ORIGINAL ARTICLE
CUMULATIVE RISK AND ASSOCIATED FACTORS FOR FALL-RELATED FRACTURES IN STROKE SURVIVORS AFTER DISCHARGE FROM REHABILITATION WARDS: A RETROSPECTIVE STUDY WITH A 6-YEAR FOLLOW-UP
Masashi KUMAGAI, OT, PHD1,2, Yohei OTAKA, MD, PHD1,3, Taiki YOSHIDA, OT, MS1,4,5, Shin KITAMURA, OT, MS1,4,6, Kazuki USHIZAWA, OT, MS1,3, Naoki MORI, MD1,7, Daisuke MATSUURA, MD, PHD1, Kaoru HONAGA, MD, PHD1,8, Kunitsugu KONDO, MD, PHD1 and Eiji SHIMIZU, MD, PHD2
From the 1Department of Rehabilitation Medicine, Tokyo Bay Rehabilitation Hospital, Chiba, 2Department of Cognitive Behavioral Physiology, Chiba University Graduate School of Medicine, Chiba, 3Department of Rehabilitation Medicine I, School of Medicine, 4Faculty of Rehabilitation, School of Health Sciences, Fujita Health University, Aichi, 5Department of Health Sciences and Social Welfare, Waseda University Graduate School of Human Sciences, Saitama, 6Department of Occupational Therapy, Tokyo Metropolitan University Graduate School of Human Health Sciences, Tokyo, 7Department of Rehabilitation Medicine, Keio University School of Medicine, Tokyo and 8Department of Rehabilitation Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan
Objective: To investigate the long-term cumulative risk and factors associated with fall-related fractures in stroke survivors discharged from convalescent rehabilitation wards.
Design: Retrospective cohort study.
Participants: A total of 786 stroke survivors discharged from a rehabilitation hospital.
Methods: Data regarding fall-related fractures post-hospital discharge were collected using self-reported questionnaires. The Kaplan–Meier method was used to calculate the cumulative incidence of fall-related fractures, and risk factors were analysed using Cox proportional hazard regression analysis.
Results: Of 1,861 consecutive stroke survivors who had been discharged from hospital, 786 (42.2%) provided information concerning fall-related fractures. Duration from time of discharge to time of collection of questionnaires ranged from 1 to 6 years (mean 38.0 months). The cumulative incidence of fall-related fractures at 1-, 2-, 3-, 4-, and 5-years post-discharge was 4.2%, 7.9%, 10.8%, 12.5% and 13.7%, respectively. Cox proportional hazard regression analysis indicated that female sex (hazard ratio (HR) 1.69) and moderate lower limb paresis (HR 3.08) were significant risk factors.
Conclusion: The cumulative risk of fall-related fractures in stroke survivors post-discharge from a rehabilitation hospital was notably high. Intensive preventive intervention should be considered for female stroke survivors with moderate lower limb paresis.
LAY ABSTRACT
This study aimed to investigate the risk of fall-related fractures and associated factors in stroke survivors who had been discharged from rehabilitation wards. A questionnaire was sent by post to 1,861 post-discharge stroke survivors to investigate their experiences of fall-related fractures, to which 786 stroke survivors responded. The incidence of fall-related fractures at 1, 2, 3, 4, and 5 years post-discharge was 4.2%, 7.9%, 10.8%, 12.5% and 13.7%, respectively. The presence of moderate lower limb paresis and female sex were associated with 3.08- and 1.69-times higher risk of developing a fall-related fracture, respectively. Intensive preventive intervention should be considered for female stroke survivors with moderate lower limb paresis following discharge from rehabilitation wards.
Key words: accidental fall; bone fracture; cerebrovascular disorder; rehabilitation; risk factor.
Citation: J Rehabil Med 2022; 54: jrm00294. DOI: http://dx.doi.org/10.2340/jrm.v54.2314
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: March 14, 2022; Epub ahead of print: Jun 2, 2022; Published: Jun 29, 2022
Correspondence address: Yohei Otaka, Department of Rehabilitation Medicine I, School of Medicine, Fujita Health University 1-98 Dengakugakubo, Kutsukake, Toyoake, Aichi 470-1192, Japan. E-mail: otaka119@mac.com
Competing interests and funding: The authors have no conflicts of interest to declare.
Stroke is a major cause of death and disability. Although global age-adjusted mortality rates for stroke have decreased, the absolute number of people who have strokes annually has been reported to be increasing, along with population growth and ageing (1). In Japan, the total number of stroke survivors receiving medical treatment was estimated to be 1,179,000 in 2014, and stroke was noted as the eighth most common disease (2). Furthermore, stroke was identified as the second most common cause of long-term care in 2016 (3). Therefore, appropriate management of stroke survivors is important from both medical and social perspectives.
