ORIGINAL REPORT
ELECTROMAGNETIC INDUCTION FOR TREATMENT OF UNSPECIFIC BACK PAIN: A PROSPECTIVE RANDOMIZED SHAM-CONTROLLED CLINICAL TRIAL
Manfred HARTARD, MD1, Mohamed AMINE FENNENI, PhD1,2, Stephan SCHARLA, MD3,4, Christian HARTARD, MD1, Diana HARTARD, MD1, Stephan MUELLER, MD1, Gabriela BOTTA MENDEZ, MD1 and Helmi BEN SAAD, MD, PhD2,5,6
From the 1Center for Diagnostic and Health, Munich, Germany, 2University of Sousse. Faculty of Medicine of Sousse, Laboratory of Physiology, Sousse, Tunisia, 3Ludwig-Maximilians University, Faculty of Medicine, Munich, Germany, 4Practice for Internal Medicine and Endocrinology, Bad Reichenhall, Germany, 5Laboratory of Physiology and Functional Explorations and 6Heart Failure (LR12SP09) Research Laboratory, Farhat Hached Hospital, Sousse, Tunisia
Objective: To evaluate the effects of high-energy pulsed electromagnetic fields on unspecific back pain.
Methods: A prospective, randomized, sham-controlled clinical trial with repeated measurements was performed. The study included 5 visits (V0 to V4) with 3 interventions during V1, V2 and V3. Sixty-one patients aged between 18 and 80 years with unspecific back pain (acute inflammatory diseases and specific causes were reasons for exclusion) were included. The treatment group (n = 31) received 1–2 pulses/s, with an intensity of 50 mT, and an electric field strength of at least 20 V/m on 3 consecutive weekdays for 10 min each time. The control group (n = 30) received a comparable sham therapy. Pain intensity (visual analogue scale), local oxyhaemoglobin saturation, heart rate, blood pressure, and perfusion index were evaluated before (b) and after (a) V1 and V3 interventions. Change in visual analogue scale for V1 (ChangeV1a-b) and V3 (ChangeV3a-b), and ChangeData between V3a and V1b (ChangeV3a–V1b) for the remaining data were calculated (results were mean (standard deviation) (95% confidence interval; 95% CI)).
Results: Concerning the visual analogue scale: (i) compared with the control group, the treatment group had higher ChangeV1a–b (–1.25 (1.76) (95% CI –1.91 to –0.59) vs –2.69 (1.74) (95% CI –3.33 to –2.06), respectively), and comparable -ChangeV3a–b (–0.86 (1.34) (95% CI –1.36 to –0.36) vs –1.37 (1.03) (95% CI –1.75 to 0.99), respectively); and (ii) there was a significant marked decrease in ChangeV3a–1b in the treatment group compared with the control group (–5.15 (1.56) (95% CI –5.72 to –4.57) vs –2.58 (1.68) (95% CI –3.21 to –1.96), p = 0.001, respectively). There was no significant ChangeV3a–V1b in local oxyhaemoglobin saturation, heart rate, blood pressure or perfusion index between the 2 groups and for the same group (before vs after).
Conclusion: Non-thermal, non-invasive electromagnetic induction therapy had a significant and rapid influence on unspecific back pain in the treatment group.
LAY ABSTRACT
Back pain is a health disorder of outstanding epidemiological, medical, and health economic importance. In the case of unspecific back pain, there is no clear specific cause. Electrotherapy is a physical therapy procedure using electric current for therapeutic purposes. Electromagnetic induction can influence many biological processes that are important for therapeutic interventions. A relatively new method is the use of non-invasive, very short, high-energy pulsed electromagnetic fields. Based on the literature, observations, and guidelines available up to February 2023, therapeutically successful use of electromagnetic induction appears possible, particularly in the case of high-energy pulsed electromagnetic fields. Pulsed electromagnetic fields with high-energy pulsed electromagnetic fields are therefore the logical therapeutic extension of high-energy pulsed electromagnetic fields. This study was designed to test the theory that high-ener-gy pulsed electromagnetic fields can reduce unspecific back-pain. The application of electromagnetic induction, short high-frequency and high-energy, but non-thermal, electromagnetic pulses with a magnetic flux density of approximately 50–100 mT was found to reduce unspecific back-pain in the treatment area of the treatment group.
Key words: calmodulin; microcirculation; nitric oxide; non-thermal pulsed electromagnetic field; signalling pathway; therapy.
Citation: J Rehabil Med 2023; 55: jrm00389. DOI: https://doi.org/10.2340/jrm.v55.3487
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Mar 13, 2023; Published: Apr 28, 2023
Correspondence address: Helmi Ben Saad, Laboratory of Physiology, Faculty of Medicine of Sousse, Street Mohamed Karoui, Sousse 4000, Tunisia. E-mail: helmi.bensaad@rns.tn
Back pain (i.e. pain occurring between the back of the head and the crena interglutaealis (1)) is a health disorder of outstanding epidemiological, medical, and health economic importance (1). Back pain is a particularly frequent reason for use of the healthcare system, incapacity to work, and claiming state benefits due to partial or full disability (1). In the case of unspecific back pain (UBP), no specific cause is clearly indicated (2). Among the common conservative methods for treatment of UBP, physical therapy modalities are the most used (3). Electrotherapy is a physical therapy procedure using electric current for therapeutic purposes (4). Explanations for the mechanisms for reduction of pain under electric stimuli involve an increase in central β-endorphin, hyperpolarization at the motor endplate (5), and promotion of local blood flow (6). It is notable that electric and magnetic fields, as well as electric charges and electric current are connected via Maxwell’s equations (7). Every electric current creates a magnetic field, and every magnetic field can be influenced by an electric field and vice versa (7). Therefore, a magnetically induced electrical field (electromagnetic induction; EMI) can influence many biological processes that are important for therapeutic interventions (8). EMI is the creation of an electric field when the magnetic flux changes (7).
