ORIGINAL REPORT
ULTRASOUND ASSESSMENT OF SPASTIC MUSCLES IN AMBULATORY CHRONIC STROKE SURVIVORS REVEALS FUNCTION-DEPENDENT CHANGES
Javier GONZÁLEZ-BUONOMO, MD1,2, Alexander H. PHAM, BS1, Jaskiran GHUMAN, DO3, Aila MALIK, MD, MS1, Nuray YOZBATIRAN, PT, PhD1,4, Gerard E. FRANCISCO, MD1,4, Walter R. FRONTERA, MD, PhD5 and Sheng LI, MD, PhD1,4
From the 1Department of Physical Medicine and Rehabilitation, McGovern Medical School, The University of Texas Health Science Center, and TIRR Memorial Hermann Hospital, Houston, TX, USA, 2Hospital de la Concepción San German, and Multy Medical Facilities, Ponce, Puerto Rico, 3Mount Sinai Hospital, New York, NY, 4TIRR Memorial Hermann Hospital, Houston, TX, USA and 5Department of Physical Medicine, Rehabilitation, and Sports Medicine, Department of Physiology, University of Puerto Rico School of Medicine, San Juan, Puerto Rico
Objective: To correlate ultrasound characteristics of spastic muscles with clinical and functional measurements in chronic stroke survivors.
Methods: Ultrasound assessment and clinical and functional assessments were performed in 28 ambulatory stroke survivors (12 females, mean age 57.8 ± 11.8 years, 76 ± 45 months after stroke).
Results: Muscle thickness in the affected side was decreased compared with the contralateral side (p < 0.001). The decrease was more evident in the upper limb muscles. On the affected side, the modified Heckmatt scale score was lowest (closer to normal) in the rectus femoris (RF) muscle compared with other muscles (biceps brachii (BB), flexor carpi ulnaris (FCU) and medial gastrocnemius (MG)). Muscle thickness and echogenicity of spastic muscles did not correlate with spasticity, as measured with the modified Ashworth scale (MAS), Fugl-Meyer motor assessment scores, age, or time since stroke. There was a significant negative correlation between grip strength and percentage decrease in muscle thickness for the spastic FCU muscle (r = –0.49, p = 0.008). RF muscle thickness correlated with ambulatory function (Timed Up and Go test (r = 0.44, p = 0.021) and 6-metre walk test (r = 0.41, p = 0.032)). There was no significant correlation between echogenicity and functional assessments
Conclusion: Ambulatory chronic stroke survivors had function-dependent changes in muscle thickness on the affected side. Muscle thickness and echogenicity of spastic muscles did not correlate with spasticity, Fugl-Meyer motor assessment scores, age, or time since stroke.
LAY ABSTRACT
Muscle changes occur secondary to various factors after stroke. In this study, ultrasound characteristics of spastic muscles and clinical and functional assessment were performed in 28 ambulatory chronic stroke survivors. Muscle thickness in the affected side was decreased compared with the contralateral side. The decrease was more evident in the upper limb muscles. The percentage decrease in the flexor carpi ulnaris muscle thickness correlated negatively with grip strength, while the rectus femoris muscle thickness correlated with ambulatory function. Echogenicity was lowest in the rectus femoris compared with other muscles. Muscle thickness and echogenicity of spastic muscles did not correlate with spasticity, Fugl-Meyer motor assessment scores, age, or time since stroke. These function-dependent changes in muscles on the affected side suggest an important role of voluntary muscle activation in preserving muscle mass after stroke. Stroke motor rehabilitation programmes should focus on active use of muscles on the affected side for strengthening and preserving muscle mass.
Key words: spasticity; stroke; ultrasound; muscles; function.
Citation: J Rehabil Med 2023; 55: jrm00342. DOI: https://dx.doi.org/10.2340/jrm.v54.3199
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Sep 27, 2022; Epub ahead of print: Oct 18, 2022; Published: Jan 19, 2023
Correspondence address: Sheng Li, Department of Physical Medicine and Rehabilitation, McGovern Medical School, The University of Texas Health Science Center, 1333 Moursund, Houston 77030, TX, USA. E-mail: sheng.li@uth.tmc.edu
Competing interests and funding: The authors have no conflicts of interest to declare.
According to the World Stroke Organization (WHO), stroke is the second most common cause of death and third leading cause of loss of disability-adjusted life years (DALYs) globally (1). The global cost of stroke is estimated to be approximately 721 billion US dollars (1). Stroke-induced muscle wasting and dysfunction is a commonly observed phenomenon amongst stroke survivors (2). However, most studies of this condition emphasize the role of brain injury and the neurological pathways involved and little attention has been given to the structural changes in muscles and their functional correlates (3). Several factors may contribute to alterations in skeletal muscles of stroke survivors, including poor feeding and diet, disuse and deconditioning, inflammation, denervation, and spasticity (4, 5). These mechanisms can result in muscle mass loss, muscle weakness, and impaired physical performance, a combination of changes similar to those seen in sarcopaenia (2).
