REVIEW ARTICLE
DOES REHABILITATION IMPROVE WORK PARTICIPATION IN PATIENTS WITH CHRONIC SPINAL PAIN AFTER SPINAL SURGERY: A SYSTEMATIC REVIEW
Jonas CALLENS, MSc1–3, Olivia LAVREYSEN, MSc4, Lisa GOUDMAN, PhD1,2,5–8, Ann DE SMEDT, PhD, MD1,2,7,9, Koen PUTMAN, PhD3, Dominique VAN DE VELDE, PhD10, Lode GODDERIS, PhD, MD4,11, Dries CEULEMANS, MSc10 and Maarten MOENS, PhD, MD1,2,5–8,12
From the 1STIMULUS research group, Vrije Universiteit Brussel, Jette, 2Cluster Neurosciences, Center for Neurosciences (C4N), Vrije Universiteit Brussel, Brussels, 3Interuniversity Centre for Health Economics Research (I-CHER), Department of Public Health (GEWE), Faculty of Medicine and Pharmacy, Vrije Universiteit Brussel, Jette, 4Centre for Environment and Health, Department of Public Health and Primary Care, KU Leuven (University of Leuven), Leuven, 5Pain in Motion Research Group (PAIN), Department of Physiotherapy, Human Physiology and Anatomy, Faculty of Physical Education & Physiotherapy, Vrije Universiteit Brussel, Jette, 6Department of Neurosurgery, Universitair Ziekenhuis Brussel, Jette, 7Center for Neurosciences (C4N), Vrije Universiteit Brussel, Jette, 8Research Foundation Flanders (FWO), Brussels, 9Department of Physical Medicine and Rehabilitation, Universitair Ziekenhuis Brussel, Jette, 10Faculty of Medicine and Healthcare Sciences, Department of Rehabilitation Sciences, Occupational Therapy Program, Ghent University, Ghent, 11IDEWE, External Service for Prevention and Protection at Work, Heverlee, and 12Department of Radiology, Universitair Ziekenhuis Brussel, Jette, Belgium
Objective: Patients with therapy-refractory chronic spinal pain after spinal surgery experience increased disability, resulting in substantial loss of employment and consequently lower quality of life. Despite findings that rehabilitation improves socio-economic outcomes in other chronic pain conditions, evidence for patients with chronic spinal pain after spinal surgery is limited. A systematic review was conducted to provide an overview of rehabilitation interventions and their effectiveness to improve work participation for patients with chronic spinal pain after spinal surgery.
Methods: MEDLINE (via PubMed), Scopus, Embase, and Web of Science, were systematically searched. Risk of bias was assessed using the modified Downs and Black checklist and GRADE was used to assess certainty of evidence. The review protocol was prospectively registered on PROSPERO (CRD42022346091).
Results: The search yielded 1,289 publications. Full-text screening of 48 articles resulted in the inclusion of 6 publications. The included interventions comprised multiple treatment components, consisting of back school, self-care, functional restoration, multidisciplinary rehabilitation, physiotherapy, and digital care programmes to improve work participation.
Conclusion: Rehabilitation to improve return to work for patients with chronic spinal pain after spinal surgery was supported only by low-certainty evidence. Rehabilitation therapies that are personalized and that integrate the patient’s work seem most suitable.
LAY ABSTRACT
Patients with chronic pain after previous spinal surgery experience significant disability, pain, and loss of employment. Return to work is an important treatment goal for these patients; however, there is no clear overview of which rehabilitation components can reinforce work resumption. We conducted a systematic literature review to provide an overview of the content of rehabilitation and its effectiveness. A broad variety of treatment components were revealed, all with a specific focus on work participation. Moreover, all programmes consisted of an interplay between different disciplines. Rehabilitation therapies that are personalized and that integrate the patient’s work seem most suitable.
Key words: chronic pain; failed back surgery syndrome; rehabilitation; return to work; systematic review.
Citation: J Rehabil Med 2025; 57: jrm25156. DOI: https://doi.org/10.2340/jrm.v57.25156.
Copyright: © 2025 The Author(s). Published by MJS Publishing, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
Submitted: Nov 6, 2023; Accepted after revision: Oct 1, 2024; Published: Jan 3, 2025.
Correspondence address: Maarten Moens, STIMULUS Research Group, Vrije Universiteit Brussel, Laarbeeklaan 103, Jette, BE-1090, Belgium. E-mail: stimulusresearchgroup@gmail.com
Competing interests and funding: LG is a postdoctoral research fellow funded by the Research Foundation Flanders (FWO), Belgium (project number 12ZF622N). MM is a postdoctoral research fellow funded by the FWO, Belgium (project number 1801125N), and has received speaker fees from Medtronic. DC, OL, and JC are PhD students working on a project funded by the Research Foundation Flanders (FWO-TBM project number T000821N). ADS, KP, DvdV, and LG have no relevant financial or non-financial interests to disclose.
The authors declare that this research did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors.
Globally, low back pain and neck pain are consistently presented among the top 4 leading causes of years lived with disability (1, 2). Both conditions significantly impact an individual’s health and constitute a high socioeconomic burden (3–5).When pain persists for at least 3 months, it is classified as chronic (6). Management of chronic low back pain and chronic neck pain mainly consists of conservative treatment options, i.e., pain neuroscience education, exercise therapy, physical activity, acupuncture, pharmacotherapy, or multidisciplinary rehabilitation (5, 7, 8). However, interventional treatment options, among which are epidural injections, disc decompression, nerve blocks, or intradiscal procedures, may be indicated in chronic or refractory spinal pain syndromes (9, 10). Previous research indicates that in 10–40% of spinal surgeries, depending on the exact type of surgery, pain reoccurs or persists leading to the so-called Persistent Spinal Pain Syndrome Type II (PSPS-T2) (11).