For therapeutic purposes, electric current, often in the form of pulsed electric fields (PEF), transcutaneous electrical nerve stimulation (9), or when performed invasively (i.e. implanted) (4, 10, 11), is used to relieve pain or to improve function. The use of very short PEF in the μs range (μsPEF) is relatively new (12). μsPEF can permeabilize the plasma membrane and are increasingly used in research, medicine and biotechnology (12). However, PEF, even the very short ones (e.g. μsPEF), are induced by direct contact with the tissue and can therefore cause pain and tissue damage (13). A relatively new method is the emerging non-invasive, contactless alternative to PEF, which are pulsed EMI or very short, high-energy pulsed electromagnetic fields (HE-PEMF), in which the electric field in the tissue is remotely generated by an external pulsed magnetic field, corresponding to EMI (i.e. Faraday induction) (13–15). Using an appropriate device, the voltage of the electric field in the tissue can be detected directly (7, 13). The properties of the electromagnetic pulse (i.e. frequency, duration, amplitude) determine the qualities of the magnetic and electric fields that are applied to tissue therapy (16). Based on the studies, observations, and guidelines available up to February 2023, therapeutically successful EMI appears to be possible, particularly in the case of very short and energetically sufficient intense pulsed electromagnetic fields (PEMF) (i.e. HE-PEMF) (7, 13–16). Pulsed electromagnetic fields with HE-PEMF are therefore the logical therapeutic extension of high-energy PEF.
Current reviews have shown that very short electrical pulses (μs- and ns-pulses) of high intensity have great potential for influencing intracellular structures (14, 15). Effects at the plasma membrane, intracellular, and cell survival levels were observed (14). These reviews have also reported an increased clinical application with a focus on low voltage (14, 15). Current studies have shown that locally applied electromagnetic impulses also mediate a locally significant increase in nitric oxide (8, 17, 18), which is a potent vasodilator (19, 20). Brisby et al. (21) reported that patients with chronic back pain have a nitric oxide level 3 times higher in the perifacial region compared with healthy controls. Nitric oxide is released in the endothelial cells by a calmodulin (CaM)-dependent nitric oxide synthase (22). Several studies have highlighted that CaM-dependent nitric oxide signalling is involved in the cell and tissue reaction through electrical and electromagnetic signals (23–25), and that these signals influence the development of pain in tissues and organs.
This randomized clinical trial (RCT) was designed to evaluate the effects of HE-PEMF on UBP. The null hypothesis was that the treatment group (TG) and the control group (CG) would have similar mean values of pain visual analogue scale (VAS) at the end of the intervention.
PATIENTS AND METHODS
Study design
This was a prospective, randomized, sham-controlled, single-blind, parallel group clinical study with repeated measurements before (b) and after (a) the interventions. The study was performed in a private physiotherapy centre in Munich, Germany, from February 2019 to August 2020. The study was performed according to the principles of the Declaration of Helsinki. The protocol was approved by the ethics committee of the Ludwig-Maximilians University of Munich (approval number 48/16). At the screening visit (V1), all patients were informed about the aims and risks of the treatment (e.g. acute effects: induction of phosphenes, perception, nerves or muscles excitation, and thermal effects) (26, 27). Written informed consent was obtained. Participation in the study was free of charge for the patients. Data collection was pseudonymized. Collected data were stored in electronic form on a password-protected computer and were saved on another computer with an identification number.
One part of the study was performed during the COVID-19 pandemic. All recommended preventive measures against transmission of the virus were applied (e.g. physical distance of at least 1 m from others, wearing a fitted face mask, cleaning hands frequently).
Study population
The patients were recruited in 3 ways: (i) mail campaigns to patients who attended the aforementioned private physiotherapy centre; (ii) mediation of resident doctors; and (iii) new patients presenting for the first time with a UBP complaint. If patients showed interest in participating, they were met by medical doctors or study coordinators (MH, CH, DH, SM, GBM) who gave them more detailed information about the study, and checked their eligibility for participation (pre-screening). If patients were eligible for participation, they were provided with a participant information sheet. After reviewing the study information, all questions and further clarifications were resolved by discussion with the physicians (MH, DH, GBM). The physician and patient then signed an informed consent form, and, therefore, the patient was admitted to the study and invited to V1. The study flow chart is shown in Fig. 1.
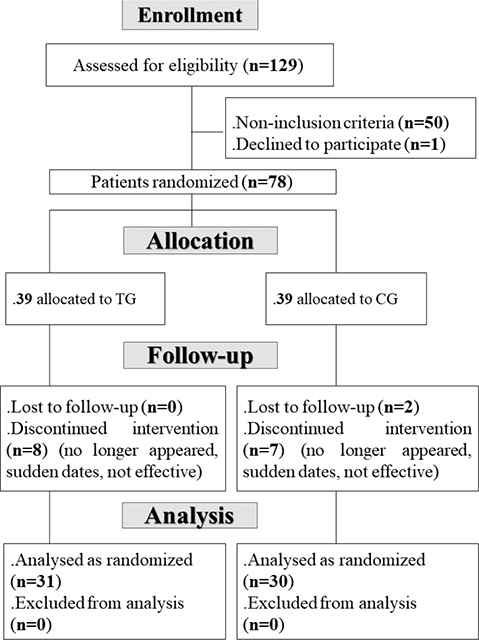
Fig. 1. Study flow chart. CG: control group; TG: treatment group.
Sample size
The sample size was estimated using the following formula (28): N = ( (r+1) (Zα/2 + Z1–β)2 σ2)/ (r d2), where:
- “Zα/2” is the normal deviate at a level of significance = 1.96 (0.05% level of significance);
- “Z1–β” is the normal deviate at 1–β% power with β% of type II error (1.03 at 85% statistical power);
- “r” ( = n1/n2,n1, and n2 are the sample sizes for the TG and CG, such N = n1 + n2) is the ratio of sample size required for the 2 groups (r = 1 gives the sample size distribution as 1:1 for the 2 groups);
- “σ” and “d” are the pooled standard deviation (SD) and the difference in pain VAS means of the 2 groups.
