ORIGINAL REPORT
INFLUENCE OF MANUAL DIAPHRAGM RELEASE TECHNIQUE COMBINED WITH INSPIRATORY MUSCLE TRAINING ON SELECTED PERSISTENT SYMPTOMS IN MEN WITH POST-COVID-19 SYNDROME: A RANDOMIZED CONTROLLED TRIAL
Ebtesam N. NAGY, PhD1, Doaa A. ELIMY, PhD2*, Ahmed Y. ALI, MD, PhD3,4, Hieba G. EZZELREGAL, MD, PhD5 and Marwa M. ELSAYED, PhD1
1Lecturer at Physical Therapy Department for Cardiovascular/Respiratory Disorder and Geriatrics, Faculty of Physical Therapy, Cairo University, Giza, Egypt, 2Lecturer at Physical Therapy Department for Basic Science, Faculty of Physical Therapy, Cairo University, Giza, Egypt, 3Assistant Professor at Internal Medicine Department, Faculty of Medicine, Cairo University, Giza, Egypt, 4Assistance Professor at Internal Medicine Department, Armed Forces College of Medicine, Cairo, Egypt, 5Assistant Professor at Chest Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Objective: To determine whether the addition of manual diaphragm release to an inspiratory muscle training programme is more effective than inspiratory muscle training alone in reducing blood pressure, dyspnoea, fatigue, and aerobic performance capacity in men with post-COVID-19 syndrome.
Design: A prospective, randomized-controlled trial.
Setting: Chest Disease Department, Outpatient Clinic, Cairo University, Egypt.
Participants: Fifty-two men with post-COVID-19 syndrome were allocated randomly to the study and control groups.
Intervention: The study group underwent diaphragm release plus inspiratory muscle training, whereas the control group received inspiratory muscle training only.
Outcome measures: All patients were assessed with the following measures at baseline and 6 weeks post-intervention: maximum static inspiratory pressure for inspiratory muscle strength, peripheral arterial blood pressure, Modified Medical Research Council scale for dyspnoea, Fatigue Severity Scale, serum lactate level, and 6-min walk test distance for aerobic performance.
Results: All outcome measures showed a significant improvement in favour of the study group (p < 0.001) over the control group. However, maximum static inspiratory pressure increased significantly, by 48.17% (p < 0.001) in the study group with no significant change in the control group.
Conclusion: Addition of manual diaphragm release to an inspiratory muscle training programme potentiates the role of inspiratory muscle training in the management of men with symptomatic post-COVID-19 syndrome.
LAY ABSTRACT
Patients with post-COVID-19 syndrome may experience a variety of symptoms that limit their ability to perform daily activities, such as breathing difficulties, diaphragmatic weakness, cardio-vascular abnormalities, fatigue, and intolerance to physical exercise. These problems may be resolved by physical therapy interventions, which may also prevent further decline. The aim of this study was to investigate the impact of adding a specific physiotherapy technique (manual diaphragm release) to inspiratory muscle training, delivered via a POWERbreath (PowerBreathe, IMT International Ltd. Southam, Warwickshire; England UK) on inspiratory muscle strength, blood pressure, dyspnoea, fatigue, lactate level, and aerobic performance capability in men with post-COVID-19 syndrome. A total of 52 men with post-COVID-19 syndrome were enrolled, and all completed the study. Twenty-six patients underwent diaphragm release plus inspiratory muscle training, whereas the other 26 received inspiratory muscle training only. The prescribed training lasted for 6 weeks. The results showed positive effects on the investigated parameters of adding the diaphragm release technique to inspiratory muscle training among these patients.
Key words: aerobic performance; COVID-19; diaphragm; dyspnoea; fatigue; maximum static inspiratory pressure.
Citation: J Rehabil Med 2022; 54: jrm00330. DOI: https://dx.doi.org/10.2340/jrm.v54.3972
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Aug 3, 2022; Epub ahead of print: Sep 19, 2022; Published: Oct 20, 2022
*Correspondence address: Doaa A. Elimy, Physical Therapy for Basic Science Department, Faculty of Physical Therapy, Cairo University, 7 Ahmed El-zyat Street, Dokki, Giza, PO 12613, Egypt. E-mail: D.Ayoub@cu.edu.eg
Competing interests and funding: The authors have no conflicts of interest to declare.
Coronavirus disease 2019 (COVID-19), or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1), is a worldwide pandemic affecting primarily the lungs, with rapid dissemination (2). The clinical symptoms range from asymptomatic to severe respiratory involvement, which can lead to respiratory failure (2) and potentially fatal pulmonary or extrapulmonary complications, particularly in patients with comorbidities such as hypertension, obesity, and diabetes (3).
Furthermore, surviving patients may experience long-term symptoms (post-acute sequelae of COVID-19) that last for days to months after the acute symptoms have subsided, such as cough, shortness of breath (4), hypoxia, depression, sleeping disorders (5), cognitive disturbance, and cardiovascular insults (6), which interfere with their functional activities.
The SARS-CoV-2 virus uses the angiotensin-converting enzyme 2 (ACE2) as an entrance receptor into pulmonary alveolar cells as well as skeletal muscles (7). The major muscle of respiration, the diaphragm, is one of the most important skeletal muscles influencing lung capacity and respiratory efficiency (8).
The majority of COVID-19 post-acute sequelae are related to diaphragm and lung dysfunction caused by a variety of factors, such as post-mechanical ventilation acquired respiratory muscle weakness (9), underlying neuromuscular effects (10), cellular damage, and a strong immune system response, which, in turn, results in elevated lactate levels due to poor oxygen diffusion in not fully recovered lungs, leading to limitations in physical functioning (11).