Loss of muscle mass, as evidenced by reduced muscle thickness, occurs early after stroke (6), and may partially recover by approximately 6 months post-stroke with therapy and exercise (4). The severity of motor impairment and functional loss can influence the degree of muscle wasting and its recovery. For example, stroke survivors who are not able to walk independently have greater muscle wasting compared with those who walk independently (7). Post-stroke spasticity is seen in up to 97% of chronic stroke survivors with moderate and severe motor impairment (8). The interaction between spasticity and weakness can result in abnormal joint posture, immobilization, disuse, and muscle wasting (9). Previous studies have investigated architectural changes in hemiplegic spastic muscles, but focused on either the upper limbs (UL) or lower limbs (LL) and did not explore the association with function (10–14). A recent systematic review of 9 studies (a total of 137 stroke subjects) revealed ultrasonography (US) evidence of reduced muscle thickness and reduced fascicle length in spastic leg muscles (14). However, the relationship of these muscle changes with weakness, spasticity, and functional performance of the LL remain unclear. Similarly, architectural changes in UL muscles in stroke survivors have been reported, although the association with function has not been explored (10, 11).
Although computed tomography (CT) and magnetic resonance imaging (MRI) are considered the gold standard when measuring muscle mass, their use is not practical in the research setting due to patient discomfort, high radiation exposure (CT), and high costs. The International Working Group on Sarcopenia recommends using dual-energy X-ray absorptiometry (DXA), while the European Working Group on Sarcopenia (15) recommends either bioelectrical impedance analysis (BIA) or DXA to measure muscle mass in elderly people. However, a recent review showed that the use of US to quantify muscle mass with measurements of muscle thickness in thigh muscles of healthy elderly subjects had high concordance with DXA measurements (16). Because of the easy availability and potential value of US in the assessment of muscle structure in sarcopaenia (17), US was used in the current study to evaluate changes in muscle thickness, a surrogate measure of muscle mass. US has also been used in research settings to evaluate changes in echo-intensity in stroke survivors. Echo-intensity offers quantitative assessment of alterations in muscles after stroke, such as fatty infiltration and fibrosis (18, 19). The Modified Heckmatt scoring system used for this purpose correlates with grey-scale scores and has high inter- and intra-rater reliability when assessing spastic muscles (18).
Due to the significant impact of stroke on functional activities and quality of life of survivors, it is important to investigate changes in muscle architecture and how these impact function. Therefore, the aim of the current study was to investigate the changes in spastic muscles of both UL and LL using US and to evaluate the association between these changes and measurements of function. It was hypothesized that muscles in affected limbs would show a reduction in muscle thickness and increased echogenicity and that these changes correlate with functional deficits.
METHODS
This cross-sectional study was approved by the Institutional Review Boards of the University of Texas Health Science Center at Houston and the Memorial Hermann Healthcare System. Stroke survivors provided written, informed consent prior to their participation in the studies.
Participants
A total of 28 chronic stroke survivors with spastic hemiplegia were recruited from the outpatient clinic of The Institute of Rehabilitation and Research (TIRR) Memorial Hermann in Houston, TX, USA, from February 2021 to June 2021. Inclusion criteria were: (i) at least 6 months post-stroke onset, (ii) haemorrhagic or ischaemic stroke confirmed via brain CT and/or MRI imaging studies, (iii) presence of hemiplegia with spasticity in the affected upper limb and lower limbs, and (iv) classification of household ambulator with or without assistive device usage. Exclusion criteria were: (i) inability to comprehend and follow instructions, (ii) medical instability, (iii) pregnancy, (iv) phenol injection history within the past 2 years, (v) botulinum toxin injection history within the previous 3 months, (vi) a history of peripheral polyneuropathy, neuromuscular disorders, or peripheral musculoskeletal disorders that hinder grip strength or walking ability, or (vii) current usage of intrathecal baclofen pumps.
The primary aim of this study was to investigate changes in skeletal muscle using US in stroke survivors. Muscle mass is influenced by a number of factors (15, 20), including age, sex, and systemic factors, such as exercise, nutrition, hormonal influences, and circulation. Because these factors are the same for both sides the unaffected side was used as the control for comparison purposes and not a group of healthy individuals.
Experimental procedures
Two brain injury medicine specialists performed the physical examinations, US imaging studies, evaluations of motor performance, spasticity assessment, and functional tests on the same day. All assessments were supervised by a neurorehabilitation specialist with more than 20 years of experience in the rehabilitation of stroke survivors and the evaluation of neuromuscular function. Rest was provided as needed between the various tests.
Ultrasound of selected muscles
US assessment (uSmart3200, Terason, Ormond Beach, FL) was performed on major muscle groups in the UL and LL. The UL muscles were the biceps brachii (BB) and the flexor carpi ulnaris (FCU) and the LL muscles were the rectus femoris (RF) and medial gastrocnemius (MG). The spastic (affected) and unaffected limbs were assessed for muscle thickness and fibrotic changes using the modified Heckmatt scale (10, 11). Muscles on both affected and unaffected sides were measured at fixed proportional lengths to ensure standardization. Pressure from the US probe on the skin was avoided. US gel was applied to reduce acoustic impedance and provide proper contact. During US assessment, the subjects were placed in a supine position. The elbow joint was held in full extension, or in a symmetrical position if limited by spasticity on the affected side. BB length was measured from the anterior axillary line to the elbow crease. With the probe positioned midway between the landmarks, medial and lateral motion was performed to detect the thickest muscle portion of the BB observable under US. For thickness and echogenicity measurements of the FCU, the subjects had their elbow flexed at 90° or in a symmetrical position. FCU length was measured from the medial epicondyle of the humerus at the ulnar groove to the ulnar styloid process. The US probe was then placed at the proximal one-third distance between these landmarks. For the RF, muscle length was measured from the anterior superior iliac spine to the patella. The US probe was placed transversely midway between these landmarks. For the MG, the subjects were placed in a prone position. The length of the MG was measured from the medial knee crease to the most prominent part of the medial malleolus. The US probe was then placed transversely at the proximal 30% distance between these landmarks. Images were saved for off-line analysis of muscle thickness and modified Heckmatt scale scores. The modified Heckmatt scale is a 4-point ordinal scale used to measure muscle echogenicity with ultrasound imaging. Heckmatt Grade 1 refers to normal muscle tissue, Grade 2 means increased muscle echogenicity with preserved bone echogenicity, Grade 3 is marked increase in muscle echogenicity with decreased bone echogenicity, and Grade 4 is highly increased muscle echogenicity and complete loss of bone echogenicity.