Falling is one of the most frequently encountered complications among stroke survivors living in the community as well as in hospitals (4, 5). Stroke survivors frequently experience falls in the early period following hospital discharge, with up to 73% experiencing a fall within 6 months of discharge (6–10). Long-term stroke survivors in the community also experience falls more frequently than the general population, albeit less frequently than in the early period after discharge (11–14). Falling can cause injuries, fractures, and psychological trauma, such as a fear of falling and depression, and these conditions further limit activities of daily living (5). The risk of fractures in stroke survivors is high (15–21), being 1.4–7 times higher than that of the general population (15–17, 19, 22), with most fractures reported to be due to falls (15, 17). Several studies have reported the incidence of fractures among stroke survivors (15–21); however, the incidence and factors influencing fall-related fractures post-discharge from rehabilitation wards, where individuals with impairments and disabilities are admitted following a stroke, have not been carefully investigated from a long-term perspective. Moreover, most previous studies investigating fractures in stroke survivors have included fractures that were not attributed to falls (15, 17), or the cause of the fractures was not specified (16, 18–22), with no studies having been limited to fall-related fractures. Furthermore, to our knowledge, no studies have investigated fall-related fractures in Asian countries, including Japan. Therefore, there is limited evidence concerning an appropriate fall-related fracture prevention strategy for stroke survivors post-discharge from rehabilitation wards in Japan.
This study aimed to investigate the long-term cumulative risk and factors associated with fall-related fractures in stroke survivors following discharge from rehabilitation wards.
METHODS
Study setting and design
The study was conducted at Tokyo Bay Rehabilitation Hospital, Chiba, Japan, which has specific convalescent rehabilitation wards (Kaifukuki Rehabilitation Wards; KRW). The KRW system was established in Japan in 2000 as part of a governmental insurance system for inpatient rehabilitation during the convalescent phase (23). Individuals with stroke are eligible for admission to the KRW within 2 months of stroke onset. Physical, occupational, and speech therapies for a maximum of 3 h per day, 7 times a week, can be provided as part of a comprehensive and intensive rehabilitation programme. The maximum length of stay is up to 150 days for individuals with stroke and 180 days for individuals with stroke who have severe cognitive disorders.
This retrospective cohort study comprised a cross-sectional questionnaire postal survey. The study protocol was approved by the local ethics committee (approval number 174-2). The postal questionnaire was accompanied by an explanatory document describing the study and participant consent, which stated that a reply and completion of the questionnaire from stroke survivors or their families constituted informed consent to participate in the study.
Participants
A total of 1,861 consecutive stroke survivors who had been discharged from the Tokyo Bay Rehabilitation Hospital between July 2011 and March 2017 were surveyed in April 2018. All stroke survivors who had been discharged from the hospital during the study period were included; therefore, no exclusion criteria were applied. The data from responders were included in the analyses after excluding participants with insufficient data for fall-related fractures.
Data collection
In addition to demographic information, the questionnaire included information concerning fall-related fractures that had occurred following discharge from convalescent rehabilitation wards, including the number of events (none, once, twice), the date of the first fracture(s), and the location(s) of the first fracture(s) (i.e. hip, proximal humerus, wrist, lumbar vertebrae, pelvis, rib, other, or uncertain).
To assess independent factors associated with fall-related fractures, the following data were retrieved from medical records: sex, age, premorbid modified Rankin Scale (mRS) score (24), type of stroke (infarction, intracerebral haemorrhage, subarachnoid haemorrhage), history of previous stroke (no, yes), duration from onset to admission to the KRW, length of stay in the KRW, side of paresis (right, left, bilateral, no paresis), Stroke Impairment Assessment Set (SIAS) motor score (25) as the degree of paresis at discharge, Functional Independence Measure (FIM) score (26, 27), and destination (i.e. home, nursing home, or transferred to another hospital).
The mRS has been found to be a reliable measure of disability severity, involving a 6-point scale ranging from Grade 0 to 5 (0, no symptoms; 1, no significant disability despite symptoms; 2, slight disability; 3, moderate disability; 4, moderately severe disability, and; 5, severe disability) (24).
The SIAS has previously shown a high degree of inter-observer agreement (25). The SIAS includes 2 items related to the upper limb (knee-mouth and finger-function tests) and 3 items related to the lower limb (hip-flexion, knee-extension, and foot-pat tests), with each item scored from 0 (total paresis) to 5 (no paresis).
