.3) | 348 (63.0) | 0.11 |
| Glasgow outcome score (at day of discharge) Vegetative state Severe disability Moderate disability Good recovery |
25 (3.3) 348 (45.9) 345 (45.5) 37 (4.9) |
25 (4.5) 282 (51.1) 227 (41.1) 15 (2.7) |
0.08 |
| Reason reduced function TBI TBI + extracranial injury/comorbidity Other |
372 (49.1) 254 (33.5) 132 (17.4) |
272 (49.3) 197 (35.7) 83 (15.0) |
0.46 |
| aAdmitted to OUH from 1 January 2018 to 31 December 2019. bAdmitted to OUH from 11 March 2020 to 17 August 2021. cIncludes evacuation of mass lesion (haematoma/haemorrhage), cerebrospinal fluid drainage, decompressive hemicraniectomy, repair of dura or fractured skull (duraplasty/cranioplasty) and vascular surgery. GCS: Glasgow Coma Scale score, TBI: traumatic brain injury. |
|||
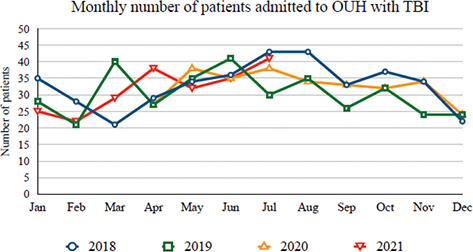
Fig. 1. Admission rate of patients hospitalized with traumatic brain injury (TBI) identified with neuroimaging (n=1,310). The dates 2018 and 2019 represent pre-pandemic years, 2020 and 2021 represent pandemic years and include patients admitted from April 2020 to July 2021 (March 2020 and August 2021 are excluded from this graph, as they were partial months).
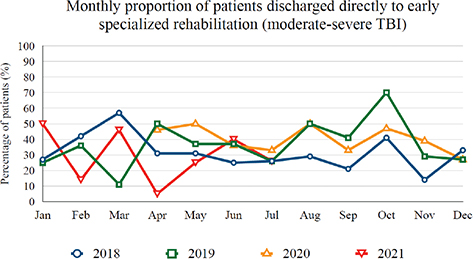
Fig. 2. Proportion of patients with moderate-severe traumatic brain injury (TBI) (n=662) who were discharged directly to early specialized rehabilitation. The dates 2018 and 2019 represent pre-pandemic years, 2020 and 2021 are pandemic years and include patients discharged in the period April 2020 to July 2021 (March 2020 and August 2021 are excluded from this graph, as they were partial months). It includes the 2 periods when the rehabilitation ward was converted to a COVID-19 ward (27 March 2020 to 4 May 2020, and 29 March 2021 to 26 April 2021).
Predictors
Tables III and IV contain a comparison of predictors for discharge directly to specialized rehabilitation between the pre-pandemic and pandemic period. Results from univariable logistic regression analysis show that strong positive predictive factors for discharge to specialized rehabilitation in both pre-pandemic and pandemic groups were: younger age, ASA-PS of 1 or 2, moderate or severe TBI, intracranial surgery, insertion of ICP-sensor (surrogate for neurointensive management), extracranial surgery, and severe disability upon discharge from acute care units due to TBI (Table III). Being male was associated with discharge to specialist rehabilitation in the pre-pandemic (OR 2.19, 95% CI 1.4, 3.42, p = 0.001), but not in the pandemic group (OR 1.55, 95% CI 0.99, 2.43, p = 0.058), similar trend was found in multivariable model (Table IV). In multivariable logistic regression analysis (Table IV), younger age (< 65 years) remained as a strong positive predictor, along with pre-injury ASA-PS of 1–2, severe disability upon discharge due to TBI in both pre-pandemic and pandemic groups. In neither group was intracranial surgery, insertion of ICP-sensor or extracranial surgery a significant predictor. Severity of the injury was a strong predictor factor in both pre-pandemic and pandemic groups, with OR for direct transfer 1.5 for moderate injury and 2.5 for severe injury in the pandemic group.