Motor impairment
Fugl-Meyer motor assessment (FMA) was used to quantify motor impairment on both the UL and LL. The FMA scores of the UL (FMA_UL) and LL (FMA_LL) were compiled separately and comprised of 66 and 34 points, respectively. Measurements were based on the ordinal 3-point scale (0 = unable to perform, 1 = partially performs, 2 = fully performs).
Spasticity
Spasticity of the UL and LL were assessed with the modified Ashworth Scale (MAS). For the UL, shoulder adductors, shoulder internal rotators, shoulder flexors, elbow extensors, elbow flexors, wrist flexors, metacarpophalangeal (MCP), proximal interphalangeal (PIP), and distal interphalangeal (DIP) joints were assessed. Spasticity of the MCP, PIP, and DIP joints was measured with the wrist in a neutral position. In the LL, hip adductors, hip flexors, hip extensors, knee flexors, knee extensors, ankle plantar flexors, and ankle invertors were assessed. After obtaining MAS values on the 6-point ordinal scale (0, 1, 1 +, 2, 3, 4), measurements were converted (0, 1, 2, 3, 4, 5) for data analysis. Composite scores of the spastic muscle groups in the UL and LL were then compiled as the UL spasticity index (SI_UL) and LL spasticity index (SI_LL), respectively. SI_UL is the sum of the highest MAS scores of shoulder, elbow, wrist, and finger joints, while SI_LL is the sum of the highest MAS scores of hip, knee, and ankle (plantar flexors or invertors) joints.
Function
Grip strength was used as a surrogate for functional performance of the UL. Handgrip strength was measured using the Jamar hydraulic dynamometer. Subjects were assessed in a neutral sitting position with the shoulder flexed and abducted at 30° in both planes. The elbow was held at 90° of flexion. If spasticity hindered motion to the desired angle on the affected side, the subjects were encouraged to attempt the most permissible angle to the best of their capabilities. The same position was obtained in the unaffected side of the subject for standardization. Subjects were instructed to grasp the dynamometer as hard as possible and grip strength was measured 3 times with a rest time of 1 min between each attempt. The mean of the 3 attempts was then calculated.
The Timed Up and Go (TUG) test and 6-minute walk test (6MWT) were used to measure functional performance of the LL.
For the TUG test, subjects were seated with their backs against a standard armchair. If ankle-foot orthotic (AFO) devices or any assistive devices were normally used by the subjects, utilization of such devices was allowed during the assessment. Subjects were instructed to rise from the chair, walk 3 m and return, and resume the original sitting position. Time was recorded from when the subjects rose from the chair to when they returned to the original position. Subjects were timed for 2 trials and the mean was recorded.
For the 6MWT, subjects were instructed to walk in a straight line at a quick, but safe and comfortable, pace along a 10-m path. The path was marked with a starting point, a 2-m mark, an 8-m mark, and a finish point. Once the patients were familiarized with this set-up, they were instructed to walk the 10-m length and time was recorded for the 6-m distance from the 2-m mark to the 8-m mark. The mean of 2 attempts at 6-m walk times was recorded.
Statistical analysis
Descriptive statistics were used for subject demographics. Paired t-tests were used to compare muscle thickness of the affected and unaffected sides. Percentage decrease in muscle thickness with reference to the value on the unaffected side was used to compare muscles in repeated measure one-way analysis of variance (ANOVA) tests. Non-parametric ANOVA tests were performed to compare modified Heckmatt scale scores. Pearson and Spearman correlations were used to look for associations between changes in spastic muscles (muscle thickness and modified Heckmatt scale) and clinical and functional variables. The significance level was set at α = 0.05.
RESULTS
Subject characteristics
A total of 28 chronic stroke subjects participated in this cross-sectional study. Their general characteristics are shown in Table I.