A comprehensive physical therapy rehabilitation approach is necessary to counter post-acute sequelae, by improving respiratory muscle function and quality of life as well as preventing further deterioration in gas exchange and other associated symptoms (12).
The International European Respiratory Society (ERS) favoured inspiratory muscle training (IMT) as an additional safe modality to the traditional pulmonary rehabilitation programme, especially for management of inspiratory muscle weakness (13). A recent study evaluated the impact of IMT on improving respiratory muscle strength and reducing dyspnoea in patients with COVID-19 (14) as well as reducing blood lactate concentration in healthy untrained individuals (15).
Direct stretching of the diaphragm using a manual diaphragm release (DR) technique promotes improvement in diaphragmatic contraction (16), pulmonary function, dyspnoea, and exercise capacity (17).
Despite the significant effect of COVID-19 on the diaphragm muscle and the benefits of the manual DR technique, the implications of using this technique in rehabilitation programs for post-COVID-19 patients have not been evaluated.
There is a lack of data regarding the combined effect of DR and IMT on post-COVID-19 syndrome persistent symptoms. We hypothesized that combining manual DR and IMT would provide a safe and effective strategy for managing dyspnoea and fatigue, which are common persistent symptoms in patients with post-COVID-19 syndrome. The aim of this study was therefore to evaluate the combined effect of manual DR and IMT compared with IMT alone on selected parameters (blood pressure, dyspnoea, high serum lactate, and fatigue levels) in men with post-acute sequelae of COVID-19 syndrome.
METHODS
Study design and setting
This 6-week, single-blinded randomized controlled trial (RCT) evaluated the combined effect of manual DR and IMT (PowerBreathe, IMT International Ltd. Southam, Warwickshire; England UK) in men with post-COVID-19 syndrome. The study commenced in August 2021 and ended in April 2022. Subjects were recruited from El Kasr-El Ainy, Chest Disease Department outpatient clinic, Cairo University, Egypt. Written informed consent was obtained from each patient. Subjects were randomized 1:1 by an independent statistician into a study group (n = 30) or a control group (n = 30) after explanation of the study details. For randomization, each patient picked an opaque sealed envelope, numbered sequentially by a researcher who was not involved in the study. The research team was aware of the allocation, while the participants and outcome analyser were blinded to the group assignment.
The study group received manual DR and IMT (POWERbreath) in addition to their prescribed medications, while the control group received IMT only, using the POWERbreath device, in addition to their prescribed medications (Fig. 1). After approval by the Faculty of Physical Therapy’s ethics committee board (number P.T.REC/012/003228) and registration with ClinicalTrials.gov (NCT04919031), all procedures were carried out in accordance with the principles of the Declaration of Helsinki and Consolidated Standards of Reporting Trials (CONSORT).
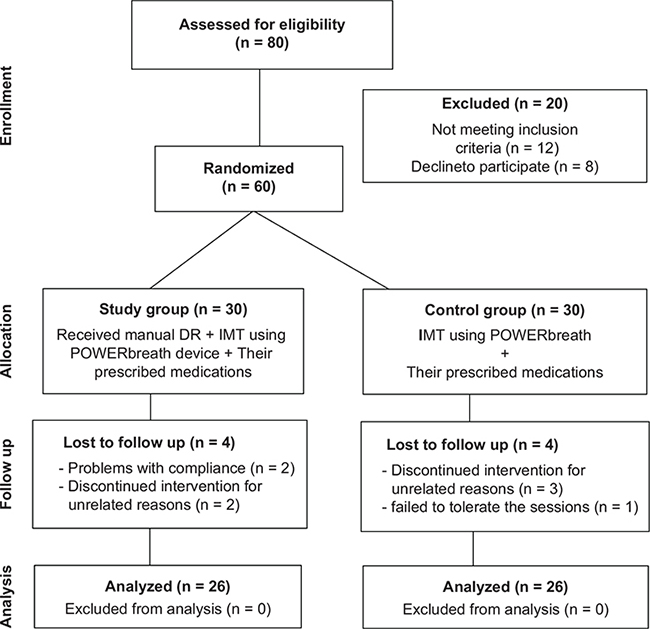
Fig. 1. Flow chart of the participants (allocation, intervention, and follow-up) according to Consolidated Standards of Reporting Trials (CONSORT) principles, with only 52 participants completing the study. DR: diaphragm release; IMT: inspiratory muscle training.
Sample size
G*Power version 3.1.9.2, Franz Faul, Uni Kiel software (http://www.gpower.hhu.de/) was used for sample size estimation of the main outcome, Maximum static inspiratory pressure (PImax), based on a pilot study of 20 men with post-COVID-19 syndrome with an effect size (d) = 0.80 at a 5% level of significance and a power of 80% (partial eta-squared (2P), within-between interaction) that determined a final sample size of 30 individuals per group with an anticipated 12% dropout rate.
Eligibility criteria
Patients with the following eligibility criteria were included in the study:
- low to moderate physical activity, according to the International Physical Activity Questionnaire (IPAQ) (18);
- mild to moderate lung fibrosis diagnosed by a physician and confirmed by high-resolution chest computed tomography (CT) (19);
- hypertension at stage II (160–179/100–109 mmHg);
- stable and non-hospitalized after COVID-19;
- men aged 30–45 years;
- body mass index (BMI) in the range 25–29.9 kg/m2;
- at least 4 weeks since the first positive COVID-19 swab at the time of screening (20).