PSPS-T2 patients are considered a heterogeneous group based on diversified aetiologies and have multilevel complaints (12). Besides pain and functional problems, these patients report a high work disability that leads to reduced work effectiveness, changes in job responsibilities, and unemployment rates of 50.9–81.5% (11, 13–17). Work participation, defined as the capability and/or opportunity to participate in the workforce and fulfil one’s work role, is consequently reduced for these patients (18). Being able to return to working activities after a period of sick leave or unemployment, otherwise known as return to work (RTW), is a common measure of work participation (18, 19). Longer periods of limited work participation due to long-term unemployment, inability to, or delayed RTW, affect a patient’s ability to maintain an independent lifestyle, impact quality of life, and simultaneously impose a high economic burden to society due to indirect costs (20–23). The literature reports that 61–87% of PSPS-T2 patients are within the working age range (24, 25). This, in combination with the high unemployment rates, emphasizes the need for improvements in work participation as realistic and major treatment goals (26). Management of PSPS-T2 aims to reduce pain and improve functioning and has been the subject of considerable research. Spinal cord stimulation, rehabilitation, psychological therapy, and minimal invasive procedures are considered to be the most effective, while poor evidence is presented for the efficacy of pharmacological therapies and reoperations (27–31). Despite the fact that optimal medical management is effective to decrease pain and improve disability, PSPS-T2 patients do not achieve successful work participation (15, 27–31). Although there is strong evidence that working can reverse the long-term negative effects of unemployment on health and well-being, detailed studies on interventions for work participation and their efficacy are currently lacking (20, 23, 32–34). It should thus be imperative to search for those interventions or therapies that are most effective to facilitate work participation.
To our knowledge, a review on nonsurgical, nonpharmacological, multidisciplinary rehabilitation to improve work participation (e.g., job coaching, ergonomics, pain management, vocational therapy, etc.) for PSPS-T2 patients has not yet been conducted. This systematic review therefore presents an up-to-date overview of the current body of literature on rehabilitation interventions to improve work participation, and their effectiveness for PSPS-T2 patients.
METHODS
This systematic review adheres to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement recommendations (35). The protocol was prospectively registered in the PROSPERO database (CRD42022346091).
Search strategy and eligibility criteria
The search strategy was developed according to the PICO (Population–Intervention–Comparison(s)–Outcome) framework (36). The population was defined as chronic spinal pain patients with previous spinal surgery, the intervention as nonsurgical, nonpharmacological rehabilitation and the outcome as work participation. The intervention could be compared to standard care, no intervention, or any other type of intervention.
MEDLINE (via PubMed), Embase, Scopus, and Web of Science were searched from inception to 1 September 2023 to identify potentially relevant studies. Additionally, reference lists were checked and citation tracking was performed to identify all relevant studies (36). The complete search strategy for each database is detailed in Table SI.
All studies were screened against predetermined inclusion criteria as presented in Table I. The following inclusion criteria were used: (i) experimental, quasi-experimental, and observational studies with and without a control group; (ii) population including adults (≥ 18 years) with a history of at least 1 spinal surgery, currently experiencing chronic back and/or neck pain (≥ 3 months); (iii) nonsurgical, noninvasive, nonpharmacological rehabilitation intervention(s) or rehabilitation programme(s); (iv) work-related outcome(s) relating to work participation (e.g., RTW, time to RTW, work-capacity score(s), sick leave, absenteeism, employment status, work disability, etc.); (v) in English, French, Dutch, and German languages. Studies enrolling participants receiving any type of pharmacological/surgical therapy as an intervention were exluded. Finally, studies that were available only in abstract format were excluded.
In the case of a mixed population of chronic spinal pain patients with and without a history of spinal surgery, a study was considered and study results on the spinal surgery history subgroup were extracted whenever possible. If a study contained missing outcomes of interest, the authors were contacted.
The employment status of participants after any rehabilitation intervention was used as the primary outcome parameter. Secondary outcomes included time between the end of rehabilitation and start of return to employment, absenteeism, sick leave, work-ability scores, and work disability.
Data extraction and analysis
All search results were exported to EndNote (EndNote v. X9, Thomson Reuters, San Francisco, CA, USA), where duplicates were removed. The title and abstract of the unique results were independently reviewed by 2 reviewers on the inclusion and exclusion criteria using Rayyan software (Rayyan Systems Inc; https://www.rayyan.ai/) (37). Full-text publications of potentially relevant references were obtained and independently assessed by 2 reviewers. Relevant data from included studies were extracted by 1 reviewer and verified by a second using an a-priori developed data extraction form comprising first author, publication year, study design, population, intervention, comparator/co-intervention, work-related outcomes, and summary of results. The work-related outcomes were presented as originally reported, without any conversion to a standardized measure. Any discrepancies in the initial or full text screening and data extraction were addressed during a consensus meeting with both reviewers and decided by a third reviewer. The methodological quality of the retained publications was independently assessed using the modified Downs and Black quality assessment checklist (Table SII). The Downs and Black checklist was designed for evaluation of both randomized and non-randomized comparative studies (38). Studies are scored on 27 items concerning reporting, external validity, internal validity (confounding), and power. This implies that the maximal scores for randomized, non-randomized, and non-controlled studies are respectively 28, 25, or 20. The following suggested cut-off scores were used: “Excellent” (26–28); “Good” (20–25); “Fair” (15–19); and “Poor” (≤ 14) (39). To avoid the selective reporting of study findings, studies were not excluded based on the results of the quality and risk of bias assessment. The GRADE methodology was used to assess the certainty of evidence (40).
RESULTS
Study selection
The search yielded a total of 1,289 potentially relevant records: 403 for Embase, 330 for Web of Science, 290 for PubMed, and 266 for Scopus. A detailed overview of the literature search is provided in Fig. 1.