The FIM is a standardized and useful assessment tool for measuring independence in daily living, comprising motor and cognitive domains (26, 27). The motor domain includes 13 items, with scores ranging from 13 to 91 points. The cognitive domain includes 5 items, with scores ranging from 5 to 35 points. Each item is scored on a scale of 1–7 points, resulting in a total of 18 items and total scores ranging from 18 to 126 points. A higher score indicates a greater degree of independence.
Statistical analysis
Following a descriptive analysis of fall-related fractures, the Kaplan–Meier method was used to analyse the cumulative incidence rates of the first fall-related fractures post-discharge. The observation period was defined as the period from the time of hospital discharge to completion of the questionnaire. For those who had died, the observation period was from the time of hospital discharge to the time of death. To explore factors related to the risk of fall-related fractures, bivariate Cox proportional hazards regression analyses were performed for the variables described in the data collection subsection above. Subsequently, a multivariate Cox proportional hazards regression analysis of variables that showed significance in the bivariate analysis was performed. Multicollinearity between independent variables was assessed using Pearson’s product moment correlation coefficients or Spearman’s rank correlation coefficients, depending on the type of variable. We excluded 1 of the 2 variables that showed a high correlation (r ≥ 0.8). For motor paresis and activities of daily living, non-linear correlations might exist (20–22, 28, 29). Therefore, we categorized the SIAS and FIM motor scores in the regression analyses. For the SIAS scores, we calculated the total scores of the upper (ranging from 0 to 10 points) and lower limbs (ranging from 0 to 15 points) and we classified them into 5 severity categories, as follows: upper limb, 0–1, 2–3, 4–5, 6–7, 8–9, and 10 (no paresis) points; lower limb, 0–2, 3–5, 6–8, 9–11, 12–14, and 15 (no paresis) points. In individuals with bilateral paresis, the score on the weaker side was considered for this analysis. The total FIM motor score was classified into 7 categories, as follows: 13–25, 26–38, 39–51, 52–64, 65–77, 78–90, and 91 (totally independent) points. Independence of gait at discharge was defined as an FIM walking score ≥ 6 points.
Stata/MP 15.1 (StataCorp LLC, College Station, TX, USA) software was used for statistical analyses. p-values < 0.05 were considered statistically significant.
RESULTS
Of 1,861 individuals surveyed, there were 817 responses to the questionnaire, and data concerning fall-related fractures were obtained from 786 (42.2%) individuals. Participant characteristics are listed in Table I. Duration since discharge from the KRW ranged from 1 to 6 years with a mean of 38.0 (standard deviation (SD), 19.0) months. The total observation period was 2,490 person/years. The survey revealed that 78 individuals had died since discharge; and 85, 153, 165, 89, 166, and 45 individuals had a mRS grade of 0, 1, 2, 3, 4, and 5, respectively.
| Characteristics | |
| Age at admission, years, mean (SD) | 68.4 (13.5) |
| Sex, female/male, n | 307/479 |
| Premorbid modified Rankin Scale, 0/1/2/3/4/5, n* | 429/75/51/37/16/0 |
| History of stroke, no/yes, n | 675/111 |
| Type of stroke, infarction/intracerebral haemorrhage/subarachnoid haemorrhage, n | 470/257/59 |
| Duration from onset to admission, days, mean (SD) | 35.1 (13.7) |
| Length of stay in convalescent rehabilitation ward, days, mean (SD) | 92.3 (44.7) |
| Duration after discharge, months, mean (SD) | 38.0 (19.0) |
| Side of paresis, right/left/both/none, n† | 310/286/41/89 |
| Motor score on SIAS at discharge, median (IQR) | |
| Total upper limb function score‡ | 8 (4–10) |
| Total lower limb function score§ | 12 (8–15) |
| Independence of gait at discharge, independent/non-independent, n** | 475/282 |
| FIM at discharge, median (IQR) | |
| Total score†† | 107 (80–119) |
| Total score of motor items‡‡ | 78 (60–87) |
| Total score of cognitive items§§ | 30 (22–34) |
| Discharge destination, home/nursing home/transferred to another hospital, n | 698/73/15 |
| Number of missing values: *n = 178, †n = 60, ‡n = 77, §n = 83, **n = 29, ††n = 30, ‡‡n = 29, §§n = 30. | |
| FIM: Functional Independence Measure; IQR: interquartile range; n: number; SIAS: Stroke Impairment Assessment Set; SD: standard deviation. | |
Table II shows the details of fall-related fractures. In total, 99 fall-related fracture events occurred during the 2,490 person/year observation period. Among the 86 individuals who experienced fall-related fractures, 73 experienced 1 fracture event and 13 experienced 2 fracture events after discharge from the KRW. The incidence rate of fall-related fracture events was 39.7 events/1,000 person-years. The first fall-related fracture occurred at a mean (SD) of 18.4 (15.6) months after discharge. Among 90 fracture locations, in which first fall-related fractures were noted, the hip was most frequently involved (38.9%, n = 35), followed by the proximal humerus (14.4%, n = 13) and the wrist (10.0%, n = 9). Most hip and proximal humerus fractures occurred on the more-affected side than on the less-affected side (hip, 32: 3, respectively; proximal humerus, 13: 0, respectively). However, wrist fractures occurred more frequently on the less-affected side than on the more-affected side (wrist, 6: 3, respectively).