Patients were excluded if they had: cardiac disease, chronic respiratory disease, active infection, severe endocrine or metabolic diseases, cognitive impairment, other disabilities that interfered with the intervention, or other reasons determined by the physician that made the participants ineligible for participation. Further exclusion criteria were: red flag indicators, such as chest pain, critical drop in oxygen saturation, musculoskeletal or neurological limitations; unconscious patients; other previous comorbidities, besides hypertension or positive COVID-19 test; and participation in a clinical study or other research in the previous 30 days.
A total of 80 non-hospitalized men with post-COVID-19 syndrome were recruited, immediately after hospital discharge (their length of hospital stay was (interquartile range (IQR) 7–10) days), with 20 patients excluded (12 did not meet the inclusion criteria, 8 declined to participate). Sixty patients were randomly assigned to the study (n = 30) and control groups (n = 30), but only 52 (26 patients in each group) completed the study and were included in the data analysis (8 patients dropped out; 6 did not attend follow up and 2 discontinued for unrelated reasons) (Fig. 1). Dropout reasons were reported, and data was analysed using the intention-to-treat (ITT) principle (21). During their involvement in the study all subjects were instructed not to change their pharmaceutical therapy (unless it was considered by the researcher that it would not affect the results).
The length of the patients’ hospital stays for acute COVID infection was 7–10 days (IQR). All patients were treated for 1–3 weeks (IQR 7–27 days) according to the certified post-discharge medication protocol recommended by El Kasr-El Ainy, Chest Disease Department outpatient clinic, Cairo University, Egypt, as following: corticosteroid (prednisolone 20 mg for 1 week, then reduced to 5 mg for an additional 5 days), apixaban 2.5 mg for six weeks, pantoprazole 40 mg for nine days, colchicine 0.5 mg twice daily for 3 days, then once daily for 27 days, zinc (1 capsule daily for 21 days), and vitamin C (75–90 mg daily for 21 days).
Measurements
The following parameters were assessed at baseline and at the end of the intervention, after gathering basic information (sex, age, marital status, family history, co-morbidities, physical anthropometry indexes (BMI, heart rate (HR), blood pressure, etc.) and all participants were screened to ensure that they met the inclusion criteria.
Primary outcome
Maximum static inspiratory pressure. The inspiratory muscle strength was assessed by measuring the PImax via a valid hand-held mouth pressure meter (PowerBreathe, IMT International Ltd. Southam, Warwickshire; England UK) in which the participant stood in an upright position, took a deep inspiration starting from the residual volume through the mouthpiece, and held the inspiration for 20–30 s for 5 trials, and the highest PImax (cmH2O) was recorded (22). At baseline and at the final training session, the same research member took measurements for both groups.
Secondary outcomes
Modified Medical Research Council scale. The self-rating Modified Medical Research Council (MMRC) scale measured the degree of breathlessness that poses a challenge during activities of daily living, scoring from 0 (no breathlessness except during strenuous exercise) to 4 (too breathless when dressing or moving) (23).
Six-minute walk test distance. The participants’ aerobic performance capacity was assessed by submaximal exercise testing (6-min walk test distance; 6-MWTD) in which the walked distance in 6 min along an indoor flat 35-m corridor was calculated and interpreted (poor prognosis if the walked distance is 300 m or less) (24).
Arterial blood pressure. Resting systolic and diastolic blood pressures (mmHg) were measured (at 08.00–09.00 AM) with a mercury sphygmomanometer (Yuwell, Yunyang Industrial Park, Danyang city, Jiangsu, P.R. China, 212300) in which the cuff was wrapped around the participant’s left arm. The participant was recommended not to smoke, eat, or to drink caffeine, and to avoid stress, for at least 2 h before measuring blood pressure.
Fatigue Severity Scale. The impact of fatigue was evaluated with the Fatigue Severity Scale (FSS), which comprises 9 statements rated on a scale of 1 to 7, with the patient either agreeing or disagreeing. Low values indicated strong disagreement, while the highest values indicated strong agreement. The overall score of less than 36 represented the best possible score, while 36 or more was the worst (25).
Serum lactate level. A blood sample (5 ml) was drawn from the right antecubital vein, collected in a vial at 09.00–11.00 h and stored on ice to be analysed in a central laboratory using a lactate oxidase catalysed reaction (Cobas Integra 800, Roche Diagnostics Mannheim city, Baden-Württemberg state, Germany). A normal blood lactate level is 0.5–1 mmol/L, and hyperlactataemia without metabolic acidosis occurs when the lactate level remains mildly to moderately elevated and gradually increases to 2–4 mmol/L (26).
Intervention
Manual DR method. Only 1 researcher (a physiotherapist) who had 12 years of experience treating respiratory patients applied this technique. The participants in the study group received only 18 sessions of manual DR, (3 sessions/week) for 6 weeks, in which the application was performed in 2 sets of 10 deep breaths, with a 1-min interval between them and the DR session was over 3 min long.
The participant was instructed to relax in a supine position (on the treatment bed) while the researcher stood beyond the subject’s head, applying gentle bilateral upward and lateral pulling up of the participant’s underside seventh to tenth rib costal cartilages (the contact points of the researcher’s hand during the technique were the pisiform, hypothenar region, and the last three fingers). The ribs elevation was applied throughout the participant’s breathing in. As the participant breathed out, the rib out pulling by the researcher went deeper toward the seventh to tenth rib inner costal margin (Fig. 2), which progressively increased over the subsequent sessions (17).