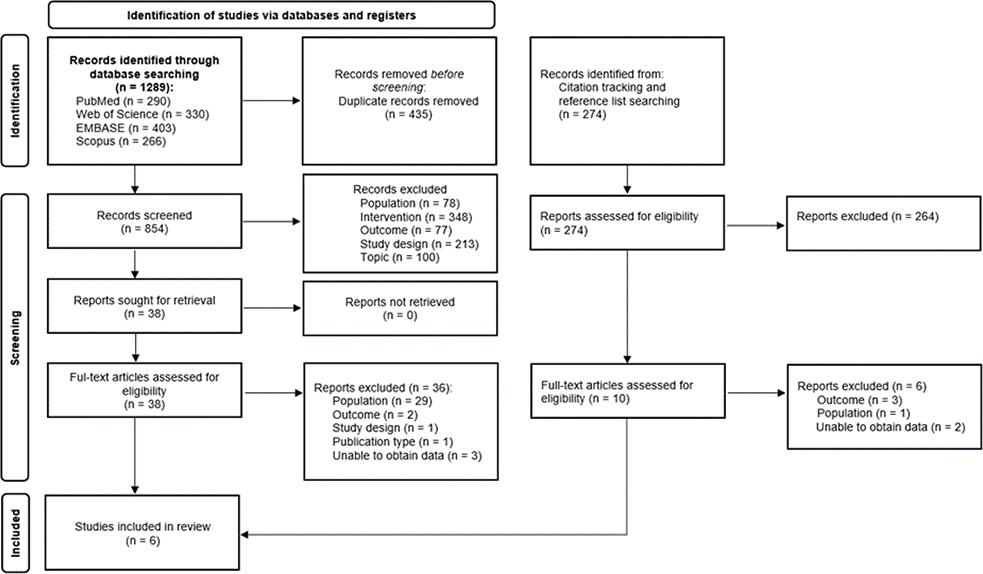
Fig. 1. Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) study flow diagram.
After removal of duplicates (n = 435), 854 unique records remained. Subsequently, title and abstract were screened, leading to the exclusion of 816 records. The main reasons for exclusion were (a) not evaluating a rehabilitation intervention (n = 348); (b) not evaluating work-related outcomes (n = 77); (c) study population not consisting of chronic spinal pain patients with previous spinal surgery (n = 78). Other studies were excluded due to study design, publication type, language, or topic (n = 313).
After exclusion, 38 articles were retrieved for full-text evaluation. Citation tracking and reference list searching revealed another 274 potential records and resulted in an additional 10 articles for full-text evaluation. Thus, 48 publications were comprehensively screened in full, whereafter 6 publications were retrieved for inclusion in this systematic review (41–46). For 5 potential records with a mixed population of patients with and without PSPS-T2, the authors were contacted. This led to the exclusion of all 5 records due to not being able to obtain work-related outcome results on the PSPS-T2 subgroup. Only a narrative synthesis was performed due to the limited number of included studies and presence of between-study heterogeneity, which precluded grouping of studies and meta-analysis.
Study characteristics
The characteristics for the included studies are detailed in Table II. The included studies were published between 1994 and 2023. Three studies were conducted in France, 2 in the USA and 1 in Switzerland. One randomized controlled trial (RCT) (45), 2 retrospective case series (41, 44), 1 prospective case series (43) and 2 prospective cohort studies (42, 46) were included. All studies recruited a mixed population including both participants with and without PSPS-T2. The total number of PSPS-T2 patients ranged between 18 and 119, totalling 240 patients who followed rehabilitation. In each study, the PSPS-T2 subgroup was a minority compared with the total study sample, comprising 10–30% of all participants. The mean age ranged between 40 and 43.8 years, and study populations were mostly male dominated.
| Study | Population, n (amount of patients with a previous surgery (i.e., PSPS-T2 patients)) | Intervention | Comparators | Outcomes |
| Porteau-Cassard et al., retrospective case-series (41) | 144 (18), mean age 43.8 ± 9.7; sex 50% male. | Back school programme (physical therapy, material handling techniques and occupational therapy) 5-day time period Providing team of rheumatologist, occupational therapist, manutention expert, physiotherapists and dietitian |
No comparators | Days off work (mean+SD): At baseline: 78.5 ± 82.4 At 6-month follow-up: 36±75.8 |
| Burke et al., prospective cohort study (42) | 397 (119), mean age 36 (T), 37 (C); sex 78% male (T), 81% male (C) | Functional restoration programme (strength testing, educational programme and work simulation) Minimum of 1 week time period Providing team not specified |
Comparator group received no treatment | RTW rate: At 6-month follow-up: 54% (T), 32% (C) At 12-month follow-up: 72% (T), 14% (C) |
| Poulain et al., prospective case series (43) | 105 (35), median age 44; sex 45% male | Functional restoration programme (physical exercise, relaxation, education and CBT) 4-week time period, 5 days a week, 6 h a day Providing team of physician, psychologist, physiotherapists, ergonomist, social worker and dietitian |
No comparators. | RTW: Prior back surgery is a non-significant variable predicting long-term RTW after intervention (p > 0.1) |
| Tavares-Figueiredo et al., retrospective case series (44) | 99 (15), mean age 40.8 ± 10.0; sex 63.6% male | Validated self-care programme (physical and educational approaches) 3-week time period, 5 days a week, 8 h a day Providing team of physical and occupational medicine physician, rehabilitation physician, rheumatologist, social worker, physical and occupational therapists, nurses, psychologist, dietitian and pain physician |
No comparators | Work status (proportion of participants working/not working): At baseline: 5/10 At 6-month follow-up: 4/4 (MD = 7) At 12-month follow-up: 3/3 (MD = 9) |
| Cui et al., randomized controlled trial (45). | 140 (15), median age 50.5 (T), 54.5 (C); sex 32% male 74.3% (T), 64.3% (C) within working age * |
Tailored digital care programme (exercise, education and CBT) 8-week time period, 3 days a week, 20 min a day Providing team of physical therapists |
Comparator group received evidence-based in-person physiotherapy | Work status (proportion of participants working/not working): At baseline: 4/5 (T), 4/2 (C) At 8-week follow-up: 2/3 (T), 2/1 (C) (MD = 7) |
| Ibrahim et al., prospective cohort study (46) | 201 (38), mean age 40.0; sex 59% male | Multidisciplinary biopsychosocial rehabilitation programme 4-week time period, 5 days a week, 100 h total Providing team of rheumatologist, rehabilitation physician, pain specialist, psychiatrist, physiotherapists, occupational therapists and psychologist |
No comparators | Work status (proportion of participants working/not working): At baseline: 9/27 (MD = 2) At programme end: 10/23 (MD = 5) At 6-month follow-up: 11/13 (MD = 14) At 18-month follow-up: 13/7 (MD = 18) |
| T: treatment group, C: comparator group, RTW: return to work, CBT: cognitive-behavioural therapy, MD: missing data. * Data reported for the entire study population. |
||||
All 6 studies described an intervention that combined a physical and educational component. Most studies included a diagnostic evaluation as the starting point of the intervention (42, 44–46). The studies by Poulain et al. and Ibrahim et al. were the only to include a management component (43, 46). A work-related component was described in 2 studies; Burke et al. included a work-simulation, and Ibrahim et al. specified workplace adaptations and workplace visits (42, 46). All studies, except 1, individually adapted specific components of the intervention programme to address patient capacity (45, 46), goals (41, 46), or work situation (41–43, 46). The study by Tavares-Figueiredo et al. (44) did not describe any adaptations but used the diagnostic evaluation to establish individual therapy objectives.
