REVIEW ARTICLE
SCREENING CUTOFF VALUES TO IDENTIFY THE RISK OF FALLS AFTER STROKE: A SCOPING REVIEW
Daisuke MATSUMOTO, OTR1, Takaaki FUJITA, OTR, PhD1, Ryuichi KASAHARA, RPT2, Kenji TSUCHIYA, OTR, PhD3 and Kazuaki IOKAWA, OTR, PhD1
From the 1Department of Occupational Therapy, School of Health Sciences, Fukushima Medical University, Fukushima, 2Department of Rehabilitation, Kita-Fukushima Medical Center, Date, and 3Faculty of Health Sciences, Nagano University of Health and Medicine, Nagano, Japan
Objective: The present scoping review aimed to summarize and determine the accuracy of the variables and cutoff values reported to date for identifying fall risk in patients with stroke and identify the commonalities, limitations, and clinical implications.
Methods: Articles published by the end of 2023 were searched using PubMed, Cumulative Index of Nursing and Allied Health Literature, and Scopus electronic databases. Two reviewers created a search formula, searched the databases, and conducted primary and secondary screenings.
Results: This review included 21 articles. The most commonly used individual indicator for identifying fall risk after stroke was the Berg Balance Scale; the cutoff values were relatively consistent, ranging between 46.5 and 50.5 points (area under the curve: 0.72–0.81). For the Timed Up and Go test and Falls Efficacy Scale-International, the cutoff values were in the range of 15–19 s and 27–29 points, respectively, and were relatively consistent across the articles. However, the area under the curve values were low (0.66–0.70 and 0.68–0.71, respectively).
Conclusion: Among various assessments, the Berg Balance Scale is the most extensively studied tool, with established cutoff values associated with falls risk. It serves as a reliable indicator for detecting fall risk, especially in community-dwelling individuals with chronic stroke.
LAY ABSTRACT
Falls are common in people with stroke and often lead to injury, fear of falling, and limitation of daily activities. Effective fall prevention requires prognostication to identify high-risk individuals. The present review summarized the accuracy of rehabilitation-related assessments and cutoff values reported to date for identifying fall risk in patients with stroke. The results showed that cutoff values of 46.5 to 50.5 points on the Berg Balance Scale was a relatively good predictor of falls in community-dwelling individuals with chronic stroke. Cutoff values for other assessments varied widely between previous studies or had insufficient predictive accuracy. We also found that few studies calculated a cutoff value to identify fall risk using cognitive function as an indicator. In conclusion, this review identifies certain limits of accuracy and contributes to the appropriate use of cutoff values in clinical practice.
Key words: Berg Balance Scale; Falls Efficacy Scale-International; fall risk; stroke; Timed Up and Go test.
Citation: J Rehabil Med 2024; 56: jrm40560. DOI: https://doi.org/10.2340/jrm.v56.40560.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
Submitted: Apr 12, 2024; Accepted after revision: Oct 3, 2024; Published: Oct 24, 2024
Correspondence address: Takaaki Fujita, Department of Occupational Therapy, School of Health Sciences, Fukushima Medical University, 10-6 Sakaemachi, Fukushima City, Fukushima 960-8516, Japan. E-mail: t-fujita@fmu.ac.jp
Competing interests and funding: The authors have no conflicts of interest to declare.
Stroke is the second leading cause of disability and death worldwide, posing a huge burden at both individual and societal levels (1). According to international guidelines for fall prevention, stroke is one of the common medical conditions associated with a higher fall risk, warranting increase supervision when there are more impairments following a stroke (2). In particular, impaired balance, poor lower limb motor function, visuospatial hemineglect, and a history of falls are associated with a high risk of falling (3, 4). Approximately half of patients with stroke experience at least 1 fall in the first year after a stroke, and falls are 7 times more prevalent in this population than among healthy individuals (5). Thus, after a stroke, patients have a significantly higher risk of fracture (6, 7), with falls often leading to limitations in activities of daily living (8). Furthermore, the fear of falling is reportedly associated with a reduced quality of life (9). Therefore, it is critical to prevent falls in such patients.
The number of prevalent cases of stroke worldwide is huge, exceeding 100 million (10). Therefore, it may not be feasible to provide fall prevention interventions to all stroke patients, and effective fall prevention requires prognostication to identify high-risk individuals. A previous review on fall risk prediction in patients with stroke has indicated that there are several multivariable risk prediction models (11). However, while predictive models with many variables and complex algorithms tend to be more accurate, they are less likely to be widely used in clinical practice (12). Therefore, a predictive model should be simple, with a single-variable cutoff value, for clinical feasibility. A review by Lee et al. (13) of fall screening assessments for older community-dwelling individuals and inpatients reported that 12.3 s is a useful cutoff value for the Timed Up and Go (TUG) test. However, to the best of our knowledge, no review has summarized the cutoff values for predicting falls in patients after stroke. A scoping review is suitable for providing overviews of a broad research field and identifying knowledge gaps. Therefore, the purpose of this scoping review was to (i) summarize and determine the accuracy of the variables and cutoff values reported to date that can be used to identify fall risk in patients with stroke, and (ii) identify the commonalities, limitations, and clinical implications of the cutoff values.