| Variable | Pre-pandemica | Pandemicb | ||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age strata, years 18–29 30–49 50–64 65–79 80+ |
1 0.69 0.65 0.21 0.04 |
0.30, 1.43 0.32, 1.33 0.09, 0.48 0.01, 0.20 |
0.314 0.238 < .001 < .001 |
1 1.44 0.92 0.29 0.04 |
0.58, 3.57 0.37, 2.29 0.10, 0.80 0.01, 0.41 |
0.433 0.860 0.017 0.006 |
| Sex Female Male |
1 1.75 |
1.00, 3.07 |
0.051 |
1 0.80 |
0.43, 1.49 |
0.484 |
| Pre-injury ASA 1–2 3–4 |
1 0.38 |
0.20, 0.74 |
0.004 |
0.35 |
0.18, 0.69 |
0.002 |
| Head injury severity score Mild Moderate Severe |
1 2.13 4.67 |
1.12, 4.06 1.95, 11.20 |
0.021 0.001 |
1 1.50 2.57 |
0.70, 3.20 0.96, 6.89 |
0.297 0.062 |
| Intracranial surgery No Yes |
1 0.78 |
0.39, 1.55 |
0.479 |
1 0.79 |
0.36, 1.71 |
0.547 |
| ICP-sensor No Yes |
1 1.04 |
0.46, 2.31 |
0.479 |
1 2.28 |
0.91, 5.73 |
0.081 |
| Extracranial surgery No Yes |
1 1.24 |
0.60, 2.55 |
0.563 |
1 1.78 |
0.78, 4.08 |
0.172 |
| GOS at discharge Moderate disability Severe disability Vegetative state |
1 9.03 1.86 |
4.65, 17.52 0.48, 7.24 |
< .001 0.374 |
1 8.89 1.34 |
4.09, 19.3 0.33, 5.51 |
< .001 0.686 |
| Reason reduced GOS Other TBI TBI + extracranial injury/comorbidity |
1 3.44 1.16 |
1.21, 9.80 0.44, 3.05 |
0.021 0.764 |
1 5.03 1.10 |
1.39, 18.3 0.33, 3.60 |
0.014 0.879 |
| aPre-pandemic: 718 patients included analysis. Missing 40 patients; 37 discharged as GOS GR, 3 discharged as GOS NA. The Hosmer and Lemeshow goodness-of-fit test was not significant indicating a satisfactory fit of the model (χ2 = 7.15, df = 8, p = 0.52). The area under the ROC curve was 0.88 (95% CI: 0.86–0.91) indicating a good discriminative ability. bPandemic: 534 patients included analysis. Missing 18 patients; 15 discharged as GOS GR, 3 discharged as GOS NA. The Hosmer and Lemeshow goodness-of-fit test was not significant indicating a satisfactory fit of the model (χ2 = 3.12, df = 8, p = 0.93). The area under the ROC curve was 0.89 (95% CI: 0.86–0.92) indicating a good discriminative ability. ASA-PS: American Society of Anaesthesiologists Physical Status Classification System, 95% CI: 95% confidence interval, GCS: Glasgow Coma Scale score, GOS: Glasgow Outcome Score, ICP: intracranial pressure, OR: odds ratio, TBI: traumatic brain injury; GR: good recovery; NA: not available; ROC: Receiver Operating Characteristic. |
||||||
DISCUSSION
This study examined the consequences for patients regarding early specialized rehabilitation following acute TBI during an 18-month period during which COVID-19 infections spread throughout south-east Norway. The continuous rehabilitation pathway for patients with moderate and severe TBI was maintained in 2020 and 2021. The TBI patient composition did not change due to the impact of the pandemic, and acute care management appears to have been maintained at OUH.
There is consensus that the COVID-19 pandemic in its early phases contributed to changes in neurosurgical referrals for TBI worldwide. Retrospective single-centre studies comparing TBI referral volumes during lockdown with previous years have shown a decrease in the incidence of TBI referrals and admission during lockdown periods (1–7, 19). In contrast, no change was found in TBI referrals in the study material. One reason could be the length of period included in this study. We report here on a prolonged period with abnormal preparedness level at the hospital, while other studies report on shorter lockdown periods. Despite seeing decreases in TBI referrals and admissions associated with the implementation of lockdowns, several studies show that the relaxation of such measures was accompanied by increases in TBIs (2, 7, 20).
