ORIGINAL REPORT
PATIENTS’ EXPECTATIONS BEFORE INITIATION OF INTRATHECAL BACLOFEN TREATMENT: A LONGITUDINAL STUDY WITH 1-YEAR FOLLOW-UP
STINA GUNNARSSON, RN, MSC1, DAG LEMMING, MD, PHD2, SIW ALEHAGEN, RNM, PHD3, A. TOMMY BERGENHEIM, MD, PHD4, BJÖRN GERDLE, MD, PHD2 AND KERSTI SAMUELSSON, OT, PHD1
From the 1Department of Rehabilitation Medicine and Department of Health, Medicine and Caring Sciences, Linköping University, 2Pain and Rehabilitation Centre and Department of Health, Medicine and Caring Sciences, Linköping University, 3Division of Nursing Sciences and Reproductive Health and Department of Health, Medicine and Caring Sciences, Linköping University, Linköping and 4Department of Clinical Sciences, Neurosciences, Umeå University, Umeå, Sweden
Objective: To investigate patients’ expectations, met/unmet expectations and satisfaction with intrathecal baclofen treatment in relation to effect on spasticity, pain intensity, sleep quality, occupational performance, well-being and self-efficacy.
Design: A prospective longitudinal study with follow-up at 1 year.
Patients: Consecutive patients, age ≥ 18 years with a disabling spasticity of cerebral or spinal origin selected for intrathecal baclofen treatment at 2 university hospitals in Sweden were included. From August 2016 to June 2019, 35 patients began intrathecal baclofen treatment; 29 patients were included and completed the study.
Methods: Baseline and 1-year follow-up included assessment of spasticity by physiotherapists, a semi-structured interview regarding occupational performance using the Canadian Occupational Performance Measure and a questionnaire.
Results: Overall satisfaction with treatment and satisfaction with occupational performance were reported as moderate. Important variables that explained satisfaction with occupational performance were improvements in performance, expectations and performance before treatment. Patients had higher expectations compared with the 1-year outcomes regarding occupational performance, spasticity, pain intensity and sleep quality, although improvements were reported.
Conclusion: A thorough discussion of goal setting with intrathecal baclofen treatment before implantation is necessary to give patients individual and realistic expectations.
LAY ABSTRACT
Spasticity is a common complication for patients with various neurological conditions, such as spinal cord injury, acquired brain injury, cerebral palsy and multiple sclerosis. For patients with disabling spasticity, intrathecal baclofen is an effective and satisfactory treatment. Dissatisfaction with treatment has been described, but has not been thoroughly evaluated. Therefore, this study examined patients’ expectations of effects on occupational performance, spasticity, pain intensity and sleep quality. Patients were followed-up after 1 year on the treatment, and results and satisfaction were reported. Patients improved, but not as much as they had expected. As a group, they were moderately satisfied, and important variables for satisfaction were found to be improvements in performance, expectations and initial performance. These results highlight the need to discuss patients’ expectations and goals with this treatment even more thoroughly and to address realistic expectations.
Key words: activity; disability; expectations; intrathecal baclofen treatment; proxy; satisfaction; spasticity.
Citation: J Rehabil Med 2023; 55: jrm00371. DOI: https://dx.doi.org/10.2340/jrm.v55.3424
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Dec 13, 2022; Published: Jan 31, 2022
Correspondence address: Stina Gunnarsson, Department of Rehabilitation Medicine, Linköping University, SE-581 85 Linköping, Sweden. E-mail: stina.gunnarsson@regionostergotland.se
Competing interests and funding: Disclosures. SG reports payment for lectures and presentations from Medtronic. The authors have no other conflicts of interest to declare.
Spasticity is often present in patients with various neurological conditions, such as spinal cord injury, acquired brain injury, cerebral palsy and multiple sclerosis (1). It has been estimated that approximately 12–27% of patients with spasticity have a disabling spasticity (2). Spasticity and accompanying pain and stiffness interfere with comfort, activities of daily living, sleep, bowel and bladder care, sexual relationships, mobility and positioning, and caregiver assistance (3–5). Intrathecal baclofen (ITB) is a well-established treatment for patients with a disabling spasticity of spinal or cerebral origin shown to be effective in reducing spasticity, pain and stiffness, and improving sleep, comfort as well as function (6–10). Before ITB treatment is initiated, a discussion about the treatment takes place to provide information on expectations and goal setting (10). Thereafter, a lumbar intrathecal injection of baclofen, either as a single bolus injection or as a continuous trial, is administered as a screening test to verify an adequate and desirable effect (11). ITB treatment requires life-long hospital follow-up for refills and dose adjustments. In addition, infections, complications related to the catheter and pump or other adverse events may occur (12, 13).