| Characteristics | |
| Subjects, n | 28 |
| Age, years, mean (SD) | 57.4 (11.9) |
| Sex, male/female, n | 12 M/16 F |
| Hemiplegia, left/right, n | 14 L/14 R |
| Time from stroke onset, months, mean (SD) | 76 (45) |
| Usage of ankle-foot orthosis, n (%) | 19/28 (67.9) |
| Usage of ambulatory assistive device (cane or walker), n (%) | 13/28 (48.6) |
| Subjects with prior BoNT injections, n (%) | 25 (89.3) |
| Times of BoNT injection, mean (range) | 9 (1 ~ 25) |
| Number of subjects with prior phenol injections, n (%) | 4 (14.3) |
| Times of phenol injection, mean (range) | 1.5 (1 ~ 2) |
| Total dose of last BoNT injection, mean (range) | 520 (300 ~ 600) |
| Biceps brachii Subjects with prior BoNT injection, n Dose unit, mean (range) |
17 57.7 (25 ~ 100) |
| Flexor carpi ulnaris Subjects with prior BoNT injection, n Dose, unit, mean (range) |
11 39.1 (12.5 ~ 75) |
| Rectus femoris Subjects with prior BoNT injection, n Dose, unit, mean (range) |
3 65.0 (25 ~ 100) |
| Medial gastrocnemius Subjects with prior BoNT injection, n Dose, unit, mean (range) |
16 96.7 (25 ~ 200) |
| Onabotulinum toxin A was used in most subjects. Dose of other botulinum toxins was converted to Onabotulinum toxin A (37). M: male; F: female; BoNT: botulinum toxin; SD: standard deviation; L: left; R: right. |
|
Ultrasonography assessment of spastic muscles
Figs 1 and 2 show representative US images of spastic muscles in the UL and LL, respectively. A reduction in muscle thickness of both UL and LL muscles in the affected limb was found compared with the unaffected limb (p < 0.001 for all comparisons).
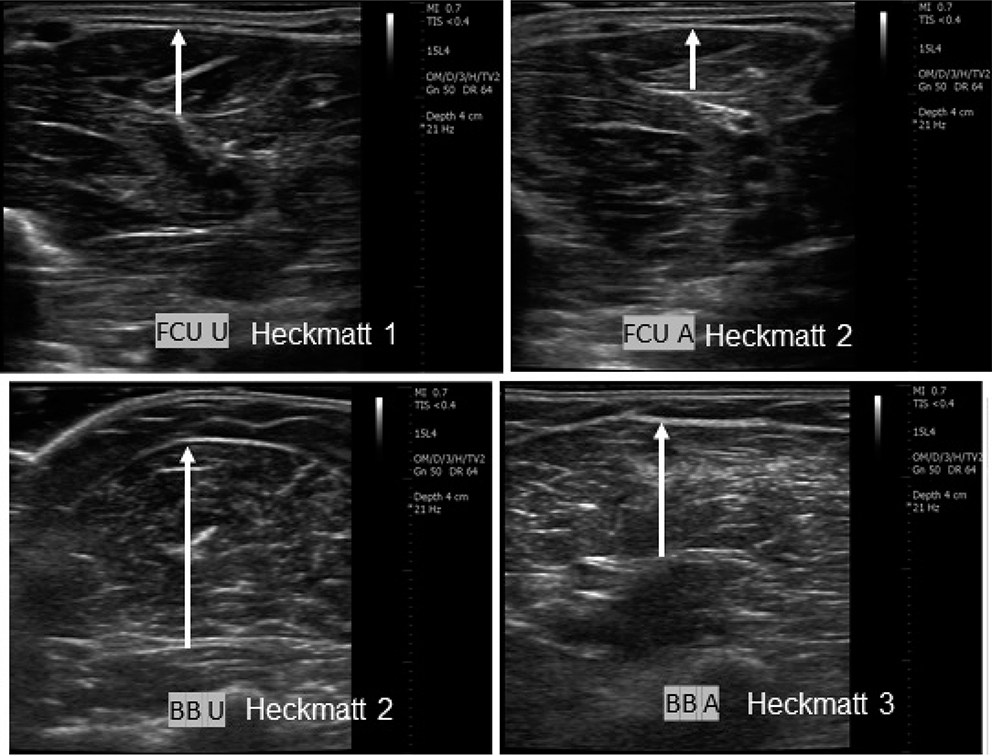
Fig. 1. Representative ultrasound images of spastic muscles of the upper limb (FCU: flexor carpi ulnaris; BB: biceps brachii). Each muscle pair corresponds to the affected (A) and unaffected (U) sides of the same subject. Images from 2 different subjects are shown. The length of the white arrows represents muscle thickness. Modified Heckmatt scale shows increased echogenicity in affected spastic muscles.
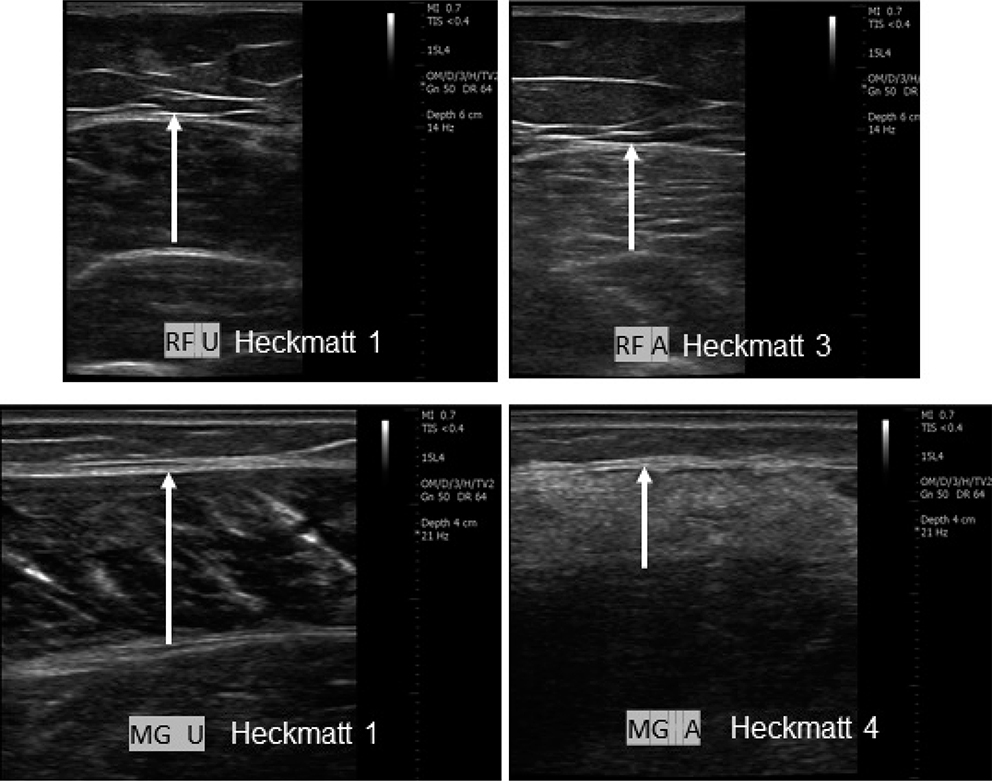
Fig. 2. Representative ultrasound images of spastic muscles of the lower limb (RF: rectus femoris; MG: medial gastrocnemius). Each muscle image pair corresponds to the affected (A) and unaffected (U) sides of the same subject. Images from 2 different subjects are shown. The length of the white arrows represents muscle thickness. Modified Heckmatt scale shows increased echogenicity in affected spastic muscles.