Given the pioneer character of this study, “σ” and “d” values were obtained from a previous randomized, single-blind, placebo-controlled clinical trial aiming to study the effectiveness of pulsed electromagnetic therapy in patients with chronic lower back pain (29). Four weeks post-therapy, the means (SDs) of pain assessed using an 11-point numerical rating scale (NRS) in the TG (i.e. 17 patients with lower back pain receiving pulsed electromagnetic therapy) and CG (19 patients with lower back pain receiving placebo) were 4.5 (1.2) and 5.4 (2.3), respectively. In this case the “σ” (i.e. common SD assumed to be the same in the 2 groups) is equal to 1.75 ( = (1.2+2.3)/2), and “d” (i.e. the difference between the 2 means) is equal to 0.9 ( = 5.4–4.5).
Insertion of the aforementioned data into the formula gave a sample of 68 patients (34 in each group). Assumption of 40% loss during the 5 visits gave a revised sample of 113 participants ( = 68/(1–0.40)).
Study protocol
Table I presents the study design. The latter includes the following 5 visits (V):
- V0: during this visit, the following actions were performed: (i) each patient was given a screening number; (ii) an anamnesis was drawn-up; (iii) a physical examination was carried out with a focus on the musculoskeletal system; (iv) anthropometric data (i.e. height, weight) were measured; (v) vital parameters (i.e. systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate, oxyhaemoglobin saturation (SpO2), perfusion index) were measured; and (vi) pain VAS was recorded (30). Once all inclusion/non-inclusion criteria were met, the date for the randomization visit (V1), and the first intervention were set. V0 and V1 were mostly on the same day.
- V1: at this visit, patients were randomly assigned (according to a random list created by means of an online random generator (i.e. random group generator of PubMed, available at http://www.pubmed.de/tools/zufallsgenerator/?no_cache = 1)) to receive first intervention (V1), either electrotherapy (i.e. TG or sham therapy (i.e. CG). Before the first intervention (V1b), the following evaluations were performed: medical history, physical examination, vital signs, VAS, and pulse oximetry. After the first intervention (V1a), only VAS scores were evaluated. A randomizing schema finalized the assignment of the patients into TG or CG. A simple randomization list with chronological. The physician, who undertakes the randomization, completes a list of every new patient’s name, with time and date, and records the randomizing number in the patient folder. For economic reason, the physician was not blinded.
- V2 and V3: at these visits, the TG received the second and the third interventions, respectively. Before the second (V2b) and the third (V3b) interventions, medical history was recorded, physical examination was performed, and VAS scores were evaluated. After the second (V2a) and third (V3a) interventions, only VAS scores were evaluated.
- V4: 1 day after the last intervention, a telephone call was made for the final follow-up of patient safety, to determine the sustainability, and to evaluate the VAS scores.
Each 2 consecutive interventions were separated by 1–3 nights. Patients were blinded to the intervention details of the study. The attending physician was informed about whether it was verum or placebo.
Inclusion, non-inclusion and exclusion criteria
Only patients aged 18–80 years with an UBP within the previous 24 h, determined by VAS, were included. Only patients with back pain for which no cause could be advanced using simple clinical means to explain the current symptoms were included (31). The following non-inclusion criteria were applied: contraindications of EMI in specific back pain (e.g. ankylosing spondylitis) (1), diseases according to the “red flags” (32), specific diseases of the spine, inflammatory spondylopathies, diseases of the internal organs (e.g. renal pelvic inflammation), vertebral collapse due to osteoporosis or following an accident, a malignant tumour, diseases with specific origins (e.g. muscles, intervertebral discs, nerve roots), acute inflammatory diseases (1), patients with implanted metallic or electronic objects (e.g. pacemaker, defibrillator, pumps), pregnancy, and large tattoos. Absence during a session was considered as a protocol violation and led to exclusion from the final analysis. All patients were asked to maintain their conventional medical therapy throughout the study.
Unspecific back pain diagnosis and localization
The criteria of the Federal Health Report of the Robert Koch Institute (1), based on the European clinical guidelines on chronic UBP (32), were used in this study. Diseases with “red and yellow flags” were excluded. The localization of back pain (e.g. upper shoulder/cervical spine, middle back (known as thoracic back), lower back (known as lumbar region)) was noted.
Collected data
Height (m) was determined by Harpenden® stadiometer, and weight (kg) was measured by a calibrated mechanical patient scale (SECA® 711) (220 kg). Body mass index (BMI, kg/m2) was calculated. SBP and DBP (mmHg) and heart-rate (bpm) were measured after a 5-min period of rest in a sitting position. A “digital blood-pressure monitor” (model UH-707plus, A&D Co, Ltd. Saitama, Japan) was used on the left upper arm. SpO2 (%) was determined by a finger pulse oximeter (Beijing Choice Electronic Technology Co., Ltd, Beijing, China). The perfusion index was carried out with a wireless pulse oximeter (Gravis Computervertriebs-GmbH, Ernst-Reuter-Platz 8, 10587 Berlin, Germany). Pain score was evaluated using a VAS (30). The scale varied from 0 (no pain) to 10 (unbearable pain) on a 10-cm long scale (30).
Intervention
For the TG, the device used was a “magnetic pulse generator” (Device: Papimi_Series, Asclipios; Athens, Greece; User Manuals Version 23 2015) (Fig. 2). During the application, the charge of a capacitor (approximately 40,000 V) was transferred to a treatment coil via a spark gap. The coil was made of copper with a high cross-section (approximately 30 mm2) to withstand high currents, and insulated with a plastic material with a thickness of approximately 5 mm (33).