METHODS
We established a search formula, searched databases, and conducted primary and secondary screenings based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis extension for Scoping Reviews (PRISMA-ScR) (14). The eligibility criteria were as follows: (i) the participants had experienced a stroke; (ii) the study calculated a cutoff value to identify fall risk; (iii) the data included the area under the curve (AUC), indicating that accuracy, sensitivity, and specificity were calculated; and (iv) studies published up to 31 December 2023. The exclusion criteria were as follows: (i) study of falls during acute hospital stay, (ii) review articles, (iii) conference proceedings, and (iv) papers written in languages other than English. The first exclusion criterion was established because the situation during acute hospitalization differs significantly from the subacute and chronic phases, as movement is severely restricted under medical supervision.
The article search was conducted on 3 January 2024, using PubMed, Cumulative Index of Nursing and Allied Health Literature (CINAHL), and Scopus electronic databases. The search formula was developed in consultation with the authors and occupational therapists (DM and TF): ([stroke] OR [cerebrovascular diseases] OR [CVA]) AND ([falls] OR [accidental fall]) AND ([cut off] OR [cutoff]). Articles extracted from the databases were imported into EndNote X7 (Clarivate PLC, Philadelphia, PA, USA), and duplicates were removed. For the primary screening, 2 reviewers (DM and TF) independently checked the titles and abstracts. Subsequently, they conducted a full-text search to determine the articles to include based on the eligibility criteria (secondary screening). Disagreements during the primary and secondary screenings were resolved through discussion.
The 2 reviewers independently extracted the following data for integration from the accepted articles: first author name, publication year, country/region, stage of stroke recovery (acute, subacute, chronic), main inclusion criteria, sample size, duration of the fall survey, environmental indicators and their cutoff values, AUC, sensitivity, and specificity. For practicality, data with cutoff sensitivities and specificities of ≥ 50% were primarily integrated.
RESULTS
Study selection
In total, 199 articles were extracted from the database search; 110 were identified after eliminating duplicates. Primary screening resulted in the exclusion of 75 articles. After secondary screening of the remaining 35 articles, 19 articles that met the eligibility criteria were selected. The reference lists of the accepted articles were also checked, and primary and secondary screenings were conducted again for the relevant literature; 2 additional articles meeting the eligibility criteria were identified. Finally, 21 articles (15–35) were selected for this scoping review (Fig. 1).
Fig. 1. PRISMA flow diagram. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Study characteristics
The included studies were conducted in 10 countries: the United States, Japan and Korea (4 articles each), Turkey and Brazil (2 articles), and the Czech Republic, China, Sweden, Hong Kong, and Taiwan (1 article each). Many of the studies (16 articles) had a sample size of < 100 (n = 27–99), 4 articles had a sample size between 100 and 200, and only 1 article had a sample size of > 200 (Table I). In terms of fall occurrences, 6 studies used inpatient fall records, 3 studies recorded falls prospectively, and 11 studies used a history of falls (Table II). Thirteen studies identified 1 or more falls as a fall group, 5 identified 2 or more falls, and 2 analysed both. The percentage of fallers ranged from 13% to 61%. The AUCs ranged from 0.61 to 0.92, and many studies reported cutoff values associated with AUCs below 0.7 (Table II). Assessments and cutoff values with an AUC of 0.7 or higher, which is generally considered an acceptable level (36) and clinically useful, are tabulated in Table SI.