| Items | |
| Respondent, participants/family/facility staff, n | 410/375/1 |
| Experience of fall-related fracture(s), none/once/twice, n | 700/73/13 |
| Time from discharge to the first fall-related fracture, months, mean (SD)* | 18.4 (15.6) |
| Location of first fall-related fracture after discharge, n† | |
| Hip (more-affected side/less-affected side) | 35 (32/3) |
| Proximal humerus (more-affected side/less-affected side) | 13 (13/0) |
| Wrist (more-affected side/less-affected side) | 9 (3/6) |
| Lumbar vertebrae | 8 |
| Pelvis | 5 |
| Rib | 4 |
| Other | 15 |
| Uncertain | 1 |
| *The date of the event was missing in 4 individuals. | |
| †Four individuals had 2 fractures during the first fall-related fracture event. | |
| SD: standard deviation; n: number. | |
After excluding 4 individuals with insufficient information regarding the date of fall-related fractures (new total, n = 782), the cumulative incidence rates (annual incidence rates) of fall-related fractures at 1, 2, 3, 4, and 5 years after discharge were 4.2 (4.2)%, 7.9 (3.7)%, 10.8 (2.9)%, 12.5 (1.7)%, and 13.7 (1.2)%, respectively. The Kaplan–Meier curve is shown in Fig. 1A.
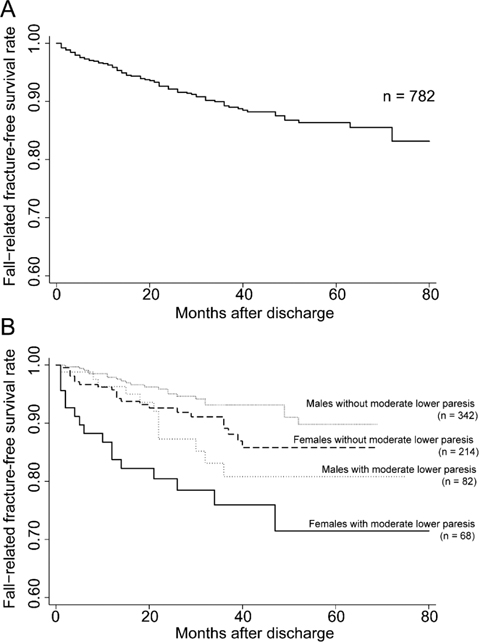
Fig. 1. Kaplan–Meier survival curve for freedom from fall-related fractures after discharge in participants with stroke. (A) Survival curve for all participants and (B) survival curves according to participant sex and severity of lower motor paresis. Y-axes show the percentage of participants who did not suffer fall-related fractures and x-axes show the time from discharge from convalescent rehabilitation wards. Moderate paresis, participants with Stroke Impairment Assessment Set (SIAS) lower limb scores ranging from 6 to 11 points.
The bivariate Cox proportional hazards regression analyses showed significant differences in 8 variables; namely, sex, age, premorbid mRS grade, length of stay, upper limb function on the SIAS, lower limb function on the SIAS, independence of gait, and total score of cognitive items in the FIM (Table III). The total scores of upper and lower limb functions on the SIAS were significantly correlated with each other (r = 0.87, p < 0.001); therefore, only lower limb function on the SIAS, which was considered to be more strongly associated with fall-related fractures, was used as an independent variable in the multivariable Cox proportional hazards regression analysis.