In the UL, the affected BB and FCU muscles showed a decrease of mean thickness of 21.4% and 26.1% compared with the unaffected side, respectively. In the LL, the affected RF and MG showed a decrease in muscle thickness of an mean of 11.6% and 16.9%, respectively. No significant difference in reduction in muscle thickness was seen between muscles of the same limb. The mean reduction in muscle thickness was significantly greater in the UL compared with the LL (F[1,27] = 15.708, p < 0.001) (Fig. 3A).
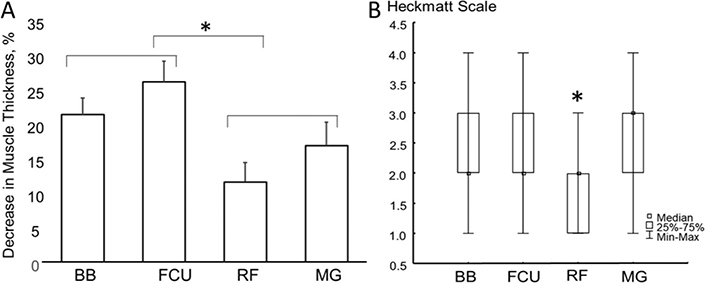
Fig. 3. (A) Percentage decrease in muscle thickness of the affected side with reference to the unaffected side in muscles of the upper and lower limb (BB: biceps brachii; FCU: flexor carpi ulnaris; RF: rectus femoris; MG: medial gastrocnemius). No significant difference in reduction in muscle thickness was seen between muscles of the same limb. A significantly greater decrease in muscle thickness was observed in upper limb compared with lower limb muscles (p < 0.001). (B) Box and whisker plot of modified Heckmatt scale of muscles on the affected side. The modified Heckmatt scale score was significantly lower in the RF than in other muscles (p < 0.001). *Statistically significant difference.
As expected, modified Heckmatt scale scores were lower on the unaffected side compared with the affected side. As shown in Figs 1 and 2, echogenicity modified (Heckmatt scale) was variable among muscles on the affected side. The modified Heckmatt scale scores for the RF of the affected side was significantly lower compared with other muscles on the affected side (ANOVA χ2 (N = 28, df = 3) = 23.31, p < 0.001) (Fig. 3B).
Correlation between muscle thickness, echogenicity and other variables
As shown in Table II, the percentage decrease in the FCU muscle thickness on the affected side had a significant negative correlation with grip strength (r = –0.49, p = 0.008). With regards to the LL, there was a significant positive correlation between the percentage decrease in the RF muscle thickness and the TUG test (r = 0.43, p = 0.021) as well as with the 6MWT (r = 0.41, p = 0.032). Muscle thickness did not correlate with age, time since stroke, FMA, or spasticity. As shown in Table I, the majority of subjects received prior botulinum toxin (BoNT) injections with various numbers of injections to different target muscles and among subjects. Muscle thickness did not correlate with the number of injections for BB and MG.
| Biceps brachii | Flexor carpi ulnaris | Rectus femoris | Medial gastrocnemius | |
| Age | –0.08 (p = 0.69) | 0.07 (p = 0.728) | 0.20 (p = 0.301) | 0.01 (p = 0.979) |
| Time since stroke | 0.36 (p = 0.061) | 0.34 (p = 0.079) | –0.01 (p = 0.958) | 0.23 (p = 0.246) |
| Fugl-Meyer motor assessment | –0.09 (p = 0.63) | –0.24 (p = 0.218) | –0.18 (p = 0.355) | –0.04 (p = 0.847) |
| Spasticity Index | –0.13 (p = 0.51) | 0.19 (p = 0.326) | –0.09 (p = 0.660) | –0.03 (p = 0.888) |
| Grip strength | –0.11 (p = 0.27) | –0.49 (p = 0.008)* | ||
| Timed Up and Go test | 0.43 (p = 0.020)* | 0.09 (p = 0.634) | ||
| 6-metre walk test | 0.41 (p = 0.032)* | 0.09 (p = 0.646) | ||
| Statistical significance in bold*. | ||||
Overall, there was no significant correlation between muscle echogenicity and age, time since stroke, FMA scores, spasticity, grip strength, or functional tests (Table III). The only exception was a positive and significant correlation between BB echogenicity and time since stroke.