Four studies described inpatient rehabilitation programmes, whereas only 1 study examined an outpatient programme (41–44, 46). The RCT by Cui et al. (45), on the other hand, compared inpatient rehabilitation with an outpatient digital programme. The length of the different interventions ranged from 1 to 8 weeks, from 20 min per day to 8 hours per day, and from 2 to 5 days a week. The study of Ibrahim et al. additionally included a refresher course at 6 months following completion of the intervention.
In most studies, a multidisciplinary team provided the rehabilitation, composed of both physicians and paramedical healthcare professionals, namely physiotherapists, occupational therapists, dietitians, social workers, psychologists, or nurses (41–44, 46). The study by Cui et al. described a monodisciplinary team of physical therapists (45).
Only 2 studies included a comparator group. The study by Burke et al. compared rehabilitation with no treatment, and the RCT by Cui et al. described conventional physiotherapy as comparator (42, 45). The other included studies were cohort studies and case series lacking a control group.
Risk of bias and quality
The methodological quality scores based on the modified Downs and Black checklist are presented in Table III. The observational studies by Porteau-Cassard et al. (41), Poulain et al. (43), and Tavares-Figueiredo et al. (46) were scored as “poor” (respectively 12, 13, and 12/28) with considerable risk of bias towards sampling, loss to follow-up and missing data. The studies by Burke et al. (42) and Ibrahim et al. were scored as “fair” (respectively 16 and 17/28), and the RCT by Cui et al. (45) was scored as “good” (24/28), although risk of bias toward blinding is present.
The level of evidence for the included studies was downgraded due to concerns regarding indirectness for all studies, the lack of blinding for the RCT by Cui et al., and additionally due to risk of selection bias and missing data/loss to follow-up in the observational studies (Table SIII).
Work-related outcomes
Four out of 6 studies reported on the employment status both before and after the intervention by providing the absolute number of working participants with PSPS-T2 or a RTW rate (42, 44, 45). In the study by Burke et al., PSPS-T2 patients receiving rehabilitation were compared with PSPS-T2 patients not receiving any treatment (42). Rehabilitation led to a higher amount of PSPS-T2 patients who achieved successful RTW at 6 months (54% vs 32%) and at 1 year (72% vs 14%) (42). Patients with a previous spinal fusion were more than 5 times as likely to achieve RTW if they received rehabilitation compared with no rehabilitation at 1 year (p = 0.004) (42). Patients with a previous laminectomy who followed rehabilitation were 1.5 more likely to return to work after 6 months compared with those without rehabilitation (42). Ibrahim et al. reported on an increase in the work rate of participants after rehabilitation immediately following the programme, and at 6-month and 18-month follow-up (missing data n = 18) (46). Likewise, in the study by Tavares-Figueiredo et al. there was an increase in the percentage of participants at work after rehabilitation at 6-month and 12-month follow-up (missing data n = 9) (44). Cui et al. (45) reported on a total of 15 participants; however, in both the intervention and control group there was no change in employment status before vs after rehabilitation (missing data n = 7). In the case-series by Poulain et al., having prior spinal surgery was reported as a non-significant variable in predicting long-term RTW after rehabilitation (p > 0.1) (43). The case-series by Porteau-Cassard et al. was the only study to present data on sick leave and reported a non-significant difference in the number of days off work at 6 months after the intervention, compared with baseline (78.5 ± 82.4 to 36 ± 5.8), which did not change between the 6-month and 12-month follow-up (41).
DISCUSSION
This systematic review provides an overview of the literature on rehabilitation therapy to improve work participation, and its effectiveness in PSPS-T2 patients. An extensive search in well-known databases resulted in 6 relevant studies. Our findings suggest that nonsurgical, nonpharmacological rehabilitation may increase work participation, but the evidence is uncertain.
Despite this uncertainty, there are important take-home messages from our results that can influence daily clinical practice. The included studies are in line with previous research in chronic pain populations, stating that multidisciplinary and multicomponent interventions are more effective than monodisciplinary and single-component counterparts for work-related outcomes, especially in patients who failed to show improvements after surgical interventions (47–49). This could explain why the study by Cui et al. (45), investigating a monodisciplinary intervention, presents negative results, while the other studies indicate positive effects (41–44, 46). Furthermore, therapy intensity (i.e., amount of hours of therapy per day) in the study by Cui et al. is significantly lower compared with the other programmes (20 to 30 min per day, 1 day per week) (45). However, the medical literature remains inconclusive on the intensity and duration of rehabilitation needed to achieve the best effects (50, 51). Similarly, there is no clear consensus on whether group-based or individual rehabilitation is more effective, whether in- or outpatient rehabilitation is preferable, and whether in-person or digital rehabilitation is more beneficial (52). This might highlight the relevance of a programme tailored to a patient’s preferences, needs, and abilities, described in all the included studies in our study (by, e.g., adaptations of exercises, addressing patient-specific shortcomings, personal and meaningful goalsetting, etc.). Research on work participation supports this personalized approach as it minimizes the risk that essential personal, social, or work-related information will be bypassed (26, 53, 54). On the other hand, evidence on personalized rehabilitation to improve work participation compared with usual care or standard treatment is still scarce (52, 55). Surprisingly, only 4 studies include a diagnostic or assessment component and just 2 studies have a management component, both of which are essential to tailor to patient preferences and abilities (41, 43).