| Variable | Hazard ratio | 95% confidence interval | p-value |
| Female sex | 1.82 | 1.18–2.82 | 0.006 |
| Age at admission, years | 1.03 | 1.01–1.05 | 0.001 |
| Premorbid modified Rankin Scale† | |||
| Grade 0 | Reference | ||
| Grade 1 | 1.81 | 0.90–3.65 | 0.094 |
| Grade 2 | 2.57 | 1.24–5.34 | 0.011 |
| Grade 3 | 1.64 | 0.58–4.62 | 0.346 |
| Grade 4 | 0.82 | 0.11–6.02 | 0.850 |
| Grade 5 | – | – | – |
| Type of stroke | |||
| Infarction | Reference | ||
| Intracerebral haemorrhage | 1.13 | 0.72–1.78 | 0.582 |
| Subarachnoid haemorrhage | 0.47 | 0.14–1.51 | 0.208 |
| Duration from onset to admission, days | 0.99 | 0.97–1.01 | 0.609 |
| Length of stay in convalescent rehabilitation ward, days | 1.00 | 1.00–1.01 | 0.011 |
| History of stroke | 1.35 | 0.76–2.40 | 0.303 |
| Side of paresis‡ | |||
| Right | Reference | ||
| Left | 1.58 | 0.97–2.59 | 0.065 |
| Both | 0.56 | 0.13–2.37 | 0.436 |
| Total upper limb function score on the SIAS§ | |||
| Score 10 | Reference | ||
| Score 8–9 | 1.72 | 0.78–3.75 | 0.173 |
| Score 6–7 | 4.12 | 1.81–9.41 | 0.001 |
| Score 4–5 | 4.02 | 1.76–9.18 | 0.001 |
| Score 2–3 | 1.97 | 0.77–4.99 | 0.153 |
| Score 0–1 | 2.81 | 1.19–6.63 | 0.018 |
| Total lower limb function score on the SIAS** | |||
| Score 15 | Reference | ||
| Score 12–14 | 1.19 | 0.57–2.47 | 0.639 |
| Score 9–11 | 3.43 | 1.63–7.21 | 0.001 |
| Score 6–8 | 3.04 | 1.40–6.56 | 0.005 |
| Score 3–5 | 2.09 | 0.86–5.05 | 0.100 |
| Score 0–2 | 2.37 | 0.94–5.96 | 0.065 |
| Independence of gait†† | |||
| Independent | Reference | ||
| Non-independent | 2.01 | 1.29–3.13 | 0.002 |
| Total score of motor items in the FIM‡‡ | |||
| Score 91 | Reference | ||
| Score 78–90 | 1.10 | 0.33–3.63 | 0.864 |
| Score 65–77 | 1.50 | 0.43–5.20 | 0.518 |
| Score 52–64 | 2.33 | 0.65–8.26 | 0.190 |
| Score 39–51 | 3.06 | 0.81–11.58 | 0.098 |
| Score 26–38 | 2.98 | 0.80–11.04 | 0.101 |
| Score 13–25 | 0.89 | 0.14–5.38 | 0.907 |
| Total score of cognitive items in the FIM§§ | 0.97 | 0.94–0.99 | 0.029 |
| Destination | |||
| Home | Reference | ||
| Nursing home | 1.19 | 0.55–2.61 | 0.648 |
| Different hospital | 2.17 | 0.53–8.89 | 0.278 |
| *Of 786 participants, 4 had missing dates concerning their fall-related fractures. | |||
| Number of missing values: †n = 178, ‡n = 149, §n = 77, **n = 83, ††n = 29, ‡‡n = 29, §§n = 30 | |||
| FIM: Functional Independence Measure; SIAS: Stroke Impairment Assessment Set. | |||
After excluding 201 participants with insufficient clinical variable data (including 25 participants with fall-related fractures), female sex (hazard ratio (HR), 1.69; 95% confidence interval (95% CI), 1.00–2.87; p = 0.049), a total lower limb function score on the SIAS of 9–11 points (HR 3.08; 95% CI, 1.28–7.38; p = 0.012), and a total lower limb function score on the SIAS of 6–8 points (HR 3.05; 95% CI, 1.18–7.87, p = 0.021; Table IV) were found to be significant risk factors for fall-related fractures.
The Kaplan–Meier curves stratified according to sex and the presence of moderate paresis (total SIAS lower limb score ranging from 6 to 11 points) are shown in Fig. 1B. The cumulative incidence rates (annual incidence rates) of fall-related fractures in women with moderate lower limb paresis at 1, 2, 3, 4, and 5 years after discharge were 16.3 (16.3)%, 19.6 (3.3)%, 24.1 (4.5)%, 28.6 (4.5)%, and 28.6 (0.0)%, respectively.
DISCUSSION
To our knowledge, this is the first study to elucidate the cumulative risk of fall-related fractures in stroke survivors after discharge from the KRW. Within this study cohort, hip fractures occurred most frequently, followed by proximal humerus fractures. Female sex and moderate paresis of the lower limbs were significantly associated with the risk of fall-related fractures.