| Author (year) | Country | Post-stroke stage | Sample size | Main selection criteria of participants | ||
| Stroke type | Physical function | Cognitive function | ||||
| Alenazi et al. (2018) (15) | United States | Chronic | 181 | The ability to walk > 10 m without an orthotic device | MMSE ≥ 24 | |
| Alzayer et al. (2009) (16) | United States | Chronic | 44 | The ability to walk 10 m independently with or without an assistive device | The ability to follow 3-step commands | |
| An et al. (2014) (17) | Korea | Chronic | 72 | The ability to walk more than 10 m without a walking aid | MMSE ≥ 24 | |
| An et al. (2017) (18) | Korea | Chronic | 57 | The ability to walk more than 10 m without a walking aid | MMSE > 24 | |
| Belgen et al. (2006) (19) | United States | Chronic | 50 | The ability to walk 10 m with no physical assistance with or without any assistive device | The ability to follow 3-step commands | |
| Beninato et al. (2009) (20) | United States | Chronic | 27 | The ability to ambulate independently at least 10 m with or without an assistive device | The ability to follow 3-step commands | |
| Faria-Fortini et al. (2021) (21) | Brazil | Chronic | 105 | Primary or recurrent unilateral stroke | The ability to walk 10 m with or without an assistive device | Except for MMSE < 13 or 18 or 26 (education-adjusted cutoff scores) |
| Fiedorová et al. (2022) (22) | Czech Republic | Subacute | 84 | Primary ischaemic stroke | FAC 3–5, and the ability to stand without support for 5 min | Except for severe phatic disorder |
| Huo et al. (2009) (23) | China | Chronic | 27 | The ability to walk independently with or without a cane | Except for higher cortical dysfunction or severe impair-ment of speech | |
| Kızılkaya et al. (2023) (24) | Turkey | Chronic | 39 | First unilateral anterior circulation stroke | The ability to walk at least 10 m without assistance, and BRS 3–6 | MMSE ≥ 24 |
| Lee et al. (2021) (25) | Korea | Unclear | 227 | FAC > 2 | ||
| Maeda et al. (2009) (26) | Japan | Chronic | 72 | |||
| Park et al. (2018) (27) | Korea | Chronic | 99 | Except for MMSE-K < 18 | ||
| Persson et al. (2011) (28) | Sweden | Acute | 96 | First-ever stroke | Except for diagnosis of dementia or severe psychiatric diseases | |
| Pinto et al. (2014) (29) | Brazil | Chronic | 150 | Ischaemic or haemorrhagic stroke | The ability to understand the tests | |
| Sahin et al. (2019) (30) | Turkey | Chronic | 50 | The ability to stand for 2 min unassisted, and walk unassisted or assisted (with cane) 6 m | MMSE ≥ 24 | |
| Takatori et al. (2009) (31) | Japan | Subacute, chronic | 60 | The ability to stand unassisted for at least 1 min | MMSE ≥ 24, and no severe higher brain function disorders | |
| Takatori et al. (2009) (32) | Japan | Subacute, chronic | 76 | The ability to stand unassisted for at least 1 min | MMSE ≥ 24, and no severe higher brain function disorders | |
| Tsang et al. (2013) (33) | Hong Kong | Chronic | 106 | The ability to understand verbal instructions | ||
| Yamasaki et al. (2023) (34) | Japan | Subacute | 33 | First stroke involving the vertebrobasilar territory | Presence of ataxic symptoms in one upper or lower limb, and BRS ≥ 5 | No cognitive impairment, or higher brain dysfunction |
| Zou et al. (2021) (35) | Taiwan | Chronic | 30 | Single and unilateral stroke | The ability to walk independently over a distance of 10 m without walking aids or orthoses | No cognitive impairments or aphasia |
| MMSE: Mini-Mental State Examination; FAC: Functional Ambulation Categories; BRS: Brunnstrom Recovery Stage; MMSE-K: Mini-Mental State Examination-Korean version. | ||||||
| Author (year) | Fall monitoring | Falling groups | Predictor | Cutoff | AUC | Sens | Spec | |
| Period | Setting | |||||||
| Alenazi et al. (15) | 42 weeks | Community | ≥ 2 falls (n = 24) vs 1 or no fall (n = 157) | FRT | 18.15 | 0.66 | 76% | 56% |
| PHQ-9 | 2.5 | 0.62 | 60% | 65% | ||||
| Alzayer et al. (16) | Last 6 months | Community | ≥ 2 falls (n = 10) vs 1 or no fall (n = 34) | BBS | 52 | 0.67 | 90% | 41% |
| An et al. (17) | Last 12 months | Unclear | ≥ 2 falls (n = 44) vs non-faller (n = 28) | POMA | 12.5 | 0.78 | 72% | 74% |
| An et al. (18) | Last 6 months | Unclear | Faller (n = 25) vs non-faller (n = 32) | DGI-4 | 9.5 | 0.77 | 68% | 59% |
| DGI-8 | 16.5 | 0.78 | 60% | 72% | ||||
| Belgen et al. (19) | Last 6 months | Community | ≥ 2 falls (n = 11) vs 1 or no fall (n = 39) | BBS | 52 | 0.72 | 91% | 42% |
| Faller (n = 20) vs non-faller (n = 30) | Swedish FES | 17.5 | 0.71 | 90% | 53% | |||