The current study did not find a decline in the direct pathway to specialized rehabilitation. In contrast, the results show that the positive trend from 2015 to 2019 reported by Tverdal et al. (14) continued in 2020–21. At the start of the pandemic, the discharge may even have been accelerated because of the pressure in the ICU and neurosurgical wards. In addition, an explanation could be the medical staffs’ willingness to make extra efforts at the onset of the pandemic. These findings reinforce the feasibility and preparedness of the rehabilitation ward to manage patients with TBI during a pandemic. Maintaining rehabilitation pathways can have major consequences for the individual patient as well as potential socio-economic consequences. The early specialized rehabilitation ward is essential to durable outcomes, as they prevent readmissions and promote hospital bed-availability.
In previous years, falls and RTAs predominated as the main cause of TBI worldwide (21–24). The restrictions on movement imposed by COVID-19 lockdown measures resulted in people staying at home. Working from home and school closures resulted in global reductions in road traffic levels, leading to a decrease in TBIs due to RTAs. However, during the latter stages of lockdown, studies from India and the United States show that rates of RTA associated TBIs increased to levels above those experienced before COVID-19 (8, 25, 26). The current study did not find any major change in mechanism of injury, except for electric scooters. In 2020, electric scooters became publicly available in the city centre of Oslo (unrelated to the pandemic), meanwhile electric scooters also gained popularity in the private market. Before the pandemic, electric scooters were available to a small extent; however, COVID-19 infection control and preventive measures recommended avoiding public transport and may have strengthened their use even further. Fall was the dominant trauma mechanism, similar to the European population (21, 27). The current study also found no change in length of stay in the ICU and/or neurosurgery ward despite pressure to discharge patients due to an increase in the number of COVID-19 patients. One reason may be that the current analyses were based on a longer preparedness level period of 18 months and variation in admissions occured due to COVID-19 in this period.
No significant epidemiological changes in TBI resulting from the pandemic were found in this study; however, the study supports the substantial variability in TBI admittance to the trauma hospitals. Evidence of significant sex differences is limited, and there is a conflicting picture (10, 25, 26), probably due to regional variation. The study by Algattas et al. (6) found no differences in the mean and median age of neurotrauma patients, but significant differences in age distribution. This highlights the need for an accurate analysis of the age distribution of TBI. Despite stratifying by different age groups, no significant change in TBI by age before and during the pandemic was found. In addition, this study did not include patients younger than 18 years of age. A difference in ASA-PS score was found, with higher scores in the pandemic group; however, the study does not explain whether this is affected by the pandemic or is an overall trend.
At the start of the pandemic, the aim of keeping hospital admissions to a minimum led to the suspension of elective surgical procedures and an emphasis on surgical prioritization. The current study reports the referral patterns, management and direct pathway to early specialized rehabilitation from a level 1 trauma centre that covers the south-east region of Norway representing more than half of the Norwegian population. The data show that TBI continued to be treated with the highest priority and its neurosurgical management remained largely unchanged. This is in contrast to other studies showing that TBI was more likely to be managed conservatively during the pandemic (8, 9, 26). Although stretched, this could reflect that the healthcare system at OUH was able to prioritize patients with TBI in need of neurosurgery and prioritized the rehabilitation for severe TBI by moving the early rehabilitation to the neurosurgical ward. The results must be interpreted in the light of Norway being a high-income country with a well-organized healthcare system and government-funded universal health coverage. During the pandemic, the national government chose a strategy with constantly adjusting measures of social restrictions to keep the spread of the virus under control, in order to maintain adequate healthcare services, and to prevent the healthcare system being overwhelmed. Across Europe, it also seems that TBI has been adequately treated. A multicentre study showed no increase in 30-day mortality of patients with TBI during 2020 compared with previous years (19). Yet, mortality is a crude outcome, and data on rehabilitation services in Norway indicate large regional variations (28). Hence, patients may have suffered from altered early rehabilitation in other regions of Norway, as well as downscaling of long-term rehabilitation services, with impacts on functional outcome and quality of life.