High satisfaction with ITB treatment is commonly reported; however, dissatisfaction has been reported by some patients, suggesting that complications, unmet goals and the burden of frequent baclofen refills could be reasons for lower satisfaction with treatment (6, 8, 14). As described by, for example, Bowling et al. (15), understanding of the patient’s expectations of healthcare, such as ITB treatment, has a central role in improving patient satisfaction (16). Dissatisfaction with ITB treatment in relation to expectations has been described, but not thoroughly evaluated (4).
The effects of ITB treatment regarding spasticity, pain intensity and function are well known (6–9). However, the role of expectations in relation to satisfaction with treatment is poorly documented. Hence, the aim of this study was to investigate expectations, met/unmet expectations and satisfaction with ITB treatment in relation to effects on spasticity, pain intensity, sleep quality, occupational performance, well-being and self-efficacy.
METHODS
A prospective longitudinal pre- and post-design was used to analyse the effects of routine clinical ITB treatment.
Participants
Patients aged ≥ 18 years with a disabling spasticity of cerebral or spinal origin were included after a decision to initiate ITB treatment at either of the 2 university hospitals University Hospital Linköping Sweden and Norrland University Hospital, Umeå Sweden. Non-Swedish speaking patients were excluded. From August 2016 to June 2019, 35 patients started ITB treatment and were consecutively invited to participate in the study. Six patients (17%) declined to participate or met the exclusion criteria; 29 patients were included. Sample size was calculated based on values from the Canadian Occupational Performance Measure (COPM) and the numerical rating scale (NRS) for spasticity and pain intensity, resulting in approximately 10–15 patients. Because 2 groups were analysed separately, we chose to increase the number of participants slightly.
Ethics approval was obtained from the Regional Ethics Committee. The study was conducted according to the Declaration of Helsinki (17). For patients unable to communicate, consent was obtained from a relative or trustee according to dialogue with the ethics committee.
Assessments
All the assessment methods used have moderate to good reliability and validity (18–29).
Satisfaction after 1 year of intrathecal baclofen treatment
The question on overall satisfaction with ITB treatment, as defined in the Patient Global Impression of Change (PGIC) scale, includes a balanced view of changes related to consequences of spasticity (23). Changes were rated on a 7-point scale, from 0 (very much worse) to 6 (very much improved). For the analyses, the answers were dichotomized into high satisfaction with ITB treatment, including the 2 highest scores (very much improved and much improved), vs little/no satisfaction formed by the rest of the answers.
Self-perceived occupational performance and satisfaction with performance
The COPM is a generic, individualized measure of self-perceived occupational performance whereby data are collected through a semi-structured interview (30). The first step is identification of important occupational performance problems due to spasticity, which is then scored by the patient/proxy on a rating scale ranging from 1 to 10 (1, not able to do it at all; 10, able to do it extremely well). Each occupational problem identified was classified according to the COPM manual into 3 predefined categories: self-care, productivity, and leisure. Next, satisfaction with the specific performance for each occupation was scored on a similar scale (1, not satisfied at all; 10, extremely satisfied) (30). Performance and satisfaction with performance were scored at baseline and at the 1-year follow-up. For each patient, up to 5 occupational performance problems were identified and scored, after which a mean value was calculated. In addition, at baseline, expected occupational performance after 1 year of treatment was scored on the same scale. Proxies did not score satisfaction with performance or expectations regarding this. A change in score of 2 or more points was considered clinically important (21, 26, 30).
Spasticity, pain intensity and sleep quality
Degree of spasticity was assessed by a physiotherapist using the Modified Ashworth Scale (MAS) with 6 steps, ranging from 0 to 5 (0, no increase in muscle tone; 5, affected part(s) rigid in flexion or extension) (18, 28). Muscles were assessed in the upper extremities (elbow flexors and extensors; wrist flexors and extensors) and in lower extremities (hip flexors, extensors and adductors; knee flexors and extensors; plantar flexors). MAS was reported as a mean value for upper and lower extremities, respectively.
NRS was used for self-rating of spasticity, pain intensity and sleep quality (0, no problem; 10, worst possible problem) (22, 23). In terms of spasticity and pain intensity, a 30% improvement was considered a clinically important difference (23, 24). At baseline, expectations with outcome at 1 year of treatment regarding spasticity, pain intensity and sleep quality were also scored on NRS scales.
Patient characteristics: activities of daily living, health state, general self-efficacy, and depression/anxiety
The Barthel Index was used to document levels of activities of daily living. It consists of 10 items, with a score ranging from 0 to 100; lower scores indicate a higher level of disability and increased need for assistance (29).
Health state was measured using a thermometer-like scale (EQ-VAS) as part of the EuroQoL 5 dimensions (EQ-5D) instrument. The EQ-VAS scale ranges from 0 (worst imaginable health state) to 100 (best imaginable health state) (20).
General self-efficacy was evaluated using the General Self-Efficacy (GSE) scale with 10 items rated on a 4-point scale with a total score ranging from 10 to 40; a higher score indicates higher self-efficacy (31). The Swedish version (S-GSE) was used for this study (27).