| Beninato et al. (20) | Last 6 months | Community | ≥ 2 falls (n = 9) vs 1 or no fall (n = 18) | ABC Scale | 81.1 | 0.92 | 100% | 72% |
| BBS | 49 | 0.76 | 78% | 72% | ||||
| SIS-16 | 61.7 | 0.86 | 78% | 89% | ||||
| STS | 17.9 | 0.66 | 67% | 72% | ||||
| Faria-Fortini et al. (21) | Last 6 months | Community | Faller (n = 42) vs non-faller (n = 63) | FES-International | 28 | 0.71 | 71% | 57% |
| Fiedorová et al. (22) | 6 months | Community | Faller (n = 32) vs non-faller (n = 52) | BBS | 35 | 0.66 | 44% | 89% |
| BBS | 42 | 0.62 | 56% | 67% | ||||
| FES-International | 27 | 0.69 | 81% | 56% | ||||
| FES-International | 29 | 0.68 | 72% | 64% | ||||
| SOT | 60 | 0.69 | 72% | 65% | ||||
| Huo et al. (23) | Last 12 months | Unclear | Faller (n = 7) vs no-faller (n = 20) | P-RT | 626 | 0.77 | 86% | 70% |
| Kızılkaya et al. (24) | Last 6 months | Unclear | Unclear | Turkish FAB | 21.5 | 0.75 | 84% | 61% |
| Lee et al. (25) | During hospital stay | Hospital | Faller (n = 44) vs non-faller (n = 183) | MFS | 32.5 | 0.61 | 79% | 38% |
| TUG | 18.58 | 0.69 | 78% | 55% | ||||
| Maeda et al. (26) | During hospital stay | Hospital | Faller (n = 27) vs non-faller (n = 45) | BBS | 29 | 0.81 | 80% | 78% |
| Park et al. (27) | Unclear | Community | Faller (n = 35) vs non-faller (n = 64) | ABC Scale | 63.75 | 0.69 | 41% | 92% |
| Korean FES | 66.5 | 0.68 | 70% | 64% | ||||
| Persson et al. (28) | 12 months | Unclear | Faller (n = 46) vs non-faller (n = 50) | 10MWT | 12 | 0.74 | 80% | 58% |
| BBS | 42 | 0.69 | 69% | 65% | ||||
| M-MAS UAS-95 | 50 | 0.72 | 74% | 58% | ||||
| SwePASS | 32 | 0.73 | 82% | 50% | ||||
| TUG | 15 | 0.7 | 63% | 58% | ||||
| Pinto et al. (29) | Last 12 months | Community | Faller (n = 56) vs non-faller (n = 94) | TUG | 25 | 0.66 | 36% | 90% |
| Sahin et al. (30) | Last 12 months | Unclear | Faller (n = 26) vs non-faller (n = 24) | ABC Scale | 55.31 | 0.78 | 75% | 81% |
| BBS | 46.5 | 0.81 | 75% | 77% | ||||
| BESTest | 69.44 | 0.84 | 75% | 85% | ||||
| Takatori et al. (31) | 3 months | Hospital | ≥ 2 falls (n = 15) vs 1 or no fall (n = 45) | EED | 6.3 | 0.8 | 80% | 78% |
| Takatori et al. (32) | 5 months | Hospital | Faller (n = 37) vs non-faller (n = 39) | EED | 6.1 | 0.7 | 69% | 82% |
| ≥ 2 falls (n = 21) vs 1 or no fall (n = 55) | EED | 6.3 | 0.8 | 81% | 78% | |||
| Tsang et al. (33) | Last 12 months | Community | Faller (n = 25) vs non-faller (n = 81) | BBS | 50.5 | 0.72 | 52% | 80% |
| FRT | 24.1 | 0.67 | 52% | 74% | ||||
| Mini-BESTest | 17.5 | 0.64 | 64% | 64% | ||||
| OLS: nonparetic side | 3.6 | 0.64 | 40% | 84% | ||||
| OLS: paretic side | 0.9 | 0.67 | 56% | 78% | ||||
| TUG | 19 | 0.66 | 61% | 67% | ||||
| Yamasaki et al. (34) | 1 month | Hospital | Faller (n = 10) vs non-faller (n = 23) | STV | 6.35 | 0.84 | 80% | 74% |
| Zou et al. (35) | 12 months | Hospital | Faller (n = 9) vs non-faller (n = 21) | Turn duration | 4 | 0.75 | 67% | 80% |
| Turn step | 7 | 0.73 | 56% | 85% | ||||
| AUC: area under the curve; Sens: sensitivity; Spec: specificity; FRT: Functional Reach Test; PHQ-9: Patient Health Questionnaire-9; BBS: Berg Balance Scale; POMA: Performance-Oriented Mobility Assessment; DGI: Dynamic Gait Index; FES: Falls Efficacy Scale; ABC Scale: Activities-specific Balance Confidence Scale; SIS: Stroke Impact Scale; STS: Five Times Sit to Stand Test; SOT: Sensory Organization Test; P-RT: Probe Reaction Time; FAB: Fullerton Advanced Balance Scale; MFS: Morse Fall Scale; TUG: Timed Up and Go; 10MWT: 10 Meter Walk Test; M-MAS UAS-95: Modified Motor Assessment Scale Uppsala Akademiska Sjukhus; SwePASS: Swedish version of the Postural Assessment Scale for Stroke patients; BESTest: Balance Evaluation Systems Test; EED: error in estimated distance; OLS: one-leg standing; STV: stride time variability; FES: Falls Efficacy Scale. | ||||||||
Study design and outcome characteristics
Most studies used gait- and balance-related assessments to identify patients at risk of falls (Table II). Six studies used confidence not to fall assessments (Activities-specific Balance Confidence [ABC] Scale, Falls Efficacy Scale [FES]) (19–22, 27, 30); 1 study used a comprehensive assessment (Morse Fall Scale [MFS]) (25), and 1 used depression (9-item Patient Health Questionnaire) as the measure of fall risk (15). The most used individual indicator was the Berg Balance Scale (BBS; 8 studies) (16, 19, 20, 22, 26, 28, 30, 33), followed by the TUG (4 studies) (25, 28, 29, 33), FES-related indicators (4 studies) (19, 21, 22, 27), and ABC Scale (3 studies) (20, 27, 30). None of the articles included in this review calculated a cutoff value to identify the risk of falling based on a measure of cognitive function.