| Biceps brachii | Flexor carpi ulnaris | Rectus femoris | Medial gastrocnemius | |
| Age | –0.17 (p = 0.398) | –0.03 (p = 0.865) | –0.19 (p = 0.324) | 0.14 (p = 0.471) |
| Time since stroke | 0.45 (p = 0.015)* | 0.24 (p = 0.216) | –0.15 (p = 0.445) | 0.19 (p = 0.342) |
| Fugl-Meyer motor assessment | –0.23 (p = 0.231) | –0.25 (p = 0.193) | –0.07 (p = 0.719) | –0.33 (p = 0.091) |
| Modified Ashworth Scale | 0.10 (p = 0.615) | 0.12 (p = 0.544) | 0.16 (p = 0.413) | –0.09 (p = 0.641) |
| Grip strength | –0.32 (p = 0.092) | –0.35 (p = 0.068) | ||
| Timed Up And Go test | 0.33 (p = 0.083) | 0.04 (p = 0.820) | ||
| 6-metre walk test | 0.37 (p = 0.050) | –0.01 (p = 0.984) | ||
| Statistical significance in bold*. | ||||
DISCUSSION
This study investigated US changes in UL and LL muscles of chronic stroke survivors and their association with motor function. The main findings were: (i) the thickness of muscles on the affected side was decreased significantly compared with the unaffected side, (ii) the decrease in muscle thickness was greater in the UL than in the LL, (iii) echogenicity was greater in muscles on the affected side, but did not correlate with clinical or functional measures, (iv) a decrease in muscle thickness did not correlate with age, time since with stroke, FMA, or spasticity, (v) there was a negative correlation between grip strength and the decrease in muscle thickness of the FCU, and (vi) the RF muscle thickness correlated with ambulatory function. To the best of our knowledge, this study is the first to explore changes in spastic muscles in both the UL and LL of chronic stroke survivors in relation to function.
The findings of muscle atrophy on the affected side are in agreement with previous studies of chronic stroke survivors (10–14). The current study also observed greater interlimb muscle thickness differences in the UL compared with the LL. In descending order, this difference was greater in the FCU, followed by the BB, MG, and RF. Berenpas et al. (13) also reported more atrophy in forearm flexors but, in contrast to the current findings, noted more atrophy in the LL compared with the BB (in descending order: forearm flexors (did not specify which muscle), MG, RF, and BB). Both studies showed the largest muscle thickness difference in forearm flexor muscles, suggesting that significant forearm muscle loss may be a common problem in chronic stroke patients and an important target for rehabilitation interventions.
It is worth noting that, in Berenpas et al.’s study (13) and in the current study, all patients were ambulatory stroke survivors. It is possible that forced use of the LL muscles on the affected side during ambulation could have minimized the loss of muscle mass. The greater loss in the UL in the current study can also be explained by the habitual avoidance of the use of the affected UL due to weakness, spasticity, sensory loss, and learned non-use (21). Furthermore, over time, stroke survivors may develop maladaptive movement patterns and engage in compensatory behaviours, such as using the unaffected limb to carry out an activity leading to interlimb muscle differences, as seen in the current study (21, 22). Collectively, these findings support the important role of active use of paretic muscles in the preservation of muscle mass.
Muscle thickness in this study showed significant correlations with functional performance in muscles that contribute to UL function (FCU) and LL functional performance (RF). Grip strength is commonly used as a surrogate for UL function (15, 20, 23) and the FCU is directly involved in the production of grip strength (24, 25). Similarly, in the LL, the knee extensors determine whether a stroke survivor can be an independent ambulator (26). Voluntary activation levels of the knee extensors have been shown to correlate with gait and balance, which are important determinants of performance in functional tests, such as the 6MWT and the TUG test (27). The current results and earlier reports (28, 29) showing a significant correlation between RF muscle thickness and gait performance emphasize the functional relevance of the knee extensors in activities that require LL mobility. Furthermore, it is possible that activation of the knee extensors in stroke survivors could help to minimize muscle atrophy. This could explain why the RF muscle had the lowest interlimb difference in thickness.
On the other hand, the majority of stroke subjects (19 out of 28) in this study used an ankle-foot orthosis (AFO). AFO can provide a stable base for support, reduce foot drag during the swing-through phase in gait, and aid in the transitioning from sitting to standing (30). At the same time, its use compensates partially for the function of ankle plantar flexors, including the MG. As such, it is not surprising to observe that MG muscle thickness was not correlated with gait function in this study. The absence of a significant correlation between muscle thickness and variables such as time since stroke, FMA, and spasticity may suggest that these factors are not important contributors to muscle wasting. Spasticity may interact with muscle weakness and have negative consequences on motor function (9). However, it has been shown that spasticity does not correlate with gait speed (31) or arm function (32) in chronic stroke survivors. The current results indicate that morphological changes in spastic muscles are probably not related to the neurally-mediated phenomenon of spasticity.