Depression and anxiety were self-reported using the Hospital Anxiety and Depression (HAD) scale (19). Each of the 2 sub-scales consists of items rated on a 4-point scale ranging from 0 to 21 points per sub-scale with a cut-off score >8; a high score indicates a high level of anxiety/depression.
Procedures
Information regarding diagnosis, age and date of surgery was collected from patient charts. Patients completed the assessments and questionnaires at the baseline, in the period between testing and implantation of the pump and at the follow-up assessment 1 year after implantation. For those who were unable to communicate verbally or in writing, data were collected through a relative or personal assistant (by-proxy). Baseline and 1-year follow-up assessment included a spasticity assessment by physiotherapists, a semi-structured interview regarding occupational performance of individual importance using the COPM (30) and a questionnaire with an adjusted version for answers by-proxy (Table I). The COPM interview was performed by study-specific personnel and no attempt was made to discuss or adjust unrealistically high expectations. The questionnaires were posted to the patients and returned either in person or by post.
| Pre-ITB | Post-ITB | |
| PGIC | × | |
| COPM occupational performance | ×b | × |
| COPM satisfaction with performancea | × | × |
| MAS by physiotherapist | × | × |
| Spasticity | ×b | × |
| Pain intensity | ×b | × |
| Sleep quality | ×b | × |
| Barthel index | × | |
| EQ-VAS | × | × |
| GSESa | × | × |
| HADa | × | × |
| aExcluded for proxy assessment. | ||
| bAssessments with additional data on expectations. | ||
| COPM: Canadian Occupational Performance Measure; EQ-VAS: EuroQoL visual analogue scale; GSES: General Self-Efficacy Scale; HAD: Hospital Anxiety and Depression scale; ITB: intrathecal baclofen; MAS: Modified Ashworth Scale; PGIC: Patient Global Impression of Change; post-ITB: at 1-year follow-up; pre-ITB: at baseline. | ||
Intervention
Patients with a decision to start treatment after an adequate response to the screening test were implanted with a pump (SynchroMed by Medtronic) (11). This and subsequent procedures for pump refills and dose adjustments followed clinical routines. These routines included education, information and discussion regarding procedures and treatment goals together with the healthcare team (physician, physiotherapist, occupational therapist and nurse) before the screening test.
Statistical analysis
Statistical analyses were performed using SPSS Statistics 27 (IBM, Armonk, NY, USA). Descriptive statistics are presented as medians and 25th–75th percentiles or means and standard deviation (SD) based on the type of variable. Data for the 2 subgroups, self and by-proxy, were analysed separately. The Wilcoxon matched-pairs test was used to analyse differences in scoring between baseline, expectations and 1-year follow-up. The Mann–Whitney U test was used to compare results for occupational performance among patients with high vs little/no satisfaction. Kruskal–Wallis test was used to compare changes in performance for the different occupations. Effect size was calculated using z scores. Correlations were calculated with Spearman’s rho, using the 95% bias accelerated corrected confidence interval with 1,000 bootstrap samples. The significance level was set at p ≤ 0.05.
Advanced principal component analysis was applied for multivariate correlation analyses of all the variables to detect multivariate outliers and orthogonal partial least squares (OPLS) regression for the multivariate regressions (SIMCA-P+ version 15; Umetrics, Sartorius Stedim Biotech, Umeå, Sweden). OPLS was used for analyses of satisfaction with occupational performance at 1 year using the other variables presented (except occupational performance at 1 year, although the variable was included when used to calculate the change in occupational performance and expectations in relation to performance) as regressors (X variables). The variable influence on projection (VIP) indicates the relevance of each X variable pooled over all dimensions and Y variables, the group of variables that best explain Y. VIP (VIPpred when more than 1 component was achieved) ≥ 1.0 was considered significant (32). See Appendix S1 for detailed information.
RESULTS
All patients who agreed to participate (n = 29) completed the study; some internal dropouts from the questionnaires and assessments occurred. Of the 29 participants, 9 patients answered through a proxy at baseline (pre-ITB) and at 1-year follow-up (post-ITB).
Situation at baseline
The self-reporting patients were older and less disabled and thus had less need for assistance than patients in the by-proxy group. All patients with spinal cord injury and multiple sclerosis answered by self-reporting, whereas patients with acquired brain injury and cerebral palsy were in both groups (Table II).
Results of the intrathecal baclofen intervention Satisfaction with intrathecal baclofen treatment as measured using Patient Global Impression of Change scale
Ten (56%) patients in the by-self group and 2 (22%) in the by-proxy group reported high satisfaction. Patients in the by-self group scoring high satisfaction reported a higher 1-year occupational performance compared with patients with little/no satisfaction (p = 0.036), but no difference was noted for the by-proxy group (p = 0.106).