In previous studies, most fractures (75–84%) among stroke survivors have been reported to be attributed to falls (15, 17). The incidence rate of fall-related fractures in the current study was 39.7 events/1,000-person-years, which was higher than the overall fracture risk of 22–37 events/1,000 person-years reported in previous studies concerning individuals with stroke, which included non-fall-related fractures (15, 17). This difference may have been observed because our study population was disabled to a greater degree, as all participants were eligible for inpatient rehabilitation. Compared with the general population, the incidence rate of fall-related fractures in a previous study was reported to be 17.9 events/1,000 person-years (30), which was significantly lower than the 39.7 events/1,000 person-years observed in the current study. The relative risk of fall-related fractures in the current study was 2.2 times higher than that in that previous study (30). These results are consistent with several previous studies that have reported incident fracture rates 1.4–3.8 times higher than in the general population (15–17, 19, 22, 31, 32). Considering the incidence rates in current study and in previous studies, the risk of fall-related fractures observed in this study was similarly high.
The cumulative incidence rate of fall-related fractures for the 5 years following discharge was 13.7%, and the annual incidence rates of fall-related fractures was the highest (4.2%) in the initial period (within 1 year) after discharge. This finding seemed reasonable and was consistent with those of previous studies, in which the highest risk was observed immediately after the onset of stroke (16, 19). The incident risk has been reported to be 3–4% at 1 year (15, 18, 20) and 5–6% at 2 years (17, 21, 22) after the onset of stroke. Although the time from onset was longer in the present study because the starting point of the analysis was set as discharge from the KRW, these percentages are slightly lower than those reported in the current study. As noted earlier, this disparity may be attributed to differences in study population characteristics.
According to previous reports, fractures occur most frequently in the hip (15, 17, 20, 22) and on the more-affected side (14, 15, 17, 33) in stroke survivors. The current study showed similar findings, which might have been due to a tendency for participants to fall towards the weaker side (7, 34), difficulty in protecting paralysed limbs when falling, and reduced bone density on the affected side (35). Interestingly, an opposite tendency was found in wrist fractures, which were more frequently observed on the less-affected side. This finding supports the hypothesis that individuals with stroke are less likely to sustain a wrist fracture on the affected side because they are unlikely to break a fall through stretching out the affected arm (5).
Female sex and moderate lower limb paresis were found to be significantly associated with the risk of fall-related fractures. Regarding sex, it has previously been well established that fractures occur more frequently in female stroke survivors (15–17, 19, 20, 22). Within the general population, women have a greater risk of falling than men (36). In addition, a decrease in bone mineral density after stroke has been shown to be greater in women than in men (37). These 2 factors contribute to a higher incidence of fractures in female stroke survivors.
In this study, moderate lower limb paresis was significantly and strongly associated with the risk of fall-related injuries. Individuals with mild paresis may have less bone loss and a lower fall risk compared with individuals with moderate paresis. In contrast, individuals with severe paresis may have a lower fall risk because of reduced mobility. Thus, mobile individuals with limb weakness might have a greater risk of fall-related fractures. Although the effects of motor paresis severity have not been specifically assessed in previous studies, the current findings are supported by previous studies that have reported that moderate impairments (20, 22) and disabilities (21, 28, 29) are related to an increased risk of falling. A moderate initial stroke severity, defined using the Canadian Neurological Scale, has been associated with subsequent fracture risk in stroke survivors (22). Similarly, individuals with intermediate stroke severity, as defined by the National Institutes of Health Stroke Scale, had the highest risk of fractures (20). Furthermore, previous studies have reported that moderately severe disability in individuals with stroke is associated with a risk of falling (21, 28, 29). Therefore, our findings are consistent with previous reports of an inverted U-shaped relationship between impairment/disability and the risk of fractures.
The clinical significance of this study is that female sex and moderate lower limb paresis were identified as risk factors for fall-related fractures in stroke survivors after discharge from rehabilitation wards. Thus, it is necessary to undertake appropriate approaches to prevent fall-related fractures, especially in stroke patients with these risk factors. Currently, there is no clear evidence on the prevention of fall-related fractures in stroke patients, but exercise has been shown to be effective in preventing falls (38). Therefore, continuation of rehabilitation, including exercise, after discharge from rehabilitation wards for stroke patients should be considered a preventive measure against falls and fall-related fractures. Appropriate evaluation and treatment of osteoporosis in postmenopausal women is also recommended, and should be considered a preventive intervention for fall-related fractures in women with stroke (39).