Integrating the information from the 6 studies that had BBS cutoff sensitivity and specificity values greater than 50% revealed that the cutoff and AUC values varied widely, ranging from 29 to 50.5 points and 0.62 to 0.81, respectively (Fig. 2). However, the cutoff values used in the 3 studies (20, 30, 33) that applied BBS for community-dwelling individuals with chronic stroke were relatively consistent, ranging from 46.5 to 50.5 points (AUC 0.72–0.81). In the only study examining falls during hospitalization (26), cutoff values tended to be low, at 29 points (AUC 0.81).
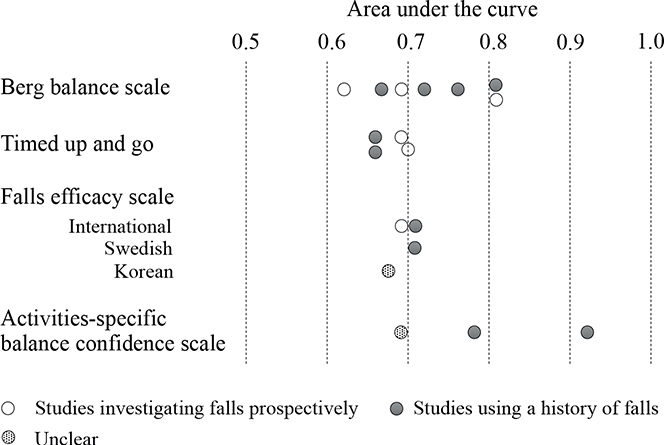
Fig. 2. Distribution of area under the curves for indicators used in 3 or more articles.
The TUG test (4 out of 21 studies) was the next most commonly used individual indicator. The cutoff values and AUCs in the 3 studies (25, 28, 33) with TUG sensitivity and specificity cutoff values greater than 50% ranged from 15 to 19 s and 0.66 to 0.7, respectively, with relatively consistent results.
FES-related assessments were used in 4 studies (19, 21, 22, 27); however, the versions used varied: 1 Korean (27), 1 Swedish (19), and 2 international versions (21, 22). The cutoff values and AUCs in the 2 studies (21, 22) using the FES-International (FES-I) were consistent, ranging from 27 to 29 and 0.68 to 0.71, respectively. The 3 studies (20, 27, 30) using the ABC Scale had a high variability of cutoff values and AUCs, ranging from 55.3 to 81.1 and 0.69 to 0.92, respectively. The highest AUC among the 21 studies was reported by Beninato et al. (20), who used the ABC Scale to identify community-dwelling individuals at risk of falling after stroke (AUC 0.92, sensitivity 100%, specificity 72%); however, the study had a small sample size of 27 and the study design was not prospective, as it investigated past falls.
DISCUSSION
This scoping review summarizes the cutoff values reported to date used to identify fall risk after stroke and examines their commonalities and limitations. Al-though the studies included in this review were conducted under various conditions using numerous measures, the range of AUCs varied from 0.61 to 0.92, with many results below 0.7, indicating certain limitations when using a single cutoff value. An existing review (11) has reported AUCs of 0.62–0.87 when using multivariate prediction models, indicating that the identification of these individuals remains a challenge.
The BBS was found to be an indicator with relatively high discriminatory power to differentiate between fall and non-fall groups in community-dwelling individuals with chronic stroke, although it was difficult to integrate the results owing to the varying conditions among the studies. From 3 different studies, the cutoff values were relatively consistent, ranging from 46.5 to 50.5 points, and the AUCs ranged from 0.72 to 0.81, above the level generally considered acceptable (AUC > 0.7) (36). Existing reviews of studies on older adults have reported BBS cutoff values of 45–51 (37) or 46–54 (38) points for identifying fall risk in older adults. The present review revealed that the BBS cutoff values were comparable among studies of community-dwelling individuals with chronic stroke. Our results also indicate that BBS cutoff values tended to be lower (29 points) when identifying inpatient fall risk. Although this finding is based on only 1 study and has limited generalizability at this stage, it is a reasonable result considering that inpatients with concern about fall are provided with the necessary assistance and monitoring whenever possible during walking.