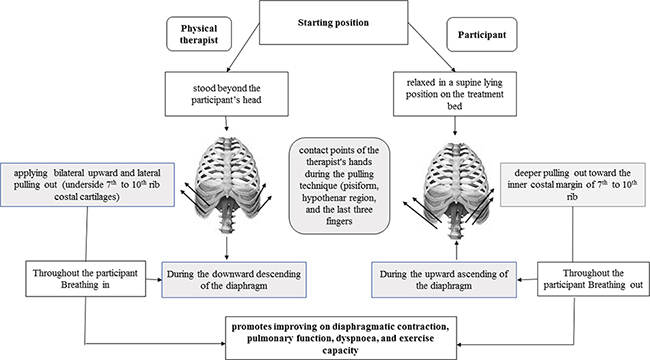
Fig. 2. Schematic diagram summarizing manual diaphragm release (DR) technique sequences and benefits.
Inspiratory muscle training via POWERbreath. After determination of the PImax for each participant, as described above, each participant was instructed to perform 2 sets of 30 dynamic inspiratory efforts (with a 2-min interval between sets) from an upright sitting position with a 4-min session length overall maximally, twice daily with a PImax workload of 60%, (3 sessions/week) for 6 weeks (27).
Since the device provides the ability to modify the applied resistance and repetitions, the training parameters were reassessed and adjusted often to ensure that the PImax workload remained at 60% throughout the trial.
Both groups continued their sessions under supervision at the outpatient clinic, Faculty of Physical Therapy, Cairo University. For the study group participants, the IMT sessions were applied immediately after application of the manual DR session.
Data analysis
All statistical analysis was performed using Statistical Package for Social Sciences (SPSS) Version 25 (IBM Corp., Armonk, NY, USA). The Shapiro–Wilk test was used to analyse the normality of data. For normally distributed data with no significant outliers or median, continuous data was presented as mean±standard deviation (SD), while categorical data was presented as absolute frequencies (N) and percentages (%). Patients, baseline characteristics were analysed by the independent samples t-test (for continuous data) and χ2 tests (for categorical data).
Mixed repeated-measures analysis of variance (ANOVA) with Bonferroni corrections was used to examine the intervention differences between the outcomes (at baseline and after 6 weeks). To analyse the differences between and within the study and control groups, the partial eta-squared (ƞ2p2) effect size was calculated. A paired t-test (pre-and post-intervention changes when ANOVA was significant) was used to investigate within-group effects, while an independent sample t-test was used to determine mean difference (MD) changes between the study and control groups (p-values less than 0.05 were considered statistically significant).
RESULTS
There were no statistically significant differences between groups in participants’ baseline characteristics (p > 0.05) (Table I). There was a 48.17% increase in the study group PImax (from 82.00 (16.86) cmH2o to 121.50 (13.52) cmH2o, p < 0.001), but no significant change in the control group (p = 0.567), with a statistically significant difference between the 2 groups (p < 0.001) (Table II). The interaction between intervention type and time was statistically significant (F (1, 58) = 28.54, p < 0.001, ƞ2 p = 0.330), while the main effect for group was also statistically significant (F (1, 58) = 66.27, p<0.001, ƞ2 p = 0.533).
| Characteristics | Study group (n=26) | Control group (n=26) | X2/t | p-value | |
| Agea (years) | Mean (SD) | 40.00 (3.36) | 39.70 (3.55) | 0.336 | 0.738 |
| Range | 30.00–45.00 | 30.00–45.00 | |||
| BMIa (kg/m²) | Mean (SD) | 27.45 (1.5) | 27.59 (1.22) | –0.407 | 0.686 |
| Range | 25.00–29.90 | 25.40–29.50 | |||
| SBPa (mmHg) | Mean (SD) | 150.00 (5.39) | 150.23 (5.53) | –0.165 | 0.869 |
| Range | 141.00–159.00 | 140.00–158.00 | |||
| DBPa (mmHg) | Mean (SD) | 94.60 (2.31) | 94.80 (2.25) | –0.339 | 0.735 |
| Range | 91.00–98.00 | 91.00–98.00 | |||
| PImaxa (cmH2O) | Mean (SD) | 82.00 (16.86) | 80.50 (17.52) | 0.338 | 0.737 |
| Range | 54.00–110.00 | 53.00–108.00 | |||
| FSSa (score) | Mean (SD) | 43.36 (5.25) | 42.47 (5.18) | 0.661 | 0.511 |
| Range | 34.90–51.82 | 33.94–51.00 | |||
| MMRC scalea (score) | Mean (SD) | 2.63 (0.60) | 2.42 (0.49) | 1.520 | 0.134 |
| Range | 1.84–3.42 | 1.63–3.20 | |||
| Serum lactate levela (mmol/L) | Mean (SD) | 1.59 (0.16) | 1.53 (0.18) | 1.335 | 0.187 |
| Range | 1.35–1.86 | 1.30–1.80 | |||
| 6-MWTDa (m) | Mean (SD) | 417.50 (19.29) | 418.50 (18.63) | –0.204 | 0.839 |
| Range | 387.00–448.00 | 385.00–452.00 | |||
| Smoking behaviourb | n (%) | ||||
| Current | 11 (36.7) | 10 (33.3) | 0.693 | 0.707 | |
| Previous | 8 (26.7) | 6 (20.0) | |||
| Never | 11(36.7) | 14 (46.7) | |||
| IPAQ scoreb | n (%) | ||||
| Low | 11 (36.7) | 13 (43.3) | 0.278 | 0.598 | |