Satisfaction with occupational performance as measured using the Canadian Occupational Performance Measure
In the by-self group, satisfaction improved significantly compared with baseline (p = 0.001); however, satisfaction was still moderate after 1 year on ITB treatment (mean, 5.19; SD, 2.50). Satisfaction improved for 13 (65%) patients according to the level of a clinically relevant change (≥ 2 point difference) (30). When analysing satisfaction at follow-up vs change in occupational performance and expectations with occupational performance in relation to 1-year follow-up, the results showed moderate to strong correlations (Figs 1 and 2) (33).
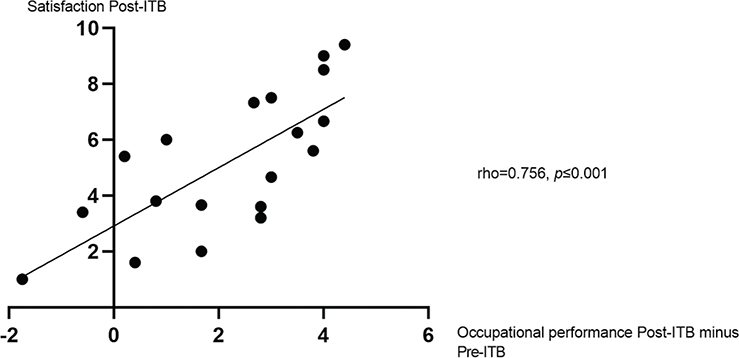
Fig. 1. Satisfaction with occupational performance post-intrathecal baclofen (ITB) in relation to occupational performance post-ITB minus pre-ITB, measured with Canadian Occupational Performance Measure (COPM). Presented with Spearman’s rho. Post-ITB, at 1-year follow-up; pre-ITB, at baseline. 95% bias accelerated corrected confidence interval, 0.484–0.894.
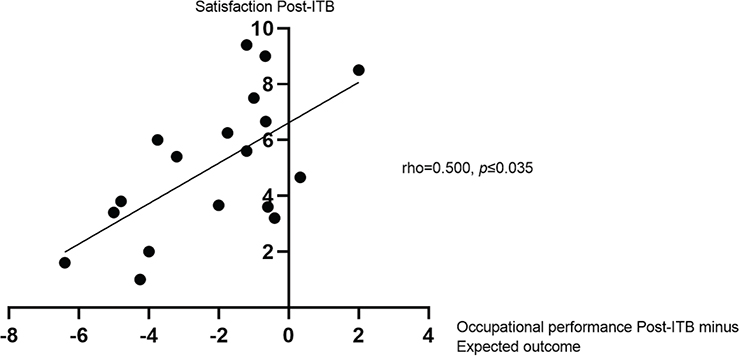
Fig. 2. Satisfaction with occupational performance in relation to occupational performance post-intrathecal baclofen (ITB) minus expected outcome, as measured with Canadian Occupational Performance Measure (COPM). Presented with Spearman’s rho. Post-ITB, at 1-year follow-up; pre-ITB, at baseline. 95% bias accelerated corrected confidence interval, 0.028–0.825.
Regression analysis was performed using satisfaction with occupational performance at 1 year as the dependent variable and the other variables as regressors (X variables) (except 1-year occupational performance, although the variable was included when used to calculate the change in occupational performance and expectations in relation to performance). The significant regression model consisting of 1 predictive component is presented in Table III. Six variables were significant regressors; the most important were change in occupational performance, expectations with occupational performance in relation to 1-year follow-up, occupational performance pre-ITB, and EQ-VAS pre-ITB. When only the 6 significant variables in Table III were included, a highly significant model (1 predictive component) was obtained with the following characteristics: R2 = 0.77, Q2 = 0.73 and cross-validated analysis of variance (CV-ANOVA), p-value 0.00003.
Expectations
The by-self and by-proxy groups both reported higher expectations in comparison with the actual outcome at 1 year regarding spasticity (p = 0.004 and p = 0.017), pain intensity (p = 0.034 and p = 0.042) and sleep quality (p = 0.004 and p = 0.042) (Table IV). Patients in the by-self group had higher expectations regarding occupational performance (p = 0.001) (Table IV).