In contrast, the primary cut-off range for the TUG identified in this study – 15–19 s – was slower than the cut-off value of 12.3 s established in previous studies for community-dwelling older adults (13, 39). Previous studies have reported that individuals with chronic stroke typically take longer to complete the TUG compared with community-dwelling older adults (40). Therefore, rehabilitation therapists should recognize that the optimal TUG cut-off values for predicting falls in older adults may not be applicable to individuals with stroke. In addition, for TUG and FES-I, the cutoff values were relatively consistent across the literature, but the AUCs were low (0.66–0.7 and 0.68–0.71, respectively), suggesting limited accuracy. Several reviews of community-dwelling older adults have noted limitations in identifying individuals at high risk of falls using TUG (41, 42). The results of this review reveal that caution should be exercised in relying solely on the cutoff values of the FES-I as well as the TUG to determine the risk of falls. Therefore, we advocate for the combined use of TUG and FES-I cutoff values alongside other indicators for fall risk.
The highest AUC in the articles used in this review was 0.92, and the indicator was the ABC scale. The results indicate a close association between falls and confidence in balance during activities. However, it is important to note that all of the studies using the ABC scale (20, 27, 30) in this review examined past falls, which is important for proper interpretation. In other words, the results of previous studies may indicate that stroke patients lose confidence in their balance due to falls. In particular, the report by Beninato et al., (20), which reported the highest AUC, was based on the multiple-fall group, who experienced 2 or more falls. It may not be surprising that multiple falls are strongly associated with reduced balance confidence. Therefore, further prospectively designed studies are needed to determine whether the cutoff value of the ABC scale can be used as a predictor of falls.
Notably, we found that the cutoff values of cognitive function for identifying fall risk are unclear. In this review, some studies used the level of cognitive function as a selection criterion for individuals; however, the number of studies was small, and it was difficult to integrate the information. However, the 2010 review by Campbell & Matthews (3) noted a poorly established role of cognitive function in falls in patients after stroke. To our best knowledge, only 1 study has used the Montreal cognitive assessment to predict falls during hospital stays in acute care hospitals (43), which was not included in this review. This is a potential gap in the literature that warrants further study.
Strength and limitations
An important finding of the present review was the certain degree of reliability and accuracy of BBS (46.5–50.5 points, AUC 0.72–0.81) in identifying fall risk among community-dwelling individuals with chronic stroke. However, a limitation of this review was that it was limited to studies in which the AUC, sensitivity, and specificity were calculated to ascertain accuracy. No searches were conducted in electronic databases other than PubMed, CINAHL, and Scopus. Therefore, our review did not include all studies that calculated the cutoff values for predicting the risk of falls. Moreover, our scoping review did not include a quality assessment of each study, which is standard practice in systematic reviews.
Conclusion
This review is the first to summarize the cutoff values for identifying the risk of falls in patients after stroke, identifying certain limits of accuracy and contributing to the appropriate use of cutoff values in clinical practice.
Among various assessments, the BBS has the best-studied cutoff values associated with falls and is a reliable indicator for detecting fall risk, especially in community-dwelling individuals with chronic stroke. Overall, however, the accuracy of single cutoff values, including TUG and FES-I, in predicting falls in stroke patients remains uncertain and therapists should be aware of the limitations of accuracy. In addition, many indicators are insufficiently validated, including cognitive functions, necessitating further research.
ACKNOWLEDGEMENT
Funding/ financial support: This work was supported by the Japan Society for the Promotion of Science KAKENHI [grant number 22K11422]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- Saini V, Guada L, Yavagal DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology 2021; 97: S6–S16. https://doi.org/10.1212/wnl.0000000000012781
- Montero-Odasso M, van der Velde N, Martin FC, Petrovic M, Tan MP, Ryg J, et al. World guidelines for falls prevention and management for older adults: a global initiative. Age Ageing 2022; 51: afac205. https://doi.org/10.1093/ageing/afac205