| Moderate | 19 (63.3) | 17 (56.7) | |||
| Lung affected (fibrosis) | n (%) | ||||
| Mild | 8 (33.3) | 10 (40) | 0.287 | 0.592 | |
| Moderate | 18 (66.7) | 16 (60) | |||
| Data represented as mean (standard deviation; SD) and range (min–max) for continuous data and N (%) for categorical data. | |||||
| aIndependent sample t-test. | |||||
| bχ2 test. | |||||
| *Statistically significant at p<0.05 according to χ2 and independent sample t-tests | |||||
| BMI: body mass index; DBP: diastolic blood pressure; FSS: Fatigue Severity Scale; IPAQ: International Physical Activity Questionnaire; PImax: maximum static inspiratory pressure; MMRC scale: Modified Medical Research Council scale; SBP: systolic blood pressure; 6-MWTD: 6-min walk test distance. | |||||
| Variables | Group | Pre-intervention | Post-intervention | Δ | % improvement | p-valuea (group × time) | p-valuea groups | p-valuea (time) | p-valueb | p-valuec |
| PImax (cmH2O) | Study group | 82.00 (16.86) |
121.50 (13.52) |
39.501 (6.29) |
48.17% ↑ | <0.001* | <0.001* | <0.001* | <0.001** | <0.001*** |
| Control group | 80.501 (7.52) |
84.001 (5.86) |
3.50 (33.12) |
4.35% ↑ | 0.567 | |||||
| SBP (mmHg) | Study group | 150.00 (5.39) |
125.00 (7.81) |
–25.00 (6.36) |
16.67% ↓ | <0.001* | <0.001* | <0.001* | <0.001** | <0.001*** |
| Control group | 150.23 (5.53) |
146.50 (5.92) |
–3.73 (9.10) |
2.48% ↓ | 0.032** | |||||
| DBP (mmHg) | Study group | 94.60 (2.31) |
77.50 (2.65) |
–17.10 (2.81) |
18.08% ↑ | <0.001* | <0.001* | <0.001* | <0.001** | <0.001*** |
| Control group | 94.80 (2.25) |
93.90 (2.43) |
–0.90 (1.18) |
0.95% ↓ | <0.01** | |||||
| Data represented as mean (standard deviation; SD). Δ: mean difference of post–pre-value (MD). ƞ2 P: partial eta squared ranges from 0 to 1 (0.01 = small, 0.06 = medium, and 0.14 = large effect-size). | ||||||||||
| aMixed repeated analysis of variance (ANOVA). | ||||||||||
| bPaired sample t-test. | ||||||||||
| cIndependent sample t-test. | ||||||||||
| *Statistically significant at p < 0.05 according to mixed repeated ANOVA. | ||||||||||
| **Statistically significant at p < 0.05 according to paired sample t-test. | ||||||||||
| ***Statistically significant at p < 0.05 according to independent sample t-test. | ||||||||||
| DBP: diastolic blood pressure; PImax: maximum static inspiratory pressure; SBP: systolic blood pressure. | ||||||||||
In terms of SBP and DBP, Table II shows a significant interaction between intervention type and time in favour of the study group (F (1, 58) = 110.04, p < 0.001, ƞ2 p = 0.655, and F (1, 58) = 847.52, p < 0.001, ƞ2 p = 0.936, respectively). There was a significant main effect for time (F (1, 58) = 200.87, p < 0.001, ƞ2 p = 0.776, F (1, 58) = 1046.33, p < 0.001, ƞ2 p = 0.947, respectively), and a significant main effect for group (F (1, 58) = 75.35, p < 0.001, ƞ2 p = 0.792, respectively).
The mean between-group differences changes in MMRC scale and FSS scores show a reduction in both variables in both the study and control group over time. Concerning MMRC scale, the mean between-group differences decreased by 48.89%, from score 2.63 (0.60) to score 1.38 (0.49), p < 0.001 in the study group and decreased by 12.81%, from score 2.42 (0.49) to score 2.11 (0.33), p < 0.01 in the control group. While FFS reduced from score 43.36 ± 5.25 to score 28.68 ± 6.01 in the study group (p < 0.001) and from score 42.47 ± 5.18 to score 39.77 ± 5.89 in the control group (p = 0.001), with a statistically significant difference in favour of the study group also observed (p < 0.001) (see Figs 3A, B).
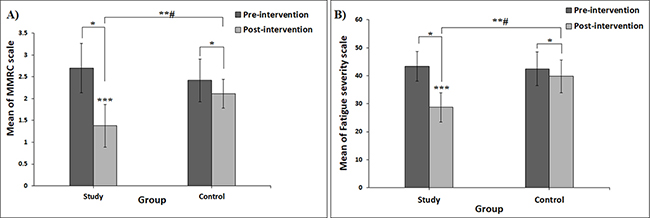
Fig. 3. Error bar charts. (A) Mean change in Modified Medical Research Council scale (MMRC) between pre- and post-intervention in both the study and control groups. (B) Mean change in Fatigue Severity Scale (FSS) between pre- and post-intervention in both the study and control groups. #Significant interaction between “intervention (group) and time” at p < 0.05. *Post-hoc Bonferroni test revealed a significant difference between pre- and post-intervention at p < 0.05. **Significant differences between post-intervention in the study group and the control group at p < 0.05. ***Significant improvement in study group compared with control group (p < 0.001).