The perspective on work disability and work participation has evolved from a narrow physical focus towards a more comprehensive biopsychosocial framework (56). Over the past decade, there has been a notable increase in the recognition of this framework (56–58). This has led to more evidence suggesting that rehabilitation for socioeconomic outcomes should be multidisciplinary and encompass the biological, psychological, and social aspects of a patient’s ability to work (56–58). The included interventions, although multidisciplinary in nature, differ from recommendations described in models stemming from the biopsychosocial framework (e.g., Sherbrooke model and ecological case management model) (56, 57, 59). It should be noted that the publication dates of the included studies range from 1994 to 2023. Therefore, it should be acknowledged that not all studies could have implemented the most recent recommendations. The interventions in each included study used 2 basic therapeutic modalities: a physical exercise component and an educational component (41–46). These components are generally considered cornerstones of rehabilitation programmes (60). In and of themselves, these components do not improve work participation (61, 62). The physical ability to perform work does not guarantee RTW and informing patients on job adaptations or self-management strategies does not effectively reduce absenteeism (61, 62). These 2 components merely represent the first step in the biopsychosocial perspective. A number of other aspects are critical for work participation. Identifying personal, social, and work-related factors (i.e., job satisfaction, worker perception, recovery expectations, self-efficacy, readiness, etc.), analysing the work-disability situation, actively involving the employer, setting and adjusting individualized objectives, considering work modifications, workplace involvement, ensuring interdisciplinary coordination, and personaliszation are necessary components for rehabilitation directed at improving work participation (63–67). Interestingly in this review, an active work-related component is described in only 2 studies. Burke et al. described a work simulation component, and Ibrahim et al. investigated advice for workplace adaptations and workplace visits, resulting in higher RTW rate and improved work rate after intervention (42, 46). Prior research and theoretical frameworks have identified active work-related components (i.e., workplace interventions, targeted vocational rehabilitation) as effective and essential to improve work participation among adults with chronic physical conditions, including chronic low back pain (20, 32, 56, 57, 59, 68–72).
Recent advances in healthcare such as novel imaging techniques (i.e., MR neurography, or SPECT/CT), the growth of minimally invasive surgical approaches, and the integration of emerging technologies (i.e., image-guided or robot-assisted surgery, virtual reality) have led to increased safety, faster recovery, and improved clinical outcomes after spinal surgery (73, 74). Despite this, and guidelines addressing the overuse of surgery in low back pain, the recent prevalence of PSPS-T2 remains high at 14.97% (75). The low to very low certainty of the evidence identified in this review, together with the fact that patients with a history of surgery are often excluded from trials on chronic low back pain, highlights the importance of high-quality clinical trials that evaluate rehabilitation for work participation in a large sample of PSPS-T2 patients (76). As these patients are more likely to incur substantial medical costs, there is a significant potential for socioeconomic improvement. Our results are a first step and a clear call for future studies to continue comprehensive research on how to increase the percentage of work participation, thereby reducing the risk of wasting scarce resources on interventions that have little effect on work participation. Both research and daily clinical practice would greatly benefit from a more transparent conceptualization of biopsychosocial rehabilitation for work-related outcomes, such as work participation. The authors advocate the use of established frameworks (i.e., biopsychosocial framework) and models (i.e., Sherbrooke model) as the foundation to guide the content and intensity of rehabilitation and recommend including active work-related intervention components. Moreover, a single outcome measure is unlikely to cover the broad concept of work participation. The authors therefore advocate using at least both a time-based (i.e., time to RTW) and status-based measure (i.e., work status or RTW status). A recent concept analysis of biopsychosocial rehabilitation for chronic low back pain in the working population identified personalization as one of the key attributes (77). Along with the results of this review, the authors recommend a personalized approach based on proper assessment when it comes to rehabilitation for work participation. Finally, considering the growth of individualized medicine, the inherent complexity of individualized approaches in chronic pain, and the findings of this review, follow-up research was initiated. A clinical trial investigating the effects of personalized biopsychosocial rehabilitation targeting RTW for PSPS-T2 patients implanted with SCS is currently ongoing (78).
Strengths and weaknesses
This systematic literature review is the first to summarize the available evidence on the effectiveness of rehabilitation to improve work participation in PSPS-T2 patients. A major strength is the predetermined systematic methodology, which ensured a comprehensive examination of the literature, while simultaneously minimizing risk of bias. An unrestricted search strategy in terms of study design, publication date, and language, as well as citation tracking, and reference list searching add to the strengths. However, this review also has some limitations. First, PSPS-T2 patients are more likely to have an extended medical treatment history and sustained periods of prolonged absence from employment, which makes finding successful treatments arduous (79, 80). In addition, a significant proportion of the literature on chronic spinal pain patients has a strong medical focus, resulting in surgical, pharmacological, or other invasive interventions (29). Therefore, the number of publications on rehabilitation for work-related outcomes in PSPS-T2 patients is potentially limited and could explain why only a small number of studies of mostly poor to fair quality and small sample size were identified. A considerable number of studies described a history of neck and/or back surgery as an exclusion criterion for patient recruitment and therefore had to be excluded from this review. This once more highlights the importance of this review and warrants the need for future research to comprehensively investigate this population, despite its potential perceived obstacles. The limited number of included studies, issues on sample size and missing data, as well as clinical heterogeneity, resulted in a downgrade of the certainty of evidence according to GRADE and precluded a meta-analysis, potentially limiting generalizability. Lastly, following full-text screening, several studies (n = 5) were excluded as the authors were unable to provide results on the work-related outcomes of the subgroup of PSPS-T2 patients as part of their included study population. However, it is uncertain whether the addition of these studies would have resulted in a non-ambiguous conclusion, based on high-quality evidence.
Conclusion
The current medical literature lacks evidence to provide recommendations regarding the effectiveness of rehabilitation focusing on work participation in PSPS-T2 patients in the short and long term. Considering the growing prevalence of chronic back pain and the increasing number of back surgeries performed worldwide, future research should continue to investigate rehabilitation options with the aim to improve professional reintegration in PSPS-T2 patients and beyond.
ACKNOWLEDGEMENTS
Ethical clearance: Not applicable as this study is based on secondary data and does not directly involve human participants.
REFERENCES
- Wu A, March L, Zheng X, Huang J, Wang X, Zhao J, et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Ann Transl Med 2020; 8: 299.