| By-self (n = 20) | By-proxy (n = 9) | |||||||
| n1 (n2) | Difference pre- vs post-ITB, p-value and effect size | Difference expected vs post-ITB, p-value and effect size | n1 (n2) | Difference pre- vs post-ITB, p-value and effect size | Difference expected vs post-ITB, p-value and effect size | |||
| COPM performance, mean (SD) | ||||||||
| Pre-ITB | (19) | 3.22 (1.15) | (9) | 3.8 (1.80) | ||||
| Expected outcome | (18) | 7.41 (1.51) | ||||||
| Post-ITB | (19) | 5.39 (2.23) | 0.001, 0.55 | 0.001, 0.54 | (9) | 5.59 (2.08) | 0.044, 0.47 | |
| COPM satisfaction, mean (SD) | ||||||||
| Pre-ITB | (19) | 2.44 (1.25) | ||||||
| Post-ITB | (19) | 5.19 (2.50) | 0.001, 0.53 | |||||
| Spasticity MAS, median (25th–75th) | ||||||||
| Upper extremities | ||||||||
| Pre-ITB | 5 (18) | 0.63 (0.19–0.94) | 6 (8) | 1.56 (1.09–2.69) | ||||
| Post-ITBa | 2 (14) | 0.38 (0–0.72) | 5 (8) | 1.75 (1.07–2.63) | 0.5 | |||
| Lower extremities | ||||||||
| Pre-ITB | 17 (18) | 1.83 (1.29–2.50) | 8 (8) | 2.17 (1.71–3.21) | ||||
| Post-ITB | 12 (14) | 0.96 (0.50–1.61) | 0.029, 0.44 | 7 (8) | 1.08 (0.75–1.75) | 0.028 | 0.59 | |
| Spasticity (NRS), median (25th–75th) | ||||||||
| Pre-ITB | 20 (20) | 6.5 (5.0–8.0) | 9 (9) | 7.0 (7.0–8.0) | ||||
| Expected outcome | 20 (20) | 2.0 (1.25–3.0) | 8 (8) | 2.0 (2.0–3.0) | ||||
| Post-ITB | 18 (18) | 5.0 (3.0–6.0) | 0.014, 0.41 | 0.004, 0.49 | 8 (8) | 6.5 (4.25–7.0) | 0.026, 0.56 | 0.017, 0.60 |
| Pain intensity (NRS), median (25th–75th) | ||||||||
| Pre-ITB | 13 (20) | 7.0 (5.0–8.0) | 7 (8) | 6.0 (5.0–7.0) | ||||
| Expected outcome | 13 (20) | 2.0 (1.5–3.0) | 7 (8) | 2.0 (0–3.0) | ||||
| Post-ITB | 12 (18) | 4.5 (1.5–5.75) | 0.009, 0.53 | 0.034, 0.43 | 7 (8) | 2.0 (1.0–6.0) | 0.033, 0.57 | 0.042, 0.54 |
| Sleep quality (NRS), median (25th–75th) | ||||||||
| Pre-ITB | 17 (19) | 7.0 (2.5–8.5) | 9 (9) | 5.0 (5.0–5.5) | ||||
| Expected outcome | 16 (18) | 1.0 (0.25–2.0) | 7 (7) | 3.0 (0.0–4.0) | ||||
| Post-ITB | 16 (18) | 2.5 (1.0–4.75) | 0.010, 0.45 | 0.006, 0.50 | 9 (9) | 4.0 (1.5–7.0) | 0.206 | 0.042, 0.54 |
| EQ-VAS, median (25th–75th) | ||||||||
| Pre-ITB | (18) | 35 (30–62.5) | (7) | 60 (40–70) | ||||
| Post-ITB | (18) | 50 (40–62.5) | 0.276 | (7) | 40 (35–50) | 0.173 | ||
| GSES, median (25th–75th) | ||||||||
| Pre-ITB | (20) | 24 (19.3–29.8) | ||||||
| Post-ITB | (18) | 26 (21.8–30.3) | 0.307 | |||||
| HAD, depression, median (25th–75th) | ||||||||
| Pre-ITB | (20) | 7.5 (4.25–10.75) | ||||||
| Post-ITB | (17) | 6.0 (3.5–8.0) | 0.168 | |||||
| HAD anxiety, median (25th–75th) | ||||||||
| Pre-ITB | (20) | 6.0 (4.0–11.0) | ||||||
| Post-ITB | (18) | 5.0 (2.0–8.25) | 0.063 | |||||
| aAt follow-up, 14 patients were assessed with MAS, only 2 of whom had spasticity in the upper extremities, therefore a comparison could not be made. | ||||||||
| COPM: Canadian Occupational Performance Measure; MAS: Modified Ashworth Scale; n1: patients who reported having the problem at baseline and hence included in the comparison; n2: number of patients who answered the questionnaire or were assessed with MAS: reported to clarify internal dropout; NRS: numerical rating scale; post-ITB: at 1-year follow-up; pre-ITB: at baseline. | ||||||||
Self-perceived occupational performance
Improvements in occupational performance were reported in both groups at follow-up (by-self, p = 0.001; by-proxy, p = 0.044) (Table IV). Eleven patients in the by-self group (55%) vs 4 (44%) in the by-proxy group had clinically important improvements.
A wide range of individually prioritized problems with occupational performance were reported based on the COPM interview. Occupations classified as self-care were the most common: 77% of all occupations identified in the by-self group and all but 1 occupation in the by-proxy group. Within the self-care category, the problems were mostly related to personal care or transfer, and only a few (n = 4) were about coping with society. Occupational problems classified within productivity (11%) were about managing the household, and 1 involved work. Occupational problems classified within leisure (12%) were about active leisure and socializing. When comparing the different occupations regarding changes in performance, there was no difference between different types of occupations; they were equally easy to achieve improvement in (p = 0.284).