The serum lactate level decreased significantly, from 1.59 (0.16) to 0.77 (0.16) mmol/L in the study group, and from 1.53 (0.18) to 1.26 (0.30) mmol/L in the control group (see Fig. 4B). However, the study group had a significantly lower mean difference in the serum lactate level (–0.82 (0.22) mmol/L, 51.57%) than the control group (–0.27 (0.20) mmol/L, 17.65%) (p < 0.001) (Fig. 4A).
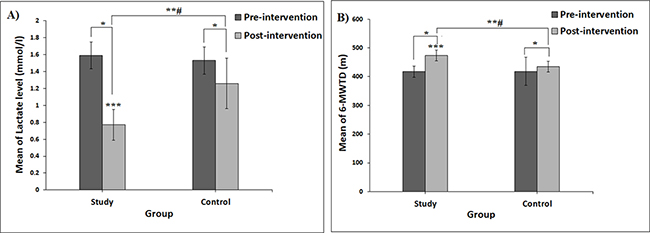
Fig. 4. Error bar charts. (A) Mean change in lactate level between pre- and post-intervention in study and control groups. (B) Mean change in 6-min walk test distance (6-MWTD) between pre- and post-intervention in study and control groups. #Significant interaction between “intervention (group) and time” at p < 0.05. *Post-hoc Bonferroni test revealed a significant difference between pre- and post-intervention at p < 0.05. **Significant differences between post-intervention in study group and control group at p < 0.05. ***Significant improvement in the study group compared with control group (p < 0.001).
The 6-MWTD estimates the functional capacity of the participants, which increased significantly in both study and control groups (from 417.50 (19.29) to 474.00 (48.69) m and from 418.50 (18.63) to 435.00 (18.17) m, respectively). Despite this, the study group showed a significantly higher improvement regarding the 6-MWTD (MD = 56.50 (48.05) m, an increase of 13.53%) than the control group (MD = 16.50 (9.52) m, an increase of 3.92%) (p < 0.001) (Fig. 4B).
DISCUSSION
The aim of this study was to investigate the effect of adding manual DR technique to an IMT programme on inspiratory muscle strength, blood pressure, dyspnoea, fatigue, serum lactate level, and aerobic performance capacity in patients with post-COVID-19 syndrome.
Patients with post-COVID-19 syndrome commonly develop long-term symptoms, as reported in a recent study, including fatigue, muscle weakness, depression, insomnia, and dyspnoea (28). However, we granted the prominence effect of IMT in COVID- 19 syndrome based on many studies, we note that manual DR added to POWERbreath provided additional clinical benefits by producing a statistically significant improvement in post-acute sequelae of COVID-19 syndrome, such as PImax, serum lactate level, dyspnoea, and 6MWTD, compared with IMT alone.
The PImax values are also considered as an indication of respiratory muscle strength. The DR technique is effective in improving the respiratory mechanics and other respiratory variables, such as PImax (29), as demonstrated in the current study.
According to Cunha et al. (30), application of IMT alone provided a non-significant change in PImax in elite swimmers, in contrast to a study by Rocha et al. (17) which found that manual DR had a significant immediate increase in PImax among patients with chronic obstructive pulmonary disease. In the current study manual DR and IMT resulted in significantly increased PImax in the study group (by 48.17%, p < 0.001) compared with the control group (p < 0.01).
A significant reduction in both systolic and diastolic blood pressure (by 0.95%, p < 0.001) was observed in the study group compared with the control group. This is consistent with Ferreira et al. (31), who demonstrated that an 8-week IMT programme with a 30% PImax load reduced daytime arterial blood pressure. Also, Farinatti et al. (32) inferred the effect of diaphragmatic stretching in people with low flexibility, which exhibited a significant impact on the sympathetic-vagal balance through boosting post-exercise vagal modulation, and consequently may reduce blood pressure and pulse rate.
We consider that the combined effect of manual DR and POWERbreath contributed to lowering the arterial blood pressure through improving respiratory muscle performance, increasing fatigue resistance (33), reducing the sympathetic outflow, and lowering the metaboreflex. Furthermore, changes in the diaphragm length-tension curve can also affect vagal and sympathetic inputs to the sinus node through cardiovascular adjustments (34).
Moreover, the findings of the current study showed a statistically significant decrease in dyspnoea in favour of the study group, in which the MMRC scale decreased by 48.9% (p < 0.001), whereas in the control group, it decreased by 12.8% (p < 0.01). This is compatible with McNarry et al. (35), who demonstrated that IMT can dramatically enhance long-term COVID patients’ dyspnoea, respiratory muscle function, and functional capacity. Furthermore, in recovered COVID-19 patients, Abodonya et al. (14) found that IMT improved pulmonary functioning, dyspnoea, and fatigue, which is also supported by Nopp et al. (36).
This improvement may be attributed to DR-induced parasympathetic system activation, which improved oxygen saturation and decreased bronchospasm, respiratory rate, and work of breathing or dyspnoea (37).
Concerning fatigue level, the current findings showed a reduction in FSS mean score by 51.57%, p < 0.001 in the study group and in the control group by 17.65%, p = 0.001, with a statistically significant difference in favour of the study group also observed (p < 0.001) which is convenient to Bosnak-Guclu et al. (38) who postulated that IMT reduced FSS scores from 42.73 ± 11.75 to 29.07 ± 13.96 in heart failure patients, p < 0.001. Feriani et al. (33) also proposed that IMT reduces respiratory muscle fatigue in patients with heart failure, by reducing respiratory muscle oxygen demand/delivery mismatch.