- Safiri S, Kolahi A-A, Hoy D, Buchbinder R, Mansournia MA, Bettampadi D, et al. Global, regional, and national burden of neck pain in the general population, 1990–2017: systematic analysis of the Global Burden of Disease Study 2017. BMJ 2020; 368: m791. https://doi.org/10.1136/bmj.m791
- Cohen SP, Hooten WM. Advances in the diagnosis and management of neck pain. BMJ 2017; 358: j3221. https://doi.org/10.1136/bmj.j3221
- Henschke N, Kamper SJ, Maher CG. The epidemiology and economic consequences of pain. Mayo Clinic Proceedings 2015; 90: 139–147. https://doi.org/10.1016/j.mayocp.2014.09.010
- Knezevic NN, Candido KD, Vlaeyen JWS, Van Zundert J, Cohen SP. Low back pain. Lancet 2021; 398: 78–92. https://doi.org/10.1016/s0140-6736(21)00733-9
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019; 160: 19–27. https://doi.org/10.1097/j.pain.0000000000001384
- Nicol V, Verdaguer C, Daste C, Bisseriex H, Lapeyre É, Lefèvre-Colau M-M, et al. Chronic low back pain: a narrative review of recent international guidelines for diagnosis and conservative treatment. J Clin Med 2023; 12: 1685.
- Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet 2017; 389: 736–747. https://doi.org/10.1016/s0140-6736(16)30970-9
- Manchikanti L, Abdi S, Atluri S, Benyamin RM, Boswell MV, Buenaventura RM, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician 2013; 16: S49–283.
- Chou R, Loeser JD, Owens DK, Rosenquist RW, Atlas SJ, Baisden J, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine 2009; 34: 1066–1077. https://doi.org/10.1097/BRS.0b013e3181a1390d
- Christelis N, Simpson B, Russo M, Stanton-Hicks M, Barolat G, Thomson S, et al. Persistent spinal pain syndrome: a proposal for failed back surgery syndrome and ICD-11. Pain Med 2021; 22: 807–818. https://doi.org/10.1093/pm/pnab015
- Alizadeh R, Sharifzadeh SR. Pathogenesis, etiology and treatment of failed back surgery syndrome. Neurochirurgie 2022; 68: 426–431. https://doi.org/10.1016/j.neuchi.2021.09.005
- Rigoard P, Billot M, Ingrand P, Durand-Zaleski I, Roulaud M, Peruzzi P, et al. How should we use multicolumn spinal cord stimulation to optimize back pain spatial neural targeting? A prospective, multicenter, randomized, double-blind, controlled trial (ESTIMET Study). Neuromodulation 2021; 24: 86–101. https://doi.org/10.1111/ner.13251
- Rigoard P, Basu S, Desai M, Taylor R, Annemans L, Tan Y, et al. Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: a multicenter randomized controlled trial. Pain 2019; 160: 1410–1420. https://doi.org/10.1097/j.pain.0000000000001510
- Naiditch N, Billot M, Goudman L, Cornet P, Roulaud M, Ounajim A, et al. Professional status of persistent spinal pain syndrome patients after spinal surgery (PSPS-T2): what really matters? A prospective study introducing the concept of “adapted professional activity” inferred from clinical, psychological and social influence. J Clin Med 2021; 10: 5055.
- Manca A, Eldabe S, Buchser E, Kumar K, Taylor RS. Relationship between health-related quality of life, pain, and functional disability in neuropathic pain patients with failed back surgery syndrome. Value Health 2010; 13: 95–102. https://doi.org/10.1111/j.1524-4733.2009.00588.x
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006; 10: 287–287. https://doi.org/10.1016/j.ejpain.2005.06.009
- Lagerveld SE, Bültmann U, Franche RL, van Dijk FJH, Vlasveld MC, van der Feltz-Cornelis CM, et al. Factors associated with work participation and work functioning in depressed workers: a systematic review. J Occup Rehabil 2010; 20: 275–292. https://doi.org/10.1007/s10926-009-9224-x
- Haafkens J, Moerman C, Schuring M, van Dijk F. Searching bibliographic databases for literature on chronic disease and work participation. Occup Med (Lond) 2005; 56: 39–45. https://doi.org/10.1093/occmed/kqi193
- Norlund A, Ropponen A, Alexanderson K. Multidisciplinary interventions: review of studies of return to work after rehabilitation for low back pain. J Rehabil Med 2009; 41: 115–121. https://doi.org/10.2340/16501977-0297
- Toye F, Seers K, Allcock N, Briggs M, Carr E, Barker K. A synthesis of qualitative research exploring the barriers to staying in work with chronic musculoskeletal pain. Disabil Rehabil 2016; 38: 566–572. https://doi.org/10.3109/09638288.2015.1049377
- Alonso-García M, Sarría-Santamera A. The economic and social burden of low back pain in Spain: a national assessment of the economic and social impact of low back pain in Spain. Spine (Phila Pa 1976) 2020; 45: E1026–e1032. https://doi.org/10.1097/brs.0000000000003476
- Waddell G, Burton A. Is work good for your health and well-being? London: TSO; 2006.