Spasticity, pain intensity and sleep quality
Spasticity in lower extremities (MAS) was reduced for both groups at follow-up; however, there was no change in spasticity in the upper extremities (Table IV). It was not possible to perform the MAS test with 3 patients; and at the follow-up, another 4 patients were not assessed. Self-rated spasticity showed significant reductions for both groups (by-self, p = 0.014; by-proxy, p = 0.026).
At baseline, 13 patients (60%) in the by-self group and 7 patients (78%) in the by-proxy group reported having pain. Pain intensity was significantly decreased at follow-up in both groups (p = 0.009 and p = 0.033).
Sleep quality was significantly improved in the by-self group (p = 0.012), but not in by-proxy group (p = 0.206).
Clinically important improvements in self-reported spasticity (NRS) were seen in 10 patients in the by-self group (50%) and 2 in the by-proxy group. Five patients in each group (38% and 71%) displayed clinically important improvements in pain intensity.
Health state, self-efficacy, depression and anxiety
No change could be seen regarding health state, self-efficacy, depression or anxiety after 1 year (Table IV).
DISCUSSION
Patients’ overall satisfaction with treatment at the 1-year follow-up was found to be moderate to low. Furthermore, the results of this study suggest that change in occupational performance, expectations with occupational performance in relation to 1-year follow-up and occupational performance pre-ITB play a key role in explaining satisfaction with occupational performance. Although improvements were seen regarding occupational performance, spasticity, pain intensity and sleep quality, patients reported higher expectations in relation to 1-year outcome.
Patients or their relatives/assistants reported a decrease in spasticity and pain intensity, and patients in the by-self group reported that their sleep quality was improved. This finding is important because sleep disturbance together with stiffness and pain associated with spasticity after spinal cord injury have been reported as the most problematic aspects affecting daily life (3). Furthermore, occupational performance improved in both groups and was focused mainly on self-care and transfer; for example, facilitated dressing, positioning in the wheelchair, transfer with or without aid and easier care. The results indicate the treatment was effective with improvements regarding both symptoms and function, which is an important basis for interpreting the results regarding expectations and satisfaction.
Overall estimated satisfaction with treatment outcome was reported as moderate by 56% in the by-self group and by 2 of 9 in the by-proxy group. The highest score (very much improved) was not reported in any of the groups, which differs from other studies reporting a high level of satisfaction (6, 8, 14, 34). When dichotomizing the PGIC scale, minimally improved was assumed to be equal with little or no satisfaction based on an assumption that treatment must be worth the effort because ITB is a symptom-relieving therapy rather than a curative treatment (5, 10). This underscores the importance of evaluating outcomes based on what is a clinically relevant difference. Regarding all outcome measures, except pain intensity for the by-proxy group (71% had a clinically important improvement), only 22–55% of the patients had a clinically important improvement.
Two versions of satisfaction measurements were included: overall satisfaction with ITB treatment as discussed earlier and satisfaction with occupational performance self-rated at baseline and at 1-year follow-up. The purpose of ITB treatment is to decrease spasticity to enable improvement in the individual patient’s specific problems and consequently reach the goal of the treatment. Hence, evaluating satisfaction with occupational performance can be seen as equivalent to evaluating the purpose of ITB treatment. In this study, with decreased spasticity and pain intensity and improved occupational performance and sleep quality, it may seem unexpected that overall satisfaction as well as satisfaction with occupational performance was not reported to be higher.
Patients with high improvement in occupational performance reported a high overall satisfaction with treatment. The meaning of occupational performance was further highlighted in the regression model, showing that the most important variables to explain satisfaction with occupational performance were related to the change in occupational performance; the difference in 1-year occupational performance compared with expectations, occupational performance before treatment and self-rated health state before treatment. What people expect to receive compared with their perceptions of what they actually receive must be acknowledged when predicting patient satisfaction and dissatisfaction with their care, treatment and health outcomes (15). Even though healthcare providers believe they thoroughly inform and discuss the goals and expected results of ITB treatment, a discrepancy between the patients’ expectations and the actual outcome is obvious from the current study. Considering expectations when evaluating treatment has been reported in other areas, such as orthopaedics and pain treatments (35, 36). Unrealistic goals, together with unmanageable mental health issues and psychosocial factors, are identified as relative contraindications for ITB treatment, but these risk factors might be modifiable through interaction with healthcare professionals (5). Both COPM, as used in this study, as well as Goal Attainment Scale (GAS) are recommended measures to use when discussing, setting and following up ITB treatment goals (12).
Study strengths and limitations
A strength of the current study is that all the methods used have been assessed as being moderate to good regarding reliability and validity. The use of proxy estimations may not be as valid as evaluation by the patients themselves, but this is the only way to capture the view of patients who are unable to communicate. Therefore, data obtained by-proxy must be analysed separately and with caution. In the proxy questionnaire, some questions were excluded because they were difficult for proxies to answer, an experience in concordance with the literature on the use of proxies (37, 38).