There was also a significant reduction in the serum lactate level in the study group (by 51.57%) and in the control group (by 17.65%); likewise, McConnell et al. (15) demonstrated a significant reduction in lactate level after the IMT program, which was associated with improvement in the endurance level (p < 0.01).
Training the respiratory muscles increases their lactic acid metabolization ability, enhancing aerobic metabolism and fatigue tolerance (39). Despite these valuable outcomes, no previous studies have evaluated the effects on fatigue or serum lactate levels of DR alone or in combination with the IMT programme.
In addition to enhancing respiratory function, diaphragmatic stretching also promotes postural function by having a biomechanical impact on distant structures, such as the cervical and lumbar spine (37). Consequently, the current results revealed a significant increase in 6-MWTD in the study group with respect to aerobic performance capability (by 13.53%, p < 0.001) and in the control group (by 3.92%, p < 0.01), which is consistent with Bosnak-Guclu et al. (38) and Rocha et al. (17) findings that the 6-MWTD increased significantly after the application of IMT or DR, respectively (p < 0.001).
The lack of studies on DR in patients with post-COVID-19 long-term symptoms encouraged us to highlight its superior effect when added to the IMT programme in overcoming these symptoms and improving the quality of life of these patients.
Furthermore, because it increases diaphragm excursion, which improves respiratory function, lowers sympathetic excitability (40), and thus reduces dyspnoea (16), DR is thought to be an indirect method of increasing muscular contraction effectiveness.
Study limitations
This has some limitations; participant selection was limited to men with moderate COVID-19 syndrome only, which may have reduced the scope of the results. Secondly, concomitant ultrasonography or electromyography measurements to assess diaphragm weakness were not available. It was also not possible to quantify the degree of peripheral muscle function improvement after intervention. The study was not sufficient to analyse different subgroups (such as age or disease severity).
CONCLUSION
This study provides evidence on the effect of DR implementation in rehabilitation programmes for post-acute sequelae of COVID-19. The results demonstrate that adding DR to the IMT programme improves long-term symptoms in hypertensive patients with post-COVID-19 syndrome, suggesting that DR should be considered for use with these patients.
Contribution statement
E.N and M.M contributed to data collection, methodology, investigation, and conceptualization. D.A, A.Y, and H.G. participated in methodology, editing, supervision, validation, and writing-original draft, and all authors read and approved the final version of the manuscript and agreed with the order of presentation of the authors.
Funding/support
This study received no specific support from public, private, or non-profit funding bodies.
ACKNOWLEDGEMENTS
The authors thank everyone who helped them with this study, particularly Ebthall Mohamed, who assisted them with data analysis and interpretation.
REFERENCES
- World Health Organization Press Conference. The World Health Organization (WHO) has officially named the disease caused by the novel coronavirus as COVID-19. 2020. [accessed on 2020 Feb 11]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- Debeuf R, Swinnen E, Plattiau T, De Smedt A, De Waele E, Roggeman S, et al. The Effect of physical therapy on impairments in COVID-19 patients from intensive care to home rehabilitation: a rapid review. J Rehabil Med 2022; 54: 8–23.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506.
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8:420–422.
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481.
- Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS, et al. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021; 76: 396–398.
- Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res 2020; 126: 1456–1474.
- Shi Z, de Vries HJ, Vlaar APJ, van der Hoeven J, Boon RA, Heunks LMA, et al. Diaphragm pathology in critically ill patients with COVID-19 and postmortem findings from 3 medical centers. JAMA Intern Med 2021; 181:122–124.
- Jonkman AH, Jansen D, Heunks LM. Novel insights in ICU-acquired respiratory muscle dysfunction: implications for clinical care. Crit Care 2017; 21:64–71.
- Farr E, Wolfe AR, Deshmukh S, Rydberg L, Soriano R, Walter JM, et al. Diaphragm dysfunction in severe COVID-19 as determined by neuromuscular ultrasound. Ann Clin Transl Neurol 2021; 8: 1745–1749.
- Velavan TP, Kieu Linh LT, Kreidenweiss A, Gabor J, Krishna S, Kremsner PG. Longitudinal monitoring of lactate in hospitalized and ambulatory COVID-19 patients. Am J Trop Med Hyg 2021; 104: 1041–1044.
- Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol 2021; 93: 1013–1022.
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188: e13–e64.
- Abodonya AM, Abdelbasset WK, Awad EA, Elalfy IE, Salem HA, Elsayed SH. Inspiratory muscle training for recovered COVID-19 patients after weaning from mechanical ventilation: a pilot control clinical study. Medicine (Baltimore) 2021; 100:13–19.
- McConnell AK & Sharpe GR. The effect of inspiratory muscle training upon maximum lactate steady-state and blood lactate concentration. Eur J Appl Physiol 2005; 94: 277–284.
- González-Álvarez FJ, Valenza MC, Torres-Sánchez I, Cabrera-Martos I, Rodríguez-Torres J, Castellote-Caballero Y. Effects of diaphragm stretching on posterior chain muscle kinematics and rib cage and abdominal excursion: a randomized controlled trial. Braz J Phys Ther 2016; 20: 405–411.
- Rocha T, Souza H, Brandão DC, Rattes C, Ribeiro L, Campos SL, et al. The manual diaphragm release technique improves diaphragmatic mobility, inspiratory capacity and exercise capacity in people with chronic obstructive pulmonary disease: a randomised trial. J Physiother 2015; 61: 182–189.