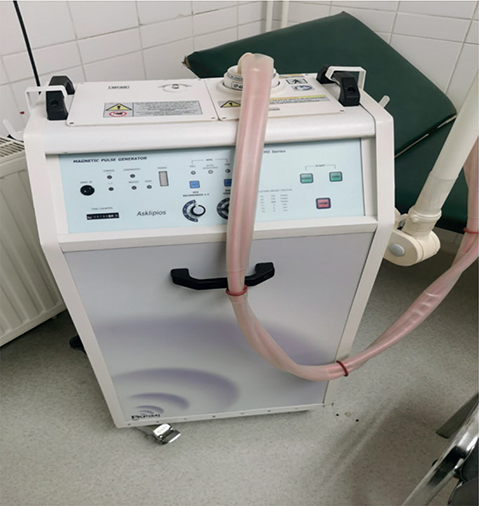
Fig. 2. Therapeutic magnetic pulse generator device (PAPIMI-SERIES, Type: ASCLIPIOS; Athens, Greece; User Manuals Version 23 2015).
The current of several thousand amperes (5,000–10,000 A) flowing through the coil creates a short electromagnetic pulse that induces electrical tension in the tissue. The device works with an intensity (magnetic flux density) of approximately 50 milli-Tesla (corresponding to approximately 40 kA/m) and with a resonance frequency range of approximately 0.3–250 MHz. The device sends 1–8 pulses/s with a pulse duration of approximately 50 µs per pulse, an energy per pulse complex of a maximum of 96 Ws, and an electric field strength of at least 20 V/m (coil with 2 loops directly on the body surface) (33).
For the CG, the placebo device was a short-circuited loop (Fig. 3). The latter produced similar sounds to the pulse generator while operating, but it did not emit a magnetic field. During the intervention, the treatment loop was initially placed over the point of maximum pain on the patient’s lower back (so called lumbar region) for 3 min, and then subsequently placed for up to 9 min. in the same position.
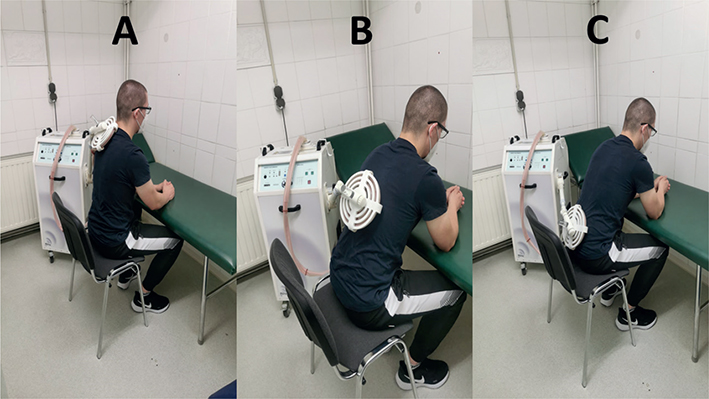
Fig. 3. Typical body position and techniques for the application of electromagnetic induction’s in patients with back pain. (A) Upper back. (B) Middle back. (C) Lower back.
Statistical analysis
The Kolmogorov–Smirnov test was used to analyse the variable distributions. All quantitative data have a normal distribution, and therefore they were expressed by their means (SDs). For VAS results, data were expressed as means (SDs) and 95% confidence interval (95% CI). All vital signs were measured throughout the study. However, only some selected data will be presented. ChangeVAS for V1 (ChangeV1a–b) and V3 (ChangeV3a–b), and ChangeData between V3a and V1b (ChangeV3a–V1b) for the remaining data were calculated. Categorical data were expressed as percentages. The Student t-test was used for comparison between the 2 groups (i.e. TG vs CG) for the (i) V3a; (ii) same intervention (ChangeV1a–b or ChangeV3a–b); and (iii) ChangeV3a–V1b. The Student t-test was also used to compare for the same group between 2 visits (i.e. V1b vs V3a; V1b vs V1a; V3b vs V3a; V3a vs V1b). Hedge’s ChangeV3a–V1bVAS value was used for effect size measurement (33). Effect sizes ≤ 0.2, approximately 0.5, approximately 0.8, and > 1.30 were described as small, medium, large, and very large, respectively (34). All mathematical computations and statistical procedures were performed using statistical software (Statistica, version 10; StatSoft, Inc., 2011, France). Significance was set at 0.05.
RESULTS
Among the 129 patients assessed for eligibility, only 78 were included in the initial sample (Fig. 1). They were allocated to the TG (n = 39) and the CG (n = 39). During follow-up, 8 patients from the TG and 9 patients from the CG discontinued the intervention. Therefore, the final TG and CG included 31 and 30 patients, respectively.
Table II describes the initial (i.e. at V0) characteristics of the 2 groups.
Table III shows the vital parameters of the 2 groups at V1b and V3a. During V3a, the 2 groups had comparable mean values of SBP, DBP, SpO2, and perfusion index, but the TG, compared with the CG, had a higher heart rate. In both groups (i.e. TG or CG), the mean values of SBP, DBP, heart-rate, SpO2, and perfusion-index were comparable between V1b and V3a.