- Inoue S, Kamiya M, Nishihara M, Arai YP, Ikemoto T, Ushida T. Prevalence, characteristics, and burden of failed back surgery syndrome: the influence of various residual symptoms on patient satisfaction and quality of life as assessed by a nationwide Internet survey in Japan. J Pain Res 2017; 10: 811–823. https://doi.org/10.2147/jpr.S129295
- Stanton EW, Chang K-E, Formanek B, Buser Z, Wang J. The incidence of failed back surgery syndrome varies between clinical setting and procedure type. J Clin Neurosci 2022; 103: 56–61. https://doi.org/10.1016/j.jocn.2022.06.027
- Goudman L, Bruzzo A, van de Sande J, Moens M. Goal identification before spinal cord stimulation: a qualitative exploration in potential candidates. Pain Pract 2020; 20: 247–254. https://doi.org/10.1111/papr.12845
- Desai MJ, Nava A, Rigoard P, Shah B, Taylor RS. Optimal medical, rehabilitation and behavioral management in the setting of failed back surgery syndrome. Neurochirurgie 2015; 61: S66–76. https://doi.org/10.1016/j.neuchi.2014.09.002
- Amirdelfan K, Webster L, Poree L, Sukul V, McRoberts P. Treatment options for failed back surgery syndrome patients with refractory chronic pain: an evidence based approach. Spine 2017; 42: S41–S52. https://doi.org/10.1097/brs.0000000000002217
- Hussain A, Erdek M. Interventional pain management for failed back surgery syndrome. Pain Pract 2014; 14: 64–78. https://doi.org/10.1111/papr.12035
- Ganty P, Sharma M. Failed back surgery syndrome: a suggested algorithm of care. Br J Pain 2012; 6: 153–161. https://doi.org/10.1177/2049463712470222
- Rigoard P, Gatzinsky K, Deneuville JP, Duyvendak W, Naiditch N, Van Buyten JP, et al. Optimizing the management and outcomes of failed back surgery syndrome: a consensus statement on definition and outlines for patient assessment. Pain Res Manag 2019; 2019. https://doi.org/10.1155/2019/3126464
- Vogel N, Schandelmaier S, Zumbrunn T, Ebrahim S, de Boer WE, Busse JW, et al. Return-to-work coordination programmes for improving return to work in workers on sick leave. Cochrane Database Syst Rev 2017; 3: Cd011618. https://doi.org/10.1002/14651858.CD011618.pub2
- Schaafsma F, Schonstein E, Whelan KM, Ulvestad E, Kenny DT, Verbeek JH. Physical conditioning programs for improving work outcomes in workers with back pain. Cochrane Database Syst Rev 2010; CD001822.pub2. https://doi.org/10.1002/14651858.CD001822.pub2
- Steenstra IA, Munhall C, Irvin E, Oranye N, Passmore S, Van Eerd D, et al. Systematic review of prognostic factors for return to work in workers with sub acute and chronic low back pain. J Occup Rehabil 2017; 27: 369–381. https://doi.org/10.1007/s10926-016-9666-x
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021; 10: 89. https://doi.org/10.1186/s13643-021-01626-4
- Thomas J, Kneale D, McKenzie JE, Brennan SE, Bhaumik S. Chapter 2: Determining the scope of the review and the questions it will address. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions. version 6.3 ed. London: Cochrane; 2022.
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan: a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. https://doi.org/10.1186/s13643-016-0384-4
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998; 52: 377–384. https://doi.org/10.1136/jech.52.6.377
- Hooper P, Jutai JW, Strong G, Russell-Minda E. Age-related macular degeneration and low-vision rehabilitation: a systematic review. Can J Ophthalmol 2008; 43: 180–187. https://doi.org/10.3129/i08-001
- Granholm A, Alhazzani W, Møller MH. Use of the GRADE approach in systematic reviews and guidelines. Br J Anaesth 2019; 123: 554–559. https://doi.org/10.1016/j.bja.2019.08.015
- Porteau-Cassard L, Zabraniecki L, Dromer C, Fournié B. A back school program at the Toulouse-Purpan teaching hospital: evaluation of 144 patients. Rev Rhum Engl Ed 1999; 66: 477–483.
- Burke SA, Harms-Constas CK, Aden PS. Return to work/work retention outcomes of a functional restoration program: a multi-center, prospective study with a comparison group. Spine 1994; 19: 1880–1885.
- Poulain C, Kernéis S, Rozenberg S, Fautrel B, Bourgeois P, Foltz V. Long-term return to work after a functional restoration program for chronic low-back pain patients: a prospective study. Eur Spine J 2010; 19: 1153–1161. https://doi.org/10.1007/s00586-010-1361-6
- Tavares Figueiredo I, Dupeyron A, Tran B, Duflos C, Julia M, Herisson C, et al. Educational self-care objectives within a functional spine restoration program: retrospective study of 104 patients. Ann Phys Rehabil Med 2016; 59: 289–293. https://doi.org/10.1016/j.rehab.2016.03.006
- Cui D, Janela D, Costa F, Molinos M, Areias AC, Moulder RG, et al. Randomized-controlled trial assessing a digital care program versus conventional physiotherapy for chronic low back pain. NPJ Digit Med 2023; 6. https://doi.org/10.1038/s41746-023-00870-3
- Ibrahim ME, Weber K, Courvoisier DS, Genevay S. Recovering the capability to work among patients with chronic low back pain after a four-week, multidisciplinary biopsychosocial rehabilitation program: 18-month follow-up study. BMC Musculoskelet Disord 2019; 20: 439. https://doi.org/10.1186/s12891-019-2831-6
- Gatchel RJ, Mayer TG. Evidence-informed management of chronic low back pain with functional restoration. Spine J 2008; 8: 65–69. https://doi.org/10.1016/j.spinee.2007.10.012
- van Tulder MW, Koes B, Malmivaara A. Outcome of non-invasive treatment modalities on back pain: an evidence-based review. Eur Spine J 2006; 15: S64–81. https://doi.org/10.1007/s00586-005-1048-6
- Waddell G, Burton A, Kendall N. Vocational rehabilitation: what works, for whom, and when? London: Department for Work and Pensions; 2008. 307 p.
- Waterschoot FPC, Dijkstra PU, Hollak N, de Vries HJ, Geertzen JHB, Reneman MF. Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. Pain 2014; 155: 179–189. https://doi.org/10.1016/j.pain.2013.10.006
- Tseli E, LoMartire R, Vixner L, Grooten WJA, Gerdle B, Äng BO. What Is the effectiveness of different duration interdisciplinary treatment programs in patients with chronic pain? A large-scale longitudinal register study. J Clin Med 2020; 9. https://doi.org/10.3390/jcm9092788
- Kamper SJ, Apeldoorn AT, Chiarotto A, Smeets RJ, Ostelo RW, Guzman J, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ 2015; 350: h444. https://doi.org/10.1136/bmj.h444
- Zaza C, Stolee P, Prkachin K. The application of goal attainment scaling in chronic pain settings. J Pain Symptom Manag 1999; 17: 55–64. https://doi.org/10.1016/s0885-3924(98)00106-7
- Hazard RG. Failed back surgery syndrome: surgical and nonsurgical approaches. Clin Orthop Relat Res 2006; 443: 228–232. https://doi.org/10.1097/01.blo.0000200230.46071.3d
- Svanholm F, Björk M, Löfgren M, Gerdle B, Hedevik H, Molander P. Work interventions within Interdisciplinary Pain Rehabilitation Programs (IPRP): frequency, patient characteristics, and association with self-rated work ability. J Pain Res 2023; 16: 421–436. https://doi.org/10.2147/jpr.S390747
- Costa-Black KM, Feuerstein M, Loisel P. Work disability models: past and present. In: Handbook of work disability: prevention and management. New York: Springer Science + Business Media; 2013: p. 71–93. https://doi.org/10.1007/978-1-4614-6214-9_6
- Loisel P, Buchbinder R, Hazard R, Keller R, Scheel I, van Tulder M, et al. Prevention of work disability due to musculoskeletal disorders: the challenge of implementing evidence. J Occup Rehabil 2005; 15: 507–524. https://doi.org/10.1007/s10926-005-8031-2
- Foster NE. Barriers and progress in the treatment of low back pain. BMC Med 2011; 9: 108. https://doi.org/10.1186/1741-7015-9-108
- Loisel P, Durand P, Abenhaim L, Gosselin L, Simard R, Turcotte J, et al. Management of occupational back pain: the Sherbrooke model. Results of a pilot and feasibility study. Occup Environ Med 1994; 51: 597–602. https://doi.org/10.1136/oem.51.9.597
- Institute of Medicine (US) Committee on Pain, Disability, and Chronic Illness Behavior, Osterweis M, Kleinman A, Mechanic D. 12. Rehabilitation approaches and issues in chronic pain. In: Pain and disability: clinical, behavioral, and public policy perspectives. Washington, DC: National Academies Press; 1987.