A limitation is that follow-up took place after 1 year and some patients may need a longer time to achieve steady state, which could affect the experienced satisfaction with treatment (39, 40). Furthermore, baclofen dose development was not followed. For study purpose neither the patient nor a physician/nurse evaluated whether a stable and sufficient dose was reached; thus, there is uncertainty regarding this. In addition, potential complications were not identified and thus not included when analysing the results.
Another limitation is the lack of complete MAS data. Reasons for internal dropouts (n = 7) were geographical distances at follow-up, lack of physiotherapist or inability to assess spasticity according to the physiotherapist’s assessment. Thus, some caution is needed when drawing conclusions from this assessment, even though the assessment is consistent with the patient-reported outcomes. In addition, no information regarding concomitant spasticity medication such as botulinum toxin was reported and therefore interpretation of the missing effect of improvements in MAS for upper extremities is lacking. Finally, due to the relatively small sample size, the generalizability might be affected.
Future research focusing on expectations should include a longer follow-up period, include baclofen dose development and follow complications over time.
CONCLUSION
Patients’ overall satisfaction with treatment was moderate to low at 1-year follow-up. Although there were improvements in several of the conditions related to daily life with improved occupational performance, satisfaction and sleep quality, there was failure to completely reach the level of the patients’ expectations of the treatment outcome. The variables that best explain 1-year satisfaction with occupational performance seem to be change in occupational performance, expectations, initial status regarding occupational performance and health state. Patients treated with ITB are a heterogeneous group regarding diagnosis, level of independence and ability to communicate, which poses a challenge when evaluating relevant treatment goals and expectations. The importance of considering expectations of the treatment outcome highlights the need to define realistic and individualized patient goals.
ACKNOWLEDGEMENTS
We thank Malin Karlsson, Eva Härkegård, Marie Lannesand and Kristin Nyman for participation in data collection.
REFERENCES
- Ward AB, Aguilar M, De Beyl Z, Gedin S, Kanovsky P, Molteni F, et al. Use of botulinum toxin type A in management of adult spasticity. A European consensus statement. Eura Medicophys 2004; 40: 83–84.
- Ertzgaard P, Anhammer M, Forsmark A. Regional disparities in botulinum toxin A (BoNT-A) therapy for spasticity in Sweden: budgetary consequences of closing the estimated treatment gap. Acta Neurol Scand 2017; 135: 366–372.
- Field-Fote EC, Furbish CL, Tripp NE, Zanca J, Dyson-Hudson T, Kirshblum S, et al. Characterizing the experience of spasticity after spinal cord injury: a national survey project of the Spinal Cord Injury Model Systems centers. Arch Phys Med Rehab 2022; 103: 764–772.
- Gunnarsson S, Samuelsson K. Patient experiences with intrathecal baclofen as a treatment for spasticity – a pilot study. Disabil Rehabil 2015; 37: 834–841.
- Saulino M, Ivanhoe CB, McGuire JR, Ridley B, Shilt JS, Boster AL. Best practices for intrathecal baclofen therapy: patient selection. Neuromodulation 2016; 19: 607–615.
- Krach LE, Nettleton A, Klempka B. Satisfaction of individuals treated long-term with continuous infusion of intrathecal baclofen by implanted programmable pump. Pediatr Rehabil 2006; 9: 210–218.
- Natale M, D’Oria S, Nero VV, Squillante E, Gentile M, Rotondo M. Long-term effects of intrathecal baclofen in multiple sclerosis. Clin Neurol Neurosur 2016; 143: 121–125.
- Plassat R, Perrouin Verbe B, Menei P, Menegalli D, Mathe JF, Richard I. Treatment of spasticity with intrathecal baclofen administration: long-term follow-up, review of 40 patients. Spinal Cord 2004; 42: 686–693.
- Sammaraiee Y, Yardley M, Keenan L, Buchanan K, Stevenson V, Farrell R. Intrathecal baclofen for multiple sclerosis related spasticity: a twenty year experience. Mult Scler Relat Disord 2019; 27:95–100.
- Biering-Soerensen B, Stevenson V, Bensmail D, Grabljevec K, Martínez Moreno M, Pucks-Faes E, et al. European expert consensus on improving patient selection for the management of disabling spasticity with intrathecal baclofen and/or botulinum toxin type A. J Rehabil Med 2022; 54: jrm00241.
- Boster AL, Bennett SE, Bilsky GS, Gudesblatt M, Koelbel SF, McManus M, et al. Best practices for intrathecal baclofen therapy: screening test. Neuromodulation 2016; 19: 616–622.
- Boster AL, Adair RL, Gooch JL, Nelson ME, Toomer A, Urquidez J, et al. Best practices for intrathecal baclofen therapy: dosing and long-term management. Neuromodulation 2016; 19: 623–631.
- Draulans N, Vermeersch K, Degraeuwe B, Meurrens T, Peers K, Nuttin B. Intrathecal baclofen in multiple sclerosis and spinal cord injury: complications and long-term dosage evolution. Clin Rehabil 2013; 27:1137–1143.