When comparing factors predicting the direct pathway to rehabilitation, univariable analysis showed several significant factors, in contrast to the multivariable model. In the multivariable model, strong positive factors in both groups were younger age, pre-injury ASA-score 1–2, severe TBI, severe disability due to TBI upon discharge, in line with a previous study (14). We cannot fully explain the variation regarding male sex, but contributing factors may be that in younger TBI patients, males are over-represented and males more often need emergency neurosurgery (29, 30). Interestingly, we found a difference in favour of the group who received neurosurgical procedure with ICP-sensor insertion in the pandemic group, although the difference was not statistically significant. Severity of the injury was a strong predictor factor in both pre-pandemic and pandemic group with OR for direct transfer 1.5 for moderate and 2.5 for severe injury in the pandemic group. TBI as reason for reduced function upon discharge was a stronger factor for the pandemic group; this may suggest a trend towards priority given to patients with severe TBI treated at the intensive unit. Possible explanatory factors for this difference can be an internal pressure at OUH to release bed capacity for COVID-19 patients at ICU, or a situation with no alternative discharge place as the receiving local hospitals did not have any available beds because of COVID-19 patients.
The strength of this study is the sample size and the use of register data minimizing the effect of selection bias due to non-response and loss to follow-up. In addition, the collection of the data was performed independently of the study. However, some limitations must be addressed, the data are pre-collected and the Oslo TBI Registry-Neurosurgery lacks detailed information, such as comorbid conditions and regarding length or intensity of rehabilitation. The current study also had no information regarding where patients were discharged to from early rehabilitation, their subsequent access to rehabilitation, or long-term outcomes.
CONCLUSION
The COVID-19 pandemic has not resulted in any notable reduction in the number of TBI referrals, or influenced the direct pathway to early, specialized rehabilitation. This study did not find evidence of a change in acute care management, although the full consequences of the pandemic for the rehabilitation of patients with TBI may not be realized for years to come.
REFERENCES
- Figueroa JM, Boddu J, Kader M, Berry K, Kumar V, Ayala V, et al. The effects of lockdown during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic on neurotrauma-related hospital admissions. World Neurosurg 2021; 146: e1–e5.
- Jayakumar N, Kennion O, Villabona AR, Paranathala M, Holliman D. Neurosurgical referral patterns during the coronavirus disease 2019 pandemic: A United Kingdom experience. World Neurosurg 2020; 144: e414–e420.
- Pinggera D, Klein B, Thomé C, Grassner L. The influence of the COVID-19 pandemic on traumatic brain injuries in Tyrol: experiences from a state under lockdown. Eur J Trauma Emerg Surg 2021; 47: 653–658.
- Sinha S, Toe KKZ, Wood E, George KJ. The impact of COVID-19 on neurosurgical head trauma referrals and admission at a tertiary neurosurgical centre. J Clin Neurosci 2021; 87: 50–54.
- Zhang M, Zhou J, Dirlikov B, Cage T, Lee M, Singh H. Impact on neurosurgical management in Level 1 trauma centers during COVID-19 shelter-in-place restrictions: the Santa Clara County experience. J Clin Neurosci 2021; 88: 128–134.
- Algattas HN, McCarthy D, Kujawski B, Agarwal N, Brown J, Forsythe RM, et al. Impact of coronavirus disease 2019 shutdown on neurotrauma volume in Pennsylvania. World Neurosurg 2021; 151: e178–e184.
- ElGhamry AN, Jayakumar N, Youssef M, Shumon S, Mitchell P. COVID-19 and changes in neurosurgical workload in the United Kingdom. World Neurosurg 2021; 148: e689–e694.
- Goyal N, Swain SK, Gupta K, Chaturvedi J, Arora RK, Sharma SK. “Locked up inside home” - Head injury patterns during coronavirus disease of 2019 pandemic. Surg Neurol Int 2020; 11: 395.
- Manivannan S, Sharouf F, Mayo I, Albaqer H, Mehrez M, Jaber H, et al. Management of neurotrauma during COVID-19: a single centre experience and lessons for the future. Brain Inj 2021; 35: 957–963.