Echogenicity can help to determine changes in the quality of muscles and supporting connective tissues. Echogenicity can be visually graded using the modified Heckmatt scale (33), and analysed quantitatively according to pixel intensity (34, 35). US assessment showed greater echogenicity in spastic muscles than those on the unaffected side. Increased echogenicity of muscle can be seen in muscle fibrosis or fatty infiltration, which results from spasticity, as reported in previous studies (34, 35). In contrast to different changes in muscle thickness in the UL and LL, the current findings of significantly less echo-intensity in the RF than in other spastic muscles (FCU, BB, MG) are noteworthy. This is probably reflective of less fibrosis in the RF. As discussed above, the RF has lowest interlimb difference in muscle thickness and has significant correlation with functional performance. Taken together, these findings suggest that voluntary activation of the knee extensors in ambulatory stroke survivors may minimize muscle atrophy and fibrosis.
Echogenicity, as measured by the modified Heckmatt scale, did not correlate with clinical and functional assessment outcomes, with the exception of the BB echogenicity and time since stroke. The modified Heckmatt scale reflects fatty infiltration and muscle fibrosis (19). The current study confirmed previous results that modified Heckmatt scale scores do not correlate with severity of post-stroke spasticity (18). Compared with changes in muscle thickness, the current study further suggests that echo-intensity changes in muscles do not affect their functional performance (6). However, the modified Heckmatt scale scoring may not be not sensitive enough to provide such correlation analyses, compared with greyscale-based echo-intensity assessment (13).
The current study highlights the usefulness of US in the evaluation of spastic muscle in stroke survivors. US allows the examiner to evaluate individual muscles in a cost-efficient manner in the clinic. Thus, it is more practical and valuable than other imaging techniques such as MRI, CT and DXA (14, 36). The current study also demonstrated that US assessment of skeletal muscle correlates with functional capacity. Its potential use to serve as an indirect measure of function and ability to perform activities of daily living should be investigated further. Finally, US could help us to identify muscles that should be the target of therapeutic and rehabilitation interventions (14).
Study limitations
The current study was not without limitations. First, only stroke subjects who were able to ambulate were tested. Because the ability to ambulate is not generalizable to the entire stroke population, we cannot extrapolate our observations to all patients. However, it is reasonable to suggest that muscle changes and functional deficits in the non-ambulatory population are likely to be even more severe. The patient population was recruited from a single institution, but the hospital has a large catchment area. This minimizes variability in acute and rehabilitation care of patients prior to the study. Another limitation was that the majority of subjects had a history of botulinum toxin (BoNT) injections, with different level of involvement among tested muscles (Table I). It is generally accepted that BoNT injections cause muscle changes, at least temporarily. However, there is no conclusive data on the level and quality of alteration to the muscles and echogenicity from chronic BoNT treatment itself. Our data on BoNT treatment will be useful for future researchers to evaluate the results of this study when more definitive findings are available. Other factors that may influence muscle mass and function, such as quality of diet, level of habitual physical activity, and other comorbidities, were not included in the current study. Finally, the study compared interlimb differences using the unaffected side as control rather than a healthy population. However, because the unaffected side may show some muscle loss the significant findings of this study are relevant.
CONCLUSION
Ambulatory stroke survivors were found to have a significant decrease in muscle thickness and increase in echogenicity on the affected side. The site and extent of reduction in muscle thickness correlated with functional performance, but not with age, time since stroke, or spasticity. These function-dependent changes in muscles on the affected side suggest an important role of voluntary muscle activation in preserving muscle mass after stroke. Stroke motor rehabilitation programmes should focus on active use of muscles on the affected side for strengthening and preserving muscle mass.
ACKNOWLEDGEMENTS
WRF’s research is partially funded by Grant S21 MD001830-04, NIMHD, NIH. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- Feigin VL, Brainin M, Norrving B, Martins S, Sacco RL, Hacke W, et al. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Intl J Stroke 2022; 17: 18–29.
- Beckwée D, Lefeber N, Bautmans I, Cuypers L, De Keersmaecker E, De Raedt S, et al. Muscle changes after stroke and their impact on recovery: time for a paradigm shift? Review and commentary. Top Stroke Rehabil 2021; 28: 104–111.
- Scherbakov N, von Haehling S, Anker SD, Dirnagl U, Doehner W. Stroke induced sarcopenia: muscle wasting and disability after stroke. Intl J Cardiol 2013; 170: 89–94.
- Su Y, Yuki M, Otsuki M. Prevalence of stroke-related sarcopenia: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 2020; 29: 105092.
- Azzollini V, Dalise S, Chisari C. How does stroke affect skeletal muscle? State of the art and rehabilitation perspective. Front Neurol 2021; 12: 797559.
- Kim JM, Tay MRJ, Rajeswaran DK, Tham SL, Lui WL, Kong KH. Changes in muscle architecture on ultrasound in patients early after stroke. NeuroRehabil 2021; 49: 565–572.
- Jang Y, Im S, Han Y, Koo H, Sohn D, Park GY. Can initial sarcopenia affect poststroke rehabilitation outcome? J Clin Neurosci 2020; 71: 113–118.
- Pundik S, McCabe J, Skelly M, Tatsuoka C, Daly JJ. Association of spasticity and motor dysfunction in chronic stroke. Ann Phys Rehabil Med 2019; 62: 397–402.
- Li S, Francisco GE, Rymer WZ. A new definition of poststroke spasticity and the interference of spasticity with motor recovery from acute to chronic stages. Neurorehabil Neural Repair 2021; 35: 601–610.
- Thielman G, Yourey L. Ultrasound imaging of upper extremity spastic muscle post-stroke and the correlation with function: a pilot study. NeuroRehabil 2019; 45: 213–220.
- Li L, Tong KY, Hu X. The effect of poststroke impairments on brachialis muscle architecture as measured by ultrasound. Arch Phys Med Rehabil 2007; 88: 243–250.