- Campbell GB, Matthews JT. An integrative review of factors associated with falls during post-stroke rehabilitation. J Nurs Scholarsh 2010; 42: 395–404. https://doi.org/10.1111/j.1547-5069.2010.01369.x
- Xie Q, Pei J, Gou L, Zhang Y, Zhong J, Su Y, et al. Risk factors for fear of falling in stroke patients: a systematic review and meta-analysis. BMJ Open 2022; 12: e056340. https://doi.org/10.1136/bmjopen-2021-056340
- Abdollahi M, Whitton N, Zand R, Dombovy M, Parnianpour M, Khalaf K, et al. A systematic review of fall risk factors in stroke survivors: towards improved assessment platforms and protocols. Front Bioeng Biotechnol 2022; 10: 910698. https://doi.org/10.3389/fbioe.2022.910698
- Zheng JQ, Lai HJ, Zheng CM, Yen YC, Lu KC, Hu CJ, et al. Association of stroke subtypes with risk of hip fracture: a population-based study in Taiwan. Arch Osteoporos 2017; 12: 104. https://doi.org/10.1007/s11657-017-0390-8
- Kumagai M, Otaka Y, Yoshida T, Kitamura S, Ushizawa K, Mori N, et al. Cumulative risk and associated factors for fall-related fractures in stroke survivors after discharge from rehabilitation wards: a retrospective study with a 6-year follow-up. J Rehabil Med 2022; 54: jrm00294. https://doi.org/10.2340/jrm.v54.2314
- Schmid AA, Rittman M. Consequences of poststroke falls: activity limitation, increased dependence, and the development of fear of falling. Am J Occup Ther 2009; 63: 310–316. https://doi.org/10.5014/ajot.63.3.310
- Kim EJ, Kim DY, Kim WH, Lee KL, Yoon YH, Park JM, et al. Fear of falling in subacute hemiplegic stroke patients: associating factors and correlations with quality of life. Ann Rehabil Med 2012; 36: 797–803. https://doi.org/10.5535/arm.2012.36.6.797
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021; 20: 795–820. https://doi.org/10.1016/S1474-4422(21)00252-0
- Walsh ME, Horgan NF, Walsh CD, Galvin R. Systematic review of risk prediction models for falls after stroke. J Epidemiol Community Health 2016; 70: 513–519. https://doi.org/10.1136/jech-2015-206475
- Stinear CM, Smith MC, Byblow WD. Prediction tools for stroke rehabilitation. Stroke 2019; 50: 3314–3322. https://doi.org/10.1161/strokeaha.119.025696
- Lee J, Geller AI, Strasser DC. Analytical review: focus on fall screening assessments. PM R 2013; 5: 609–621. https://doi.org/10.1016/j.pmrj.2013.04.001
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169: 467–473. https://doi.org/10.7326/m18-0850
- Alenazi AM, Alshehri MM, Alothman S, Rucker J, Dunning K, D’Silva LJ, et al. Functional reach, depression scores, and number of medications are associated with number of falls in people with chronic stroke. PM R 2018; 10: 806–816. https://doi.org/10.1016/j.pmrj.2017.12.005
- Alzayer L, Beninato M, Portney LG. The accuracy of individual Berg Balance Scale items compared with the total Berg score for classifying people with chronic stroke according to fall history. J Neurol Phys Ther 2009; 33: 136–143. https://doi.org/10.1097/npt.0b013e3181b51307
- An S, Lee Y, Lee G. Validity of the performance-oriented mobility assessment in predicting fall of stroke survivors: a retrospective cohort study. Tohoku J Exp Med 2014; 233: 79–87. https://doi.org/10.1620/tjem.233.79
- An SH, Jee YJ, Shin HH, Lee GC. Validity of the original and short versions of the Dynamic Gait Index in predicting falls in stroke survivors. Rehabil Nurs 2017; 42: 325–332. https://doi.org/10.1002/rnj.280
- Belgen B, Beninato M, Sullivan PE, Narielwalla K. The association of balance capacity and falls self-efficacy with history of falling in community-dwelling people with chronic stroke. Arch Phys Med Rehabil 2006; 87: 554–561. https://doi.org/10.1016/j.apmr.2005.12.027
- Beninato M, Portney LG,. Sullivan PE. Using the International Classification of Functioning, Disability and Health as a framework to examine the association between falls and clinical assessment tools in people with stroke. Phys Ther 2009; 89: 816–825. https://doi.org/10.2522/ptj.20080160
- Faria-Fortini I, Polese JC, Faria CDCM, Scianni AA, Nascimento LR, Teixeira-Salmela LF. Fall Efficacy Scale-International cut-off score discriminates fallers and non-fallers individuals who have had stroke. J Bodyw Mov Ther 2021; 26: 167–173. https://doi.org/10.1016/j.jbmt.2020.12.002
- Fiedorová I, Mrázková E, Zádrapová M, Tomášková H. Receiver operating characteristic curve analysis of the Somatosensory Organization Test, Berg Balance Scale, and Fall Efficacy Scale-International for predicting falls in discharged stroke patients. Int J Environ Res Public Health 2022; 19: 9181. https://doi.org/10.3390/ijerph19159181