- Booth ML, Ainsworth BE, Pratt MI, Ekelund UL, Yngve AG, Sallis JF, et al. International physical activity questionnaire: 12-country reliability and validity. Med sci sports Exerc 2003; 195: 3508–1381.
- Hegazy MA, Lithy RM, Abdel-Hamid HM, Wahba M, Ashoush OA, Hegazy MT, et al. COVID-19 disease outcomes: does gastrointestinal burden play a role? Clin Exp Gastroenterol 2021; 14: 199–207.
- Datta SD, Talwar A, Lee JT. A proposed framework and timeline of the spectrum of disease due to SARS-CoV-2 Infection: illness beyond acute infection and public health implications. JAMA 2020; 324: 2251–2252.
- Yang Z, Liu H, Meng F, Guan Y, Zhao M, Qu W, et al. The analysis of circadian rhythm of heart rate variability in patients with drug-resistant epilepsy. Epilepsy Res 2018; 146: 151–159.
- American Thoracic Society/European Respiratory Society. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med 2002; 166: 518–624.
- Launois C, Barbe C, Bertin E, Nardi J, Perotin JM, Dury S, et al. The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC Pulm Med 2012; 12: 61–68.
- Marek EM, Friz Y, Pohl W, Vogel P, Mückenhoff K, Kotschy-Lang N, et al. Effizienz als ein neuer Parameter zur Objektivierung der körperlichen Leistungsfähigkeit mittels 6-Minuten-Gehtest [Efficiency as a new parameter of physical fitness of patients in 6-minute-walk-test]. Rehabilitation (Stuttg) 2011; 50:118–126 (in German).
- Rossi D, Galant LH, Marroni CA. Psychometric property of fatigue severity scale and correlation with depression and quality of life in cirrhotics. Arq Gastroenterol 2017; 54: 344–348.
- Donnino MW, Andersen LW, Giberson T, Gaieski DF, Abella BS, Peberdy MA, et al. Initial lactate and lactate change in post-cardiac arrest: a multicenter validation study. Crit Care Med 2014; 42: 1804–1811.
- Maldaner V, Coutinho J, Santana ANDC, Cipriano GFB, Oliveira MC, Carrijo MM, et al. Adjunctive inspiratory muscle training for patients with COVID-19 (COVIDIMT): protocol for randomised controlled double-blind trial. BMJ Open 2021; 11: e049545.
- Mizrahi B, Shilo S, Rossman H, Kalkstein N, Marcus K, Barer Y, et al, Shamir-Stein NA, Shalev V, Zohar AE, Chodick G. Longitudinal symptom dynamics of COVID-19 infection. Nat Commun 2020; 11: 1–10.
- Marizeiro DF, Florêncio ACL, Nunes ACL, Campos NG, Lima POP. Immediate effects of diaphragmatic myofascial release on the physical and functional outcomes in sedentary women: a randomized placebo-controlled trial. J Bodyw Mov Ther 2018; 22: 924–929.
- Cunha M, Mendes F, Paciência I, Rodolfo A, Carneiro-Leão L, Rama T, et al. The effect of inspiratory muscle training on swimming performance, inspiratory muscle strength, lung function, and perceived breathlessness in elite swimmers: a randomized controlled trial. Porto Biomed J 2019; 4: e49.
- Ferreira JB, Plentz RDM, Stein C, Casali KR, Arena R, Lago PD. Inspiratory muscle training reduces blood pressure and sympathetic activity in hypertensive patients: a randomized controlled trial, Int J Cardiol 2013; 166: 61–67.
- Farinatti PT, Brandão C, Soares PP, Duarte AF. Acute effects of stretching exercise on the heart rate variability in subjects with low flexibility levels. J Strength Cond Res 2011; 25: 1579–1585.
- Feriani DJ, Coelho HJ Júnior, Scapini KB, de Moraes OA, Mostarda C, Ruberti OM, et al. Effects of inspiratory muscle exercise in the pulmonary function, autonomic modulation, and hemodynamic variables in older women with metabolic syndrome. J Exerc Rehabil 2017; 13: 218–226.
- St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol 2000; 529: 493–504.
- McNarry MA, Berg RMG, Shelley J, Hudson J, Saynor ZL, Duckers J, et al. Inspiratory muscle training enhances recovery post COVID-19: a randomised controlled trial. Eur Respir J 2022; 2: 2103101 [Epub ahead of print].
- Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Res Pract Thromb Haemost 2020; 4:1178–1191.
- Nair A, Alaparthi GK, Krishnan S, Rai S, Anand R, Acharya V, Acharya P. Comparison of diaphragmatic stretch technique and manual diaphragm release technique on diaphragmatic excursion in chronic obstructive pulmonary disease: a randomized crossover trial. Pulm Med 2019; 2019: 6364376.
- Bosnak-Guclu M, Arikan H, Savci S, Inal-Ince D, Tulumen E, Aytemir K, et al. Effects of inspiratory muscle training in patients with heart failure. Respir Med 2011; 105: 1671–1681.
- Hargreaves M, Spriet LL. Skeletal muscle energy metabolism during exercise. Nat Metab 2020; 2: 817–828.
- Arroyo-Morales M, Olea N, Martinez M, Moreno-Lorenzo C, Díaz-Rodríguez L, Hidalgo-Lozano A. Effects of myofascial release after high-intensity exercise: a randomized clinical trial. J Manipulative Physiol Ther 2008; 31: 217–223.