- Rault F, Terrier L, Leclerc A, Gilard V, Emery E, Derrey S, et al. Decreased number of deaths related to severe traumatic brain injury in intensive care unit during the first lockdown in Normandy: at least one positive side effect of the COVID-19 pandemic. Acta Neurochir 2021; 163: 1829–1836.
- Lester A, Leach P, Zaben M. The impact of the COVID-19 pandemic on traumatic brain injury management: lessons learned over the first year. World Neurosurg 2021; 156: 28–32.
- Andelic N, Bautz-Holter E, Ronning P, Olafsen K, Sigurdardottir S, Schanke AK, et al. Does an early onset and continuous chain of rehabilitation improve the long-term functional outcome of patients with severe traumatic brain injury? J Neurotrauma 2012; 29: 66–74.
- Andelic N, Ye J, Tornas S, Roe C, Lu J, Bautz-Holter E, et al. Cost-effectiveness analysis of an early-initiated, continuous chain of rehabilitation after severe traumatic brain injury. J Neurotrauma 2014; 31: 1313–1320.
- Tverdal C, Andelic N, Helseth E, Brunborg C, Rønning P, Hellstrøm T, et al. In the aftermath of acute hospitalization for traumatic brain injury: factors associated with the direct pathway into specialized rehabilitation. J Clin Med 2021; 10.
- Medinsight database. (accessed 2021 June 18). Available from: https://medinsight.no/
- Stein SC, Spettell C. The Head Injury Severity Scale (HISS): a practical classification of closed-head injury. Brain Inj 1995; 9: 437–444.
- American Society of Anesthesiologists. ASA Physical Status Classification System. (accessed 2019 August 11). Available from: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system
- Teasdale GM, Pettigrew LE, Wilson JT, Murray G, Jennett B. Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J Neurotrauma 1998; 15: 587–597.
- Grassner L, Petr O, Warner FM, Dedeciusova M, Mathis AM, Pinggera D, et al. Trends and outcomes for non-elective neurosurgical procedures in Central Europe during the COVID-19 pandemic. Sci Reports 2021; 11: 6171.
- Abdulazim A, Ebert A, Etminan N, Szabo K, Alonso A. Negative impact of the COVID-19 pandemic on admissions for intracranial hemorrhage. Frontiers Neurol 2020; 11: 584522.
- Steyerberg EW, Wiegers E, Sewalt C, Buki A, Citerio G, De Keyser V, et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol 2019; 18: 923–934.
- Brazinova A, Rehorcikova V, Taylor MS, Buckova V, Majdan M, Psota M, et al. Epidemiology of traumatic brain injury in Europe: a living systematic review. J Neurotrauma 2021; 38: 1411–1440.
- Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 2017; 16: 987–1048.
- Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 56–87.
- Karthigeyan M, Dhandapani S, Salunke P, Sahoo SK, Kataria MS, Singh A, et al. The collateral fallout of COVID19 lockdown on patients with head injury from north-west India. Acta Neurochirurg 2021; 163: 1053–1060.
- Lara-Reyna J, Yaeger KA, Rossitto CP, Camara D, Wedderburn R, Ghatan S, et al. “Staying home” – early changes in patterns of neurotrauma in New York City during the COVID-19 pandemic. World Neurosurg 2020; 143: e344–e350.
- Peeters W, van den Brande R, Polinder S, Brazinova A, Steyerberg EW, Lingsma HF, et al. Epidemiology of traumatic brain injury in Europe. Acta Neurochirurg 2015; 157: 1683–1696.
- Norwegian Directorate of Health. Coronavirus decisions and recommendations. Guide to law and regulation. [Koronavirus-beslutninger og anbefalinger. Veileder til lov og forskrift]. 2020.
- Tverdal C, Aarhus M, Andelic N, Skaansar O, Skogen K, Helseth E. Characteristics of traumatic brain injury patients with abnormal neuroimaging in Southeast Norway. Inj Epidemiol 2020; 7: 45.
- Tverdal C, Aarhus M, Rønning P, Skaansar O, Skogen K, Andelic N, et al. Incidence of emergency neurosurgical TBI procedures: a population-based study. BMC Emerg Med 2022; 22: 1.