Fig. 4 illustrates the mean ChangeV1a–b (Fig. 4A), ChangeV3a–b (Fig. 4B), and ChangeV3a–1b (Fig. 4C) of the 2 groups VAS (data were mean (SD) (95% CI)). The main findings were:
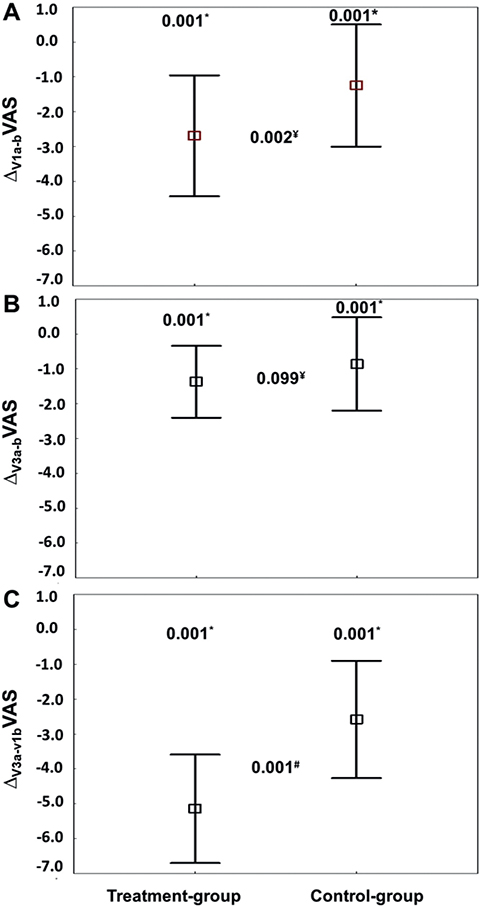
Fig. 4. Pain (visual analogue scale; VAS), at (A) the first intervention (V1), (B) the third intervention (V3), and (C) between “before V1 (V1b)” and “after V3 (V3a)” of the treatment (n = 31) and control (n = 30) groups. V1a: after the first intervention; V1b: before the first intervention; V3a:after the third intervention; V3b: before the third intervention. ChangeV1a–b = value at V1a minus value at V1b. ChangeV3a–b = value at V3a minus value at V3b. ChangeV3a–V1b = value at V3a minus value at V1b. Data were mean (95% confidence interval; 95% CI). p-values: Student t-test. *< 0.05: comparison before (b) vs after (a) (V1b vs V1a; V3b vs V3a; V3a vs V1b) for the same group. ¥< 0.05: comparison for the same intervention (ChangeV1a–b or ChangeV3a–b) between the 2 groups. #< 0.05: comparison of ChangeV3a–V1b between the 2 groups.
- Compared with V1b, at V1a VAS decreased significantly by –2.69 (1.74) (95% CI –3.33 to –2.06) (i.e. from 6.50 (1.41) (95% CI 5.98–7.02) to 3.81 (1.55) (95% CI 3.24–4.38), p = 0.001, respectively) in the TG, and by –1.25 (1.76) (95% CI –1.91 to –0.59) (i.e. from 6.02 (1.67) (95% CI 5.39–6.64) to 4.77 (2.40) (95% CI 3.87–5.66), p = 0.023, respectively) in the CG. The decrease was more marked in the TG (p = 0.002, Fig. 4A).
- Compared with V3b, at V3a VAS decreased significantly by –1.37 (1.03) (95% CI –1.75 to –0.99) (i.e. from 2.73 (1.20) (95% CI 2.26–3.17) to 1.36 (0.90) (95% CI 1.03–1.68), p = 0.001, respectively) in the TG. In the CG, VAS at V3b and V3a were comparable: the change was –0.86 (1.34) (95% CI –1.36 to –0.36) (i.e. from 4.20 (2.29) (95% CI 3.44–5.15) to 3.43 (2.16) (95% CI 2.59–4.28), p = 0.148, respectively). The decrease was similar between the 2 groups (p = 0.099) (Fig. 4B).
- Compared with V1b, at V3a VAS decreased significantly by –5.15 (1.56) (95% CI –5.72 to –4.57, p = 0.001) in the TG, and by –2.58 (1.68) (95% CI –3.21 to –1.96, p = 0.030) in the CG. The decrease was significantly marked in the TG (p = 0.001) (Fig. 4C). The ChangeV3a–V1bVAS effect size was very large (Hedges’ unbiased d = 1.566).
DISCUSSION
With the EMI application presented here, this study showed that short (50 µs) high-frequency (0.3–250 MHz) and high-energy (50 J), but non-thermal, electromagnetic pulses with a magnetic flux density of approximately 50–100 milli-Tesla can reduce pain in the treatment area in the TG.
Explanations for pain reduction using electrical stimuli range from a direct influence on cell membrane potentials through a central increase in β-endorphin (5) to a local effect on microcirculation and therefore on the supply of the tissue with oxygen and vital substrates (6, 35). Pain is a classic sign of inflammation, and sensitization by primary sensory neurones is a major mediation mechanism, transmitted mainly through a system of primary sensory neurones (35). Pharmacological control of inflammatory pain is based on 2 main strategies: (i) non-steroidal anti-inflammatory drugs targeting the inhibition of prostaglandin production, and (ii) opioids and dipyrone, which directly block nociceptor sensitization by activating the nitric oxide signalling pathway (35). A significant number of human diseases have an inflammatory component, and an important mediator of immune activation and inflammation is nitric oxide (20). Thus, back pain tends to be very complex, often with multiple causes or combinations (6, 35, 36). The nitric oxide/cyclic guanosine monophosphate signalling pathway is described in Appendix S1.
Principles of electromagnetic induction and examples of its medical application
The principles of EMI were first described by Faraday in August 1831 (7). Faraday’s law of induction is a basic law of electromagnetism that estimates the time varying magnetic field interacting with an electric circuit to produce an electromotive force (16). The law states that the induced electromotive force in any closed circuit is equal to the rate of change of the magnetic flux enclosed by the circuit (16). The strength of the magnetic field is proportional to the electric current strength of the coil (7, 16, 36). The induction voltage depends on the speed and strength of the change in the magnetic field, and on the structure and the line thickness of the coil (7, 36). The more turns a coil has, the stronger is the magnetic field it can generate. Similar to an induction coil in an induction hob, alternating current flows through the treatment loop and causes a rapidly changing magnetic field (7, 36). If the copper wire of the treatment loop is thicker, a more energetic magnetic field is built-up in the loop (7, 36). In turn, a more energetic magnetic field enables the induction of a more powerful electric field in the tissue (37).
An electromagnetic field does not generate heat in the human body (7, 37, 38). However, if the induction hits a metallic object, such as the metallic base of a pot on an induction hob, or an endoprosthesis, or a pacemaker in the human body, then the alternating magnetic field causes an electric voltage in this metallic object, which, in turn, creates an induction current that is converted into heat (7, 37, 38). Electromagnetic waves are made up of coupled electric and magnetic fields (35, 38, 39). Unlike sound waves, electromagnetic waves do not need a medium to propagate. They move in a vacuum at the speed of light, regardless of their frequency. Thus, an electromagnetic wave can always serve as a vehicle to transmit electricity into an organism without “resistance” or “loss of energy” (8). This “resistance” is well-known for a conventional, sometimes painful, application of electricity through the skin (8). The application of electromagnetic waves is normally painless (magnetic induction of electricity), even in full clothing. It is applicable in all body regions, feasible in all body positions, and always possible without any tangled cables (39).