- Mathur SN, Chu SK, McCormick Z, Chang Chien GC, Marciniak CM. Long-term intrathecal baclofen: outcomes after more than 10 years of treatment. PM R 2014; 6: 506–513.
- Bowling A, Rowe G, Lambert N, Waddington M, Mahtani KR, Kenten C, et al. The measurement of patients’ expectations for health care: a review and psychometric testing of a measure of patients’ expectations. Health Technol Assess 2012; 16: i–xii, 1–509.
- El-Haddad C, Hegazi I, Hu W. Understanding patient expectations of health care: a qualitative study. J Patient Exp 2020; 7: 1724–1731.
- World Medical Association. Medical Association Declaration of Helsinki – ethical principles for medical research involving human subjects. 1964. [cited 2022 May 5]. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
- Ansari NN, Naghdi S, Arab TK, Jalaie S. The interrater and intrarater reliability of the Modified Ashworth Scale in the assessment of muscle spasticity: limb and muscle group effect. NeuroRehabilitation 2008; 23: 231–237.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002; 52: 69–77.
- Brooks R. EuroQol: the current state of play. Health Policy 1996; 37: 53–72.
- Carswell A, McColl MA, Baptiste S, Law M, Polatajko H, Pollock N. The Canadian Occupational Performance Measure: a research and clinical literature review. Canadian journal of occupational therapy Rev Can Ergother 2004; 71: 210–222.
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005; 113: 9–19.
- Farrar JT, Troxel AB, Stott C, Duncombe P, Jensen MP. Validity, reliability, and clinical importance of change in a 0–10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther 2008; 30: 974–985.
- Farrar JT, Young JP, Jr., LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001; 94: 149–158.
- Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther 2004; 27: 26–35.
- Kjeken I, Slatkowsky-Christensen B, Kvien TK, Uhlig T. Norwegian version of the Canadian Occupational Performance Measure in patients with hand osteoarthritis: validity, responsiveness, and feasibility. Arthritis Rheum 2004; 51: 709–715.
- Love J, Moore CD, Hensing G. Validation of the Swedish translation of the General Self-Efficacy scale. Qual Life Res 2012; 21: 1249–1253.
- Meseguer-Henarejos AB, Sánchez-Meca J, López-Pina JA, Carles-Hernández R. Inter- and intra-rater reliability of the Modified Ashworth Scale: a systematic review and meta-analysis. Eur J Phys Rehabil Med 2018; 54: 576–590.
- Quinn TJ, Langhorne P, Stott DJ. Barthel index for stroke trials: development, properties, and application. Stroke 2011; 42: 1146–1151.
- Law M, Baptiste S, Carswell A, McColl M, Polatajko H, Pollock N. Canadian Occupational Performance Measure. 3rd edn. Ottawa: CAOT Publications ACE; 1998.
- Schwarzer R, Jerusalem, M. Generalized Self-Efficacy scale. In: Weinman J, Wright S, Johnston M, editors. Measures in health psychology: a user’s portfolio. Causal and control beliefs. Windsor, UK: NFER-NELSON; 1995: 35–37.
- Eriksson L. Multi- and megavariate data analysis: basic principles and applications. 3rd rev. edn. Malmö, Sweden MKS Umetrics; 2013.
- Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med 2018; 18: 91–93.
- Gunnarsson S, Alehagen S, Lemming D, Ertzgaard P, Ghaderi Berntsson S, Samuelsson K. Experiences from intrathecal baclofen treatment based on medical records and patient- and proxy-reported outcome: a multicentre study. Disabil Rehabil 2019; 41: 1037–1043.
- Mannion AF, Kämpfen S, Munzinger U, Kramers-de Quervain I. The role of patient expectations in predicting outcome after total knee arthroplasty. Arthritis Res Ther 2009; 11: R139.
- Robinson ME, Brown JL, George SZ, Edwards PS, Atchison JW, Hirsh AT, et al. Multidimensional success criteria and expectations for treatment of chronic pain: the patient perspective. Pain Med 2005; 6: 336–345.
- Duncan PW, Lai SM, Tyler D, Perera S, Reker DM, Studenski S. Evaluation of proxy responses to the Stroke Impact Scale. Stroke 2002; 33: 2593–2599.
- Sneeuw KC, Aaronson NK, Osoba D, Muller MJ, Hsu MA, Yung WK, et al. The use of significant others as proxy raters of the quality of life of patients with brain cancer. Med Care 1997; 35: 490–506.
- Gunnarsson S, Lemming D, Alehagen S, Berntsson S, Ertzgaard P, Samuelsson K. Dosing patterns in treatment of disabling spasticity with intrathecal baclofen. Rehabil Nurs 2021; 46: 315–322.
- Heetla HW, Staal MJ, Kliphuis C, Laar T. The incidence and management of tolerance in intrathecal baclofen therapy. Spinal Cord 2009; 47: 751–756.