- Monjo H, Fukumoto Y, Asai T, Kubo H, Ohshima K, Tajitsu H, et al. Differences in muscle thickness and echo intensity between stroke survivors and age- and sex-matched healthy older adults. Phys Therapy Res 2020; 23: 188–194.
- Berenpas F, Martens AM, Weerdesteyn V, Geurts AC, van Alfen N. Bilateral changes in muscle architecture of physically active people with chronic stroke: a quantitative muscle ultrasound study. Clin Neurophysiol 2017; 128: 115–122.
- Schillebeeckx F, A DEG, N DEB, Desloovere K, Verheyden G, Peers K. Muscle and tendon properties of the spastic lower leg after stroke defined by ultrasonography: a systematic review. Eur J Phys Rehabil Med 2021; 57: 495–510.
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31.
- Ticinesi A, Meschi T, Narici MV, Lauretani F, Maggio M. Muscle ultrasound and sarcopenia in older individuals: a clinical perspective. J Am Med Dir Assoc 2017; 18: 290–300.
- Perkisas S, Bastijns S, Baudry S, Bauer J, Beaudart C, Beckwée D, et al. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update. Eur Geriatr Med 2021; 12: 45–59.
- Moreta MC, Fleet A, Reebye R, McKernan G, Berger M, Farag J, et al. Reliability and validity of the modified heckmatt scale in evaluating muscle changes with ultrasound in spasticity. Arch Rehabil Res Clin Transl 2020; 2: 100071.
- Pillen S, Arts IM, Zwarts MJ. Muscle ultrasound in neuromuscular disorders. Muscle Nerve 2008; 37: 679–693.
- Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020; 21: 300–30. e2.
- Raghavan P. Upper limb motor impairment after stroke. Phys Med Rehabil Clin N Am 2015; 26: 599–610.
- Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair 2009; 23: 313–319.
- Bae JH, Kang SH, Seo KM, Kim DK, Shin HI, Shin HE. Relationship between grip and pinch strength and activities of daily living in stroke patients. Ann Rehabil Med 2015; 39: 752–762.
- Jones KE, Bawa P, McMillan AS. Recruitment of motor units in human flexor carpi ulnaris. Brain Res 1993; 602: 354–356.
- Lung BE, Siwiec RM. Anatomy, Shoulder and upper limb, forearm flexor carpi ulnaris muscle. StatPearls. Treasure Island, FL: StatPearls Publishing; 2021.
- Akazawa N, Okawa N, Tamura K, Moriyama H. Determining the cut-off value for knee extensor strength for identifying independence in gait in chronic stroke survivors. J Rehabil Med 2017; 49: 765–767.
- Yang DJ, Park SK, Uhm YH, Park SH, Chun DW, Kim JH. The correlation between muscle activity of the quadriceps and balance and gait in stroke patients. J Phys Ther Sci 2016; 28: 2289–2292.
- Maeda H, Imada K, Ishida K, Akima H. Quadriceps thickness and echo intensity predict gait independence in individuals with severe and mild hemiparetic stroke. Eur Neurol 2020; 83: 167–173.
- Akazawa N, Harada K, Okawa N, Tamura K, Hayase A, Moriyama H. Relationships between muscle mass, intramuscular adipose and fibrous tissues of the quadriceps, and gait independence in chronic stroke survivors: a cross-sectional study. Physiotherapy 2018; 104: 438–445.
- Tsuchiyama K, Mukaino M, Ohtsuka K, Matsuda F, Tanikawa H, Yamada J, et al. Effects of ankle-foot orthoses on the stability of post-stroke hemiparetic gait. Eur J Phys Rehabil Med 2022; 58: 352–362.
- Miller T, Qin L, Hung VWY, Ying MTC, Tsang CSL, Ouyang H, et al. Gait speed and spasticity are independently associated with estimated failure load in the distal tibia after stroke: an HR-pQCT study. Osteoporos Int 2022; 33: 713–724.
- Zondervan DK, Augsburger R, Bodenhoefer B, Friedman N, Reinkensmeyer DJ, Cramer SC. Machine-based, self-guided home therapy for individuals with severe arm impairment after stroke: a randomized controlled trial. Neurorehabil Neural Repair 2015; 29: 395–406.
- Heckmatt JZ, Leeman S, Dubowitz V. Ultrasound imaging in the diagnosis of muscle disease. J Pediatr 1982; 101: 656–660.
- Stecco A, Pirri C, Caro R, Raghavan P. Stiffness and echogenicity: Development of a stiffness-echogenicity matrix for clinical problem solving. Eur J Transl Myol 2019; 29: 8476.
- Yang YB, Zhang J, Leng ZP, Chen X, Song WQ. Evaluation of spasticity after stroke by using ultrasound to measure the muscle architecture parameters: a clinical study. Intl J Clin Exp Med 2014; 7: 2712–2717.
- Abe T, Loenneke JP, Young KC, Thiebaud RS, Nahar VK, Hollaway KM, et al. Validity of ultrasound prediction equations for total and regional muscularity in middle-aged and older men and women. Ultrasound Med Biol 2015; 41: 557–564.
- Woo J, Mas MF, Zhang J, Wong B, Stampas A, Francisco GE, et al. Real-world analysis of botulinum toxin (BoNT) injections in post-stroke spasticity: higher doses of BoNT and longer intervals in the early-start group. J Neurol Sci 2021; 425: 117449.