Most of the examples with regard to the application of EMI come from neurology (36, 40–43). Work is carried out with the so-called “transcranial magnetic stimulation” (42, 43). A magnetic coil placed tangentially on the skull generates a short magnetic field of 200–600 µs duration with a magnetic flux density of up to 2–3 Tesla. The current in the coil reaches more than 15,000 A. The resulting change in electric potential in the cerebral cortex near the skull causes a depolarization of neurones with triggering of action potentials (36, 42, 43). Studies carried out to date have shown results with a high level of evidence for various applications in the field of neurology (40, 41). Examples of the use of EMI in other medical specialties have, so far, been rare but promising (16, 30). It is also important to establish that these new effective treatment modalities have minimal side-effects.
Discussion of results
The rapid response in the TG was remarkably similar to the results reported in some previous studies (8, 18, 23, 25, 29, 44). First, in a randomized, double-blind, placebo-controlled study, Nelson et al. (23) reported that high-frequency PEMF reduces knee pain (by almost 60% within 3 days) in patients with early-stage knee osteoarthritis. The PEMF signal used consisted of a 7 ms burst of 6.8 MHz sinusoidal waves repeating at 1 burst/s, and an induced electrical peak field of 34 ± 8 V/m. Secondly, Lee et al. (29) observed a 38% reduction in the mean NRS pain score for back pain in the TG (i.e. PEMF group), and a 12% reduction in the CG after the first week with 3 applications per week (29). The induced electromagnetic pulses were 2-phased with a pulse width of 270 μs, a frequency of 5–10 Hz, and a magnetic flux density of 1.3–2.1 Tesla. Other authors reported some effects starting comparably quickly (18, 25, 44). First, Rohde et al. (25) reported an approximately 2.5-fold reduction in pain from breast reduction surgery within 5 h post-operation. The above study also noted that interleukin-1b (i.e. a master inflammatory cytokine) is concomitantly reduced by approximately a 2.5-fold in the wound bed (25). Secondly, Fitzsimmons et al. (44) investigated the effects of pulsed electric fields (10 ms bursts at 4,150 MHz) on chondrocytes in vitro. The applied current generates electric fields of approximately 0.1–10 mV/cm in the tissue (44). Thirty minutes after PEF, the nitric oxide content in the nutrient media was approximately 150% compared with the controls, similar to the DNA increase in the chondrocytes after 72 h (44). Thirdly, Bragin et al. (18) provided the first evidence of the acute effects of PEMF on microvascular blood-flow and cortical metabolism. A 30-min PEMF treatment resulted in dilation of the cerebral arterioles, leading to an increase in microvascular blood flow and tissue oxygenation that persisted for at least 3 h (18). Bragin et al. (18) concluded that nitric oxide mediates the effects of PEMF, and they suggested that PEMF is an effective treatment after traumatic or ischaemic brain injury (18). Using the EMI application presented in the current study, the delivered high-frequency and high-energy electromagnetic pulses exceeding 50 J contributed to a significant pain relief (18). However, the current results also identified a strong placebo effect with a significant improvement in pain reduction in the CG as well. At the end of the study, however, a significantly greater improvement was noted in the TG during intergroup comparisons. Such effects are well-documented in placebo research (5), and can also be found in studies on the effects of electromagnetic impulses on pain in the back or other parts of the body (23, 29, 45, 46).
In the current study, measurements of SpO2 and tissue perfusion did not provide conclusive results. This is may be due to the applied measurement method and the selected measurement location (i.e. index finger), which were anatomically far removed from the location of the EMI intervention. However, the measurement method applied in the current study was well suited for recording systemic influences on the microcirculation or, as in the present case, for excluding them (47, 48). Peripheral non-invasive transcutaneous measurements of oxygen saturation and tissue perfusion are used for the early assessment of the peripheral effects of central organ functions or external influencing factors (47, 48).
As already suspected and described, locally applied EMI was more likely to be responsible for an assumed CaM-dependent nitric oxide signalling for pain reduction (49–51). According to the current study hypothesis, the externally induced local EMI impulse caused significant endothelial nitric oxide excretion, followed by local vascular relaxation and locally increased blood flow and downregulation of the inflammatory cascade (49–51). Thus, the current findings on peripheral microcirculation are understandable, and they confirm that EMI has no systemic influence. The observed decreases in SBP (i.e. by 4 ± 10 mmHg in the TG, and in DBP by 5 ± 8 mmHg in the CG, Table III) are not statistically significant and have no clinical importance, since there are lower than the recommended minimal clinically important difference (52).
Compared with EMI in neurology (40–43), the design (structure and line thickness) of the coil used in the current study (36, 43) allows a higher induction power (> 50 J) or electric field strength of at least 20 V/m (coil with 2 loops directly on the body surface) (33) with a short pulse duration of approximately 50 µs/pulse. The treatment with short-pulsed (µs and ns) electric fields/pulses has developed into a promising technique having effects at the plasma membrane level, intracellular elements, as well as effects on cell survival (14, 15). An emerging non-invasive, non-contact alternative to PEFs is HE-PEMF, in which the electrical field in the tissue is remotely induced by an external pulsed magnetic field (30). Future studies may show the extent to which these properties can also influence treatment with EMI.