- Huo M, Maruyama H, Chen L. Relationship between probe reaction time during walking and falls for patients with post-stroke hemiplegia. J Phys Ther Sci 2009; 21: 349–354. https://doi.org/10.1589/JPTS.21.349
- Kızılkaya E, Köse N, Ünsal Delialioğlu S, Karakaya J, Fil Balkan A. Psychometric properties of Fullerton Advanced Balance Scale in patients with stroke. Top Stroke Rehabil 2024; 31: 145–156. https://doi.org/10.1080/10749357.2023.2235800
- Lee KB, Lee JS, Jeon IP, Choo DY, Baik MJ, Kim EH, et al. An analysis of fall incidence rate and risk factors in an inpatient rehabilitation unit: a retrospective study. Top Stroke Rehabil 2021; 28: 81–87. https://doi.org/10.1080/10749357.2020.1774723
- Maeda N, Kato J, Shimada T. Predicting the probability for fall incidence in stroke patients using the Berg Balance Scale. J Int Med Res 2009; 37: 697–704. https://doi.org/10.1177/147323000903700313
- Park EY, Lee YJ, Choi YI. The sensitivity and specificity of the Falls Efficacy Scale and the Activities-specific Balance Confidence Scale for hemiplegic stroke patients. J Phys Ther Sci 2018; 30: 741–743. https://doi.org/10.1589/jpts.28.741
- Persson CU, Hansson PO, Sunnerhagen KS. Clinical tests performed in acute stroke identify the risk of falling during the first year: postural stroke study in Gothenburg (POSTGOT). J Rehabil Med 2011; 43: 348–353. https://doi.org/10.2340/16501977-0677
- Pinto EB, Nascimento C, Marinho C, Oliveira I, Monteiro M, Castro M, Castro, et al. Risk factors associated with falls in adult patients after stroke living in the community: baseline data from a stroke cohort in Brazil. Top Stroke Rehabil 2014; 21: 220–227. https://doi.org/10.1310/tsr2103-220
- Sahin IE, Guclu-Gunduz A, Yazici G, Ozkul C, Volkan-Yazici M, Nazliel B, et al. The sensitivity and specificity of the balance evaluation systems test-BESTest in determining risk of fall in stroke patients. NeuroRehabilitation 2019; 44: 67–77. https://doi.org/10.3233/nre-182558
- Takatori K, Shomoto K, Shimada T. Relationship between self-perceived postural limits and falls among hospitalized stroke patients. J Phys Ther Sci 2009; 21: 29–35. https://doi.org/10.1589/jpts.21.29
- Takatori K, Okada Y, Shomoto K, Shimada T. Does assessing error in perceiving postural limits by testing functional reach predict likelihood of falls in hospitalized stroke patients? Clin Rehabil 2009; 23: 568–575. https://doi.org/10.1177/0269215509102957
- Tsang CS, Liao LR, Chung RC, Pang MY. Psychometric properties of the Mini-Balance Evaluation Systems Test (Mini-BESTest) in community-dwelling individuals with chronic stroke. Phys Ther 2013; 93: 1102–1115. https://doi.org/10.2522/ptj.20120454
- Yamasaki Y, Arai T, Takaishi S, Takamura H, Maruki H. Increased stride time variability is associated with a higher risk of falls in patients with ataxia after stroke. Physiother Theory Pract 2023: 1–9. https://doi.org/10.1080/09593985.2023.2286334
- Zou TE, Liang PJ, Lee SC. Turning duration and steps predict future falls in poststroke hemiplegic individuals: a preliminary cohort study. Top Stroke Rehabil 2021; 28: 33–41. https://doi.org/10.1080/10749357.2020.1760644
- Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol 2010; 5: 1315–1316. https://doi.org/10.1097/jto.0b013e3181ec173d
- Lima CA, Ricci NA, Nogueira EC, Perracini MR. The Berg Balance Scale as a clinical screening tool to predict fall risk in older adults: a systematic review. Physiotherapy 2018; 104: 383–394. https://doi.org/10.1016/j.physio.2018.02.002
- Neuls PD, Clark TL, Van Heuklon NC, Proctor JE, Kilker BJ, Bieber ME, et al. Usefulness of the Berg Balance Scale to predict falls in the elderly. J Geriatr Phys Ther 2011; 34: 3–10. https://doi.org/10.1097/jpt.0b013e3181ff2b0e
- Wrisley DM, Kumar NA. Functional gait assessment: concurrent, discriminative, and predictive validity in community-dwelling older adults. Phys Ther 2010; 90: 761–73. https://doi.org/10.2522/ptj.20090069
- Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil 2005; 86: 1641–7. https://doi.org/10.1016/j.apmr.2005.01.011
- Barry E, Galvin R, Keogh C, Horgan F, Fahey T. Is the Timed Up and Go test a useful predictor of risk of falls in community-dwelling older adults: a systematic review and meta-analysis. BMC Geriatr 2014; 14: 14. https://doi.org/10.1186/1471-2318-14-14
- Beauchet O, Fantino B, Allali G, Muir SW, Montero-Odasso M, Annweiler C. Timed Up and Go test and risk of falls in older adults: a systematic review. J Nutr Health Aging 2011; 15: 933–938. https://doi.org/10.1007/s12603-011-0062-0
- Eikenberry M, Ganley KJ, Zhang N, Kinney CL. Association between performance on an interdisciplinary stroke assessment battery and falls in patients with acute stroke in an inpatient rehabilitation facility: a retrospective cohort study. Arch Phys Med Rehabil 2019; 100: 2089–2095. https://doi.org/10.1016/j.apmr.2019.05.026