This study had several limitations. The retrospective design of the study and the relatively low response rate may have biased the results. It is possible that individuals who experienced fractures were more likely to respond; therefore, the incidence rates might have been overestimated. However, if individuals with stroke who experienced fall-related fractures had a poorer health status than individuals with stroke with a better health status, they might not have been able to reply, and the incidence might have been underestimated. Most respondents were individuals with stroke (or their families) whose cognitive function was not accurately assessed; thus, the reliability of their responses may be limited. This study was conducted at a single facility, and its findings may have limited generalizability to other institutions. The results of the Cox proportional hazards regression analysis showed a wide range of 95% CIs, which may be attributed to the variety of the characteristics among the participants or instability in the model. In future, data involving a large number of stroke survivors with specific characteristics should be collected and analysed. A prospective multicentre study with a larger sample size is required to confirm the current findings. Despite these limitations, we consider that this study provides valuable information concerning fall-related fractures in stroke survivors. In particular, we report for the first time the characteristics of individuals that have experienced a fall-related fracture after discharge from the KRW.
In conclusion, the incidence of fall-related fractures in stroke individuals after discharge from the KRW was 39.7 events/1,000 person-years, indicating a high risk. In particular, appropriate preventive interventions should be considered for female stroke survivors with moderate lower-limb paresis at discharge from rehabilitation wards.
ACKNOWLEDGEMENTS
The authors thank Yusuke Tadokoro, Kohei Moriya, Yuta Asada, Haruna Kawahara, Eriko Oya, Misaki Yamane, Kanako Ishikawa, Shota Watanabe, Sayaka Tomita, Yoko Kanaya, Mizuki Iijima, Kanako Mori, Saori Otani, Ayana Hosobuchi, Shigehiro Makino, Tomomi Yoshida, Anna Miyamoto, Narumi Watanabe, Kazuki Mori, Yuya Narita, Shogo Miyamura, Ayumi Nakamura, Miho Yasuda, Masayuki Dogan, Hiromi Sawada, Azusa Kumagai, Honoka Abe, Masafumi Sugasawa, Yuto Goto, Yasuhiro Tani, Noriaki Takahashi and Shuri Kumagai for helping with the data collection.
REFERENCES
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017; 135: e146–e603.
- Ministry of Health, Labour and Welfare. Estimated number of patients receiving medical treatment for selected diseases. 2014 Summary of patient survey. [cited 20 Feb 2021]. Available from: https://www.mhlw.go.jp/english/database/db-hss/dl/sps_2014_05.pdf
- Ministry of Health, Labour and Welfare. Summary report of comprehensive survey of living conditions 2016. [cited 20 Feb 2021]. Available from: https://www.mhlw.go.jp/english/database/db-hss/dl/report_gaikyo_2016.pdf
- Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, et al. Medical complications after stroke: a multicenter study. Stroke 2000; 31: 1223–1229.
- Weerdesteyn V, de Niet M, van Duijnhoven HJ, Geurts AC. Falls in individuals with stroke. J Rehabil Res Dev 2008; 45: 1195–1213.
- Forster A, Young J. Incidence and consequences of falls due to stroke: a systematic inquiry. BMJ 1995; 311: 83–86.
- Mackintosh SF, Hill K, Dodd KJ, Goldie P, Culham E. Falls and injury prevention should be part of every stroke rehabilitation plan. Clin Rehabil 2005; 19: 441–451.
- Alemdaroglu E, Ucan H, Topcuoglu AM, Sivas F. In-hospital predictors of falls in community-dwelling individuals after stroke in the first 6 months after a baseline evaluation: a prospective cohort study. Arch Phys Med Rehabil 2012; 93: 2244–2250.
- Mansfield A, Wong JS, McIlroy WE, Biasin L, Brunton K, Bayley M, et al. Do measures of reactive balance control predict falls in people with stroke returning to the community? Physiotherapy 2015; 101: 373–380.
- Simpson LA, Miller WC, Eng JJ. Effect of stroke on fall rate, location and predictors: a prospective comparison of older adults with and without stroke. PLoS ONE 2011; 6: e19431.
- Jorgensen L, Engstad T, Jacobsen BK. Higher incidence of falls in long-term stroke survivors than in population controls: depressive symptoms predict falls after stroke. Stroke 2002; 33: 542–547.
- Lamb SE, Ferrucci L, Volapto S, Fried LP, Guralnik JM. Risk factors for falling in home-dwelling older women with stroke: the Women’s Health and Aging Study. Stroke 2003; 34: 494–501.
- Wada N, Sohmiya M, Shimizu T, Okamoto K, Shirakura K. Clinical analysis of risk factors for falls in home-living stroke patients using functional evaluation tools. Arch Phys Med Rehabil 2007; 88: 1601–1605.
- Goto Y, Otaka Y, Suzuki K, Inoue S, Kondo K, Shimizu E. Incidence and circumstances of falls among community-dwelling ambulatory stroke survivors: a prospective study. Geriatr Gerontol Int 2019; 19: 240–244.
- Ramnemark A, Nyberg L, Borssen B, Olsson T, Gustafson Y. Fractures after stroke. Osteoporos Int 1998; 8: 92–95.