Study limitations
The current study has some limitations. First, it was better to opt for an intermediate or long-term follow-up. Secondly, the exclusion of some patients from the statistical evaluation is a source of potential bias (53). It appears that excluding patients from the analysis in randomized trials often results in biased estimates of treatment effects, but the extent and direction of bias is unpredictable (53). Thirdly, it was better to report data on success rates in each body area of the UBP (e.g. middle back (known as thoracic back) or lower back (known as lumbar region)). The fourth limitation concerns the form of the current RCT. In clinical practice, the data analysis of an RCT can be performed by using 2 complementary strategies, i.e. according to the intention to treat (ITT) principle and the per protocol (PP) analysis (54, 55). In order to investigate the effect of receiving the assigned treatment as specified in the protocol, we opted for the PP analysis (54, 55). However, it was better to use the ITT analysis to assess the effect of assigning a drug, to report its superiority, and to demonstrate conclusive effect (54, 55). Both ITT and PP analyses are essentially valid, but they have different scopes and interpretations dependent on the context (54, 55). Future larger and longer-term studies, should opt for an ITT analysis (54, 55). Fifthly, whether patients knew which group they were randomized to is a possible potential confounder. In practice, the report of information on the percentage of each group who believed they received the actual treatment would be helpful in supporting difference from placebo. However, despite the fact that the current intervention is less commonly used, the current sham intervention is “good enough” to mimic real treatment. The other limitations are the exclusive use of the NRS scale, the absence of other measurement methods for evaluating the microcirculation, and the short intervention time. Concerning the generalizability (e.g. external validity, applicability) of the current results, these findings can only be transferred to 1 population with corresponding exclusion and inclusion criteria. However, it makes sense to further investigate the use of EMI, as few or no side-effects have occurred in clinical use. If the EMI method proves successful in future, new treatment regimens with expanded treatment options could be created for both doctors and patients.
CONCLUSION
The rapid effect of EMI therapy on UBP in the current RCT is promising enough to warrant further and larger studies into the effect of EMI on pain in other indications. The current results suggest that the release of nitric oxide was part of the local biochemical metabolic pathway. Thus, follow-up examinations should also include parameters of microcirculation as well as markers of inflammation in the serum. It is therefore recommended to test whether and to what extent EMI signals can affect other parts of the body and organs.
REFERENCES
- Raspe H. Gesundheitsberichterstattung des Bundes. Rückenschmerzen 2012: 53.
- Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet 2017; 389: 736–747.
- Altinbilek T, Murat S. A comparison of application frequency of physical therapy modalities in patients with chronic mechanical low back pain. Turk J Phys Med Rehabil 2020; 66: 201–209.
- Tiktinsky R, Chen L, Narayan P. Electrotherapy: yesterday, today and tomorrow. Haemophilia 2010; 16: 126–131.
- Bender T, Nagy G, Barna I, Tefner I, Kadas E, Geher P. The effect of physical therapy on beta-endorphin levels. Eur J Appl Physiol 2007; 100: 371–382.
- Xu X, Zhang H, Yan Y, Wang J, Guo L. Effects of electrical stimulation on skin surface. Acta Mech Sin 2021; 37: 1843–1871.
- Faraday M. Experimental researches in electricity. In: London RSo, editor. Philosophical transactions of the Royal Society of London. London: Taylor; 1844, p. 331.
- Markov MS. Magnetic and electromagnetic field therapy: basic principles of application for pain relief. In: Bioelectromagnetic medicine. 1st edition. Boca Raton: ImprintCRC Press; 2004.
- Johnson MI. Resolving long-standing uncertainty about the clinical efficacy of transcutaneous electrical nerve stimulation (TENS) to relieve pain: a comprehensive review of factors influencing outcome. Medicina (Kaunas) 2021; 57.
- Ropero Pelaez FJ, Taniguchi S. The gate theory of pain revisited: modeling different pain conditions with a parsimonious neurocomputational model. Neural Plast 2016; 2016: 4131395.
- King EW, Audette K, Athman GA, Nguyen OXH, Sluka KA, Fairbanks CA. Transcutaneous electrical nerve stimulation activates peripherally located alpha-2A adrenergic receptors. Pain 2005; 115: 364–373.
- Hanna H, Denzi A, Liberti M, Andre FM, Mir LM. Electropermeabilization of inner and outer cell membranes with microsecond pulsed electric fields: quantitative study with calcium ions. Sci Rep 2017; 7: 13079.
- Miklavcic D, Novickij V, Kranjc M, Polajzer T, Haberl Meglic S, Batista Napotnik T, et al. Contactless electroporation induced by high intensity pulsed electromagnetic fields via distributed nanoelectrodes. Bioelectrochemistry 2020; 132: 107440.
- Batista Napotnik T, Rebersek M, Vernier PT, Mali B, Miklavcic D. Effects of high voltage nanosecond electric pulses on eukaryotic cells (in vitro): a systematic review. Bioelectrochemistry 2016; 110: 1–12.
- Chopinet L, Rols MP. Nanosecond electric pulses: a mini-review of the present state of the art. Bioelectrochemistry 2015; 103: 2–6.
- Gaynor JS, Hagberg S, Gurfein BT. Veterinary applications of pulsed electromagnetic field therapy. Res Vet Sci 2018; 119: 1–8.
- Pilla AA. Electromagnetic fields instantaneously modulate nitric oxide signaling in challenged biological systems. Biochem Biophys Res Commun 2012; 426: 330–333.
- Bragin DE, Statom GL, Hagberg S, Nemoto EM. Increases in microvascular perfusion and tissue oxygenation via pulsed electromagnetic fields in the healthy rat brain. J Neurosurg 2015; 122: 1239–1247.
- Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007; 15: 252–259.
- Cinelli MA, Do HT, Miley GP, Silverman RB. Inducible nitric oxide synthase: regulation, structure, and inhibition. Med Res Rev 2020; 40: 158–189.
- Brisby H, Ashley H, Diwan AD. In vivo measurement of facet joint nitric oxide in patients with chronic low back pain. Spine (Phila Pa 1976) 2007; 32: 1488–1492.
- Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol 2012; 10: 4–18.
- Nelson FR, Zvirbulis R, Pilla AA. Non-invasive electromagnetic field therapy produces rapid and substantial pain reduction in early knee osteoarthritis: a randomized double-blind pilot study. Rheumatol Int 2013; 33: 2169–2173.
- Pilla AA. Nonthermal electromagnetic fields: from first messenger to therapeutic applications. Electromagn Biol Med 2013; 32: 123–136.