- Schaafsma FG, Whelan K, van der Beek AJ, van der Es-Lambeek LC, Ojajärvi A, Verbeek JH. Physical conditioning as part of a return to work strategy to reduce sickness absence for workers with back pain. Cochrane Database Syst Rev 2013; 2013: Cd001822. https://doi.org/10.1002/14651858.CD001822.pub3
- Engers A, Jellema P, Wensing M, van der Windt DA, Grol R, van Tulder MW. Individual patient education for low back pain. Cochrane Database Syst Rev 2008; 2008: Cd004057. https://doi.org/10.1002/14651858.CD004057.pub3
- Goudman L, Bruzzo A, van de Sande J, Moens M. Goal identification before spinal cord stimulation: a qualitative exploration in potential candidates. Pain Pract 2020; 20: 247–254. https://doi.org/10.1111/papr.12845
- Bernaers L, Cnockaert E, Braeckman L, Mairiaux P, Willems TM. Disability and return to work after a multidisciplinary intervention for (sub)acute low back pain: a systematic review. Clin Rehabil 2023; 37: 964–974. https://doi.org/10.1177/02692155221146447
- Cole DC, Mondloch MV, Hogg-Johnson S. Listening to injured workers: how recovery expectations predict outcomes – a prospective study. CMAJ 2002; 166: 749–754.
- Sampere M, Gimeno D, Serra C, Plana M, López JC, Martínez JM, et al. Return to work expectations of workers on long-term non-work-related sick leave. J Occup Rehabil 2012; 22: 15–26. https://doi.org/10.1007/s10926-011-9313-5
- Michel C, Guêné V, Michon E, Roquelaure Y, Petit A. Return to work after rehabilitation in chronic low back pain workers: does the interprofessional collaboration work? J Interprof Care 2018; 32: 521–524. https://doi.org/10.1080/13561820.2018.1450231
- Verhoef JAC, Bal MI, Roelofs P, Borghouts JAJ, Roebroeck ME, Miedema HS. Effectiveness and characteristics of interventions to improve work participation in adults with chronic physical conditions: a systematic review. Disabil Rehabil 2022; 44: 1007–1022. https://doi.org/10.1080/09638288.2020.1788180
- Cullen KL, Irvin E, Collie A, Clay F, Gensby U, Jennings PA, et al. Effectiveness of workplace interventions in return-to-work for musculoskeletal, pain-related and mental health conditions: an update of the evidence and messages for practitioners. J Occup Rehabil 2018; 28: 1–15. https://doi.org/10.1007/s10926-016-9690-x
- Wehman PH, Revell WG, Kregel J, Kreutzer JS, Callahan M, Banks PD. Supported employment: an alternative model for vocational rehabilitation of persons with severe neurologic, psychiatric, or physical disability. Arch Phys Med Rehabil 1991; 72: 101–105.
- Wegrzynek PA, Wainwright E, Ravalier J. Return to work interventions for chronic pain: a systematic review. Occup Med 2020; 70: 268–277. https://doi.org/10.1093/occmed/kqaa066
- Hoefsmit N, Houkes I, Nijhuis FJ. Intervention characteristics that facilitate return to work after sickness absence: a systematic literature review. J Occup Rehabil 2012; 22: 462–477. https://doi.org/10.1007/s10926-012-9359-z
- Evans L, O’Donohoe T, Morokoff A, Drummond K. The role of spinal surgery in the treatment of low back pain. Med J Aust 2023; 218: 40–45. https://doi.org/10.5694/mja2.51788
- Wang TY, Wang MY. Advances and challenges in minimally invasive spine surgery. J Clin Med 2024; 13: 3329.
- Alshammari HS, Alshammari AS, Alshammari SA, Ahamed SS. Prevalence of chronic pain after spinal surgery: a systematic review and meta-analysis. Cureus 2023; 15: e41841. https://doi.org/10.7759/cureus.41841
- Amundsen PA, Evans DW, Rajendran D, Bright P, Bjørkli T, Eldridge S, et al. Inclusion and exclusion criteria used in non-specific low back pain trials: a review of randomised controlled trials published between 2006 and 2012. BMC Musculoskeletal Disord 2018; 19: 113. https://doi.org/10.1186/s12891-018-2034-6
- Ceulemans D, Moens M, Reneman M, Callens J, De Smedt A, Godderis L, et al. Biopsychosocial rehabilitation in the working population with chronic low back pain: a concept analysis. J Rehabil Med 2024; 56: jrm13454. https://doi.org/10.2340/jrm.v56.13454
- Moens M, Goudman L, Van de Velde D, Godderis L, Putman K, Callens J, et al. Personalised rehabilitation to improve return to work in patients with persistent spinal pain syndrome type II after spinal cord stimulation implantation: a study protocol for a 12-month randomised controlled trial – the OPERA study. Trials 2022; 23: 974. https://doi.org/10.1186/s13063-022-06895-5
- Thomson S. Failed back surgery syndrome: definition, epidemiology and demographics. Br J Pain 2013; 7: 56–59. https://doi.org/10.1177/2049463713479096
- Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007; 132: 179–188. https://doi.org/10.1016/j.pain.2007.07.028