- Kanis J, Oden A, Johnell O. Acute and long-term increase in fracture risk after hospitalization for stroke. Stroke 2001; 32: 702–706.
- Dennis MS, Lo KM, McDowall M, West T. Fractures after stroke: frequency, types, and associations. Stroke 2002; 33: 728–734.
- Brown DL, Morgenstern LB, Majersik JJ, Kleerekoper M, Lisabeth LD. Risk of fractures after stroke. Cerebrovasc Dis 2008; 25: 95–99.
- Pouwels S, Lalmohamed A, Leufkens B, de Boer A, Cooper C, van Staa T, et al. Risk of hip/femur fracture after stroke: a population-based case-control study. Stroke 2009; 40: 3281–3285.
- Lisabeth LD, Morgenstern LB, Wing JJ, Sanchez BN, Zahuranec DB, Skolarus LE, et al. Poststroke fractures in a bi-ethnic community. J Stroke Cerebrovasc Dis 2012; 21: 471–477.
- Callaly EL, Ni Chroinin D, Hannon N, Sheehan O, Marnane M, Merwick A, et al. Falls and fractures 2 years after acute stroke: the North Dublin Population Stroke Study. Age Ageing 2015; 44: 882–886.
- Kapral MK, Fang J, Alibhai SM, Cram P, Cheung AM, Casaubon LK, et al. Risk of fractures after stroke: results from the Ontario Stroke Registry. Neurology 2017; 88: 57–64.
- Miyai I, Sonoda S, Nagai S, Takayama Y, Inoue Y, Kakehi A, et al. Results of new policies for inpatient rehabilitation coverage in Japan. Neurorehabil Neural Repair 2011; 25: 540–547.
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607.
- Chino N, Sonoda S, Domen K, Saitoh E, Kimura A. Stroke Impairment Assessment Set (SIAS): a new evaluation instrument for stroke patients. Jpn J Rehabil Med 1994; 31: 119–125.
- Keith RA, Granger CV, Hamilton BB, Sherwin FS. The Functional Independence Measure: a new tool for rehabilitation. Adv Clin Rehabil 1987; 1: 6–18.
- The data management service of the uniform data system for medical rehabilitation and the center for functional assessment research: guide for use of the uniform data set for medical rehabilitation (Ver. 3.0). New York: State University of New York at Buffalo; 1990.
- Wei WE, De Silva DA, Chang HM, Yao J, Matchar DB, Young SHY, et al. Post-stroke patients with moderate function have the greatest risk of falls: a National Cohort Study. BMC Geriatr 2019; 19: 373.
- Sze KH, Wong E, Leung HY, Woo J. Falls among Chinese stroke patients during rehabilitation. Arch Phys Med Rehabil 2001; 82: 1219–1225.
- Orces CH. Emergency department visits for fall-related fractures among older adults in the USA: a retrospective cross-sectional analysis of the National Electronic Injury Surveillance System All Injury Program, 2001–2008. BMJ Open 2013; 3.
- Yuan ZC, Mo H, Guan J, He JL, Wu ZJ. Risk of hip fracture following stroke, a meta-analysis of 13 cohort studies. Osteoporos Int 2016; 27: 2673–2679.
- Luan L, Li R, Wang Z, Hou X, Gu W, Wang X, et al. Stroke increases the risk of hip fracture: a systematic review and meta-analysis. Osteoporos Int 2016; 27: 3149–3154.
- Ramnemark A, Nilsson M, Borssen B, Gustafson Y. Stroke, a major and increasing risk factor for femoral neck fracture. Stroke 2000; 31: 1572–1577.
- Hyndman D, Ashburn A, Stack E. Fall events among people with stroke living in the community: circumstances of falls and characteristics of fallers. Arch Phys Med Rehabil 2002; 83: 165–170.
- Beaupre GS, Lew HL. Bone-density changes after stroke. Am J Phys Med Rehabil 2006; 85: 464–472.
- Tinetti ME, Kumar C. The patient who falls: ”It’s always a trade-off”. JAMA 2010; 303: 258–266.
- Bainbridge NJ, Davie MW, Haddaway MJ. Bone loss after stroke over 52 weeks at os calcis: influence of sex, mobility and relation to bone density at other sites. Age Ageing 2006; 35: 127–132.
- Denissen S, Staring W, Kunkel D, Pickering RM, Lennon S, Geurts AC, et al. Interventions for preventing falls in people after stroke. Cochrane Database Syst Rev 2019; 10: CD008728.
- Tu KN, Lie JD, Wan CKV, Cameron M, Austel AG, Nguyen JK, et al. Osteoporosis: a review of treatment options. P T 2018; 43: 92–104.