- Rohde C, Chiang A, Adipoju O, Casper D, Pilla AA. Effects of pulsed electromagnetic fields on interleukin-1 beta and postoperative pain: a double-blind, placebo-controlled, pilot study in breast reduction patients. Plast Reconstr Surg 2010; 125: 1620–1629.
- Bodewein L, Schmiedchen K, Dechent D, Stunder D, Graefrath D, Winter L, et al. Systematic review on the biological effects of electric, magnetic and electromagnetic fields in the intermediate frequency range (300Hz to 1MHz). Environ Res 2019; 171: 247–259.
- Belyaev I, Dean A, Eger H, Hubmann G, Jandrisovits R, Kern M, et al. EUROPAEM EMF Guideline 2016 for the prevention, diagnosis and treatment of EMF-related health problems and illnesses. Rev Environ Health 2016; 31: 363–397.
- Serhier Z, Bendahhou K, Ben Abdelaziz A, Bennani MO. Fiche Méthodologique n°1: Comment calculer la taille d’un échantillon pour une étude observationnelle? Tunis Med 2020; 98: 1–7.
- Lee PB, Kim YC, Lim YJ, Lee CJ, Choi SS, Park SH, et al. Efficacy of pulsed electromagnetic therapy for chronic lower back pain: a randomized, double-blind, placebo-controlled study. J Int Med Res 2006; 34: 160–167.
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog scale for pain (VAS Pain), numeric rating scale for pain (NRS Pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res (Hoboken) 2011; 63: S240–252.
- Raspe H. Back pain. Berlin: Robert Koch Institute; 2012.
- Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber-Moffett J, Kovacs F, et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J 2006; 15: S192–300.
- Bonelli M, Moroder E. Non-ionizing radiation: evaluation of action values in the case of complex spectrum pulsed electromagnetic fields, AIRP – Proceedings of the national convention on radiation protection “Radiation protection in the health sector”: Bolzano, 2010.
- Olejnik S, Algina J. Measures of effect size for comparative studies: Applications, interpretations, and limitations. Contemp Educ Psychol 2000; 25: 241–286.
- Gomes FIF, Cunha FQ, Cunha TM. Peripheral nitric oxide signaling directly blocks inflammatory pain. Biochem Pharmacol 2020; 176: 113862.
- Post A, Muller MB, Engelmann M, Keck ME. Repetitive transcranial magnetic stimulation in rats: evidence for a neuroprotective effect in vitro and in vivo. Eur J Neurosci 1999; 11: 3247–3254.
- Nerreter W. Grundlagen der elektrotechnik. mit micro-cap und MATLAB. GmbH & Co. KG: Hanser-Verlag; 2020.
- Ulaby F. Fundamentals of applied electromagnetics. 5th edition. USA: Pearson Prentice Hall; 2007.
- Eschweiler G, Plewnia C, Bartels M. Gemeinsamkeiten und Unterschiede der therapeutischen transkraniellen Magnetstimulation und der Elektrokrampftherapie. Nervenheilkunde 2003; 22: 189–195.
- Leon Ruiz M, Rodriguez Sarasa ML, Sanjuan Rodriguez L, Benito-Leon J, Garcia-Albea Ristol E, Arce Arce S. Current evidence on transcranial magnetic stimulation and its potential usefulness in post-stroke neurorehabilitation: opening new doors to the treatment of cerebrovascular disease. Neurologia (Engl Ed) 2018; 33: 459–472.
- Schambra HM. Repetitive transcranial magnetic stimulation for upper extremity motor recovery: does it help? Curr Neurol Neurosci Rep 2018; 18: 97.
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 2001; 21: RC157.
- Wang Y, Liu Z, Ge X, Hu X, Cao X, Li L, et al. Neuropathic pain releasing calcitonin gene related peptide protects against stroke in rats. Am J Transl Res 2020; 12: 54–69.
- Fitzsimmons RJ, Gordon SL, Kronberg J, Ganey T, Pilla AA. A pulsing electric field (PEF) increases human chondrocyte proliferation through a transduction pathway involving nitric oxide signaling. J Orthop Res 2008; 26: 854–859.
- Elshiwi AM, Hamada HA, Mosaad D, Ragab IMA, Koura GM, Alrawaili SM. Effect of pulsed electromagnetic field on nonspecific low back pain patients: a randomized controlled trial. Braz J Phys Ther 2019; 23: 244–249.
- Lisi AJ, Scheinowitz M, Saporito R, Onorato A. A pulsed electromagnetic field therapy device for non-specific low back pain: a pilot randomized controlled trial. Pain Ther 2019; 8: 133–140.
- Lima A, Bakker J. Noninvasive monitoring of peripheral perfusion. Intensive Care Med 2005; 31: 1316–1326.
- Nitzan M, Romem A, Koppel R. Pulse oximetry: fundamentals and technology update. Med Devices (Auckl) 2014; 7: 231–239.
- Adams JA, Uryash A, Lopez JR, Sackner MA. The endothelium as a therapeutic target in diabetes: a narrative review and perspective. Front Physiol 2021; 12: 638491.
- Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 2012; 33: 829–837, 837a–837d.
- Melikian N, Seddon MD, Casadei B, Chowienczyk PJ, Shah AM. Neuronal nitric oxide synthase and human vascular regulation. Trends Cardiovasc Med 2009; 19: 256–262.
- Chan LS. Minimal clinically important difference (MCID) – adding meaning to statistical inference. Am J Public Health 2013; 103: e24–25.
- Nuesch E, Trelle S, Reichenbach S, Rutjes AW, Burgi E, Scherer M, et al. The effects of excluding patients from the analysis in randomised controlled trials: meta-epidemiological study. BMJ 2009; 339: b3244.
- McCoy CE. Understanding the intention-to-treat principle in randomized controlled trials. West J Emerg Med 2017; 18: 1075–1078.
- Tripepi G, Chesnaye NC, Dekker FW, Zoccali C, Jager KJ. Intention to treat and per protocol analysis in clinical trials. Nephrology (Carlton) 2020; 25: 513–517.