ORIGINAL REPORT
POST-INTENSIVE CARE SYNDROME PREVALENCE SIX MONTHS AFTER CRITICAL COVID-19: COMPARISON BETWEEN FIRST AND SECOND WAVES
Amandine RAPIN, MD, MHP1,2, François Constant BOYER, MD, MHP, PhD1.2, Bruno MOURVILLIER, MD, PhD3, Guillaume GIORDANO ORSINI, MD3, Claire LAUNOIS, MD, PhD4,5, Redha TAIAR, PhD6, Gaëtan DESLEE, MD, PhD4.5, Antoine GOURY, MD3 and Sandy CARAZO-MENDEZ, MD1
From the 1Centre Hospitalo-Universitaire de Reims (CHU), Hôpital Sébastopol, Département de Médecine Physique et de Réadaptation, Reims, France, 2Université de Reims Champagne-Ardenne, Faculté de Médecine, Vieillissement, Fragilité (VieFra) Reims, France, 3Centre Hospitalo-Universitaire de Reims (CHU), Hôpital Robert-Debré, Service de Réanimation médicale, Reims, France, 4Centre Hospitalo-Universitaire de Reims (CHU), Département de Médecine Pulmonaire, Reims, France, 5Université de Reims Champagne-Ardenne, Institut National de la Santé et de la Recherche Médicale Pathologies Pulmonaires et Plasticité Cellulaire (P3Cell) Unité Médicale de Recherche-S1250, Structure Fédérative de Recherche (SFR) CAP-SANTE, Reims, France and 6Université de Reims Champagne-Ardenne, Laboratoire MATériaux et Ingénieurerie Mécanique (MATIM), Reims, France
Objective: To explore the impact of improved intensive care for COVID-19 patients on the prevalence of post-intensive care syndrome (PICS).
Design: Ambispective cohort study.
Patients: Post-intensive care unit COVID-19 patients from the first and second waves of COVID-19.
Methods: Patients were evaluated at 6 months after infection. PICS was defined as the presence of a 1-min sit-to-stand test (1STS) score < 2.5th percentile or a Symbol Digit Modalities Test (SDMT) below the 2 standard deviation cut-off, or a Hospital Anxiety and Depression Scale score ≥ 11.
Results: A total of 60 patients were included (34 from wave 1 and 26 from wave 2). Intensive care unit management improved between waves, with shorter duration of orotracheal intubation (7 vs 23.5 days, p = 0.015) and intensive care unit stay (6 vs 9.5 days, p = 0.006) in wave 2. PICS was present in 51.5% of patients after wave 1 and 52% after wave 2 (p = 0.971). Female sex and diabetes were significantly associated with PICS by multivariate analysis.
Conclusion: Approximately half of post-intensive care unit COVID-19 patients have 1 or more impairments consistent with PICS at 6 months, with an impact on quality of life and participation. Improved intensive care unit management was not associated with a decrease in the prevalence of PICS. Identification of patients at risk, particularly women and diabetic patients, is essential. Further studies of underlying mechanisms and the need for rehabilitation are essential to reduce the risk of PICS.
LAY ABSTRACT
COVID-19 infection can lead to hospitalization in an intensive care unit (ICU). Some patients experience physical, mental or emotional symptoms after ICU admission, collectively termed post-intensive care syndrome (PICS). It was hypothesized that improvements in management in the ICU between the first and second waves of the COVID-19 pandemic may have had a positive impact on the prevalence of PICS. In the current study, according to the definition proposed, However, the results of this study showed that, according to the definition proposed, half of all post-ICU COVID-19 patients had PICS at 6 months after infection, with no difference between waves, despite improvements in care in the ICU. In the current study, women and patients with diabetes were most frequently affected. Improving care at the acute phase seems to be insufficient to prevent PICS post-COVID. It seems necessary to improve care programmes and follow-up after the acute phase in order to prevent and manage PICS and improve quality of life for these patients.
Key words: COVID-19; critical care; physical and rehabilitation medicine; exercise capacity; long-term consequences.
Citation: J Rehabil Med 2022; 54: jrm00339. DOI: https://dx.doi.org/10.2340/jrm.v54.4363
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Sep 12, 2022; Epub ahead of print: Oct 4, 2022; Published: Oct 28, 2022
Correspondence address: Centre Hospitalo-Universitaire de Reims, Hôpital Sébastopol, Département de Médecine Physique et de Réadaptation, 51092, Reims, France. E-mail: arapin@chu-reims.fr
Competing interests and funding: The authors have no conflicts of interest to declare.
The new severe acute respiratory syndrome coronavirus (SARS-CoV-2) and the resultant disease, COVID-19, first emerged in late 2019, and was declared a pandemic by the World Health Organization (WHO) in March 2020. The long-term consequences of SARS-CoV-2 infection are the focus of intense research activity. In addition to its pulmonary, renal and hepatic effects, SARS-CoV-2 infection is associated with vascular and neurological complications, as well as acquired neuro-muscular weakness. Survivors of severe or critical forms of COVID-19 are at risk of developing post-intensive care syndrome (PICS) (1, 2), defined as the presence of physical, cognitive or mental impairments occurring during or after a stay in the intensive care unit (ICU) (3–5). Although there is a general consensus regarding the functions affected by PICS, the measurements and cut-offs used to define them are not standardized, introducing significant heterogeneity in the literature, and making comparisons between studies difficult. Furthermore, the physiopathology of PICS unrelated to SARS-Cov-2 infection is unclear (6). These functional alterations may have long-term repercussions, including an inability to return to work for up to 60 months after ICU (7) and impaired quality of life (8).
The prevalence of PICS after ICU admission for severe forms of COVID-19 was thought to be much higher than after other diseases; namely 87–91% (4, 9), although recent studies did not report any differences (10, 11).
Given the health and social impact of PICS, recommendations have been made to prevent its occurrence. Interprofessional approaches to symptom management during critical illness, known as the ABCDEF bundle, have been proposed in guidelines to prevent short- and long-term impairments in functioning after a stay in the ICU (12, 13). Among the proposed bundles, early mobilization in the acute phase must be associated with systematic rehabilitation during the ICU stay, and after discharge.
Management of COVID-19 during the first wave of the pandemic was hampered by a lack of knowledge of the disease and its consequences, compounded by the massive influx to ICUs within a very short time-span. Marked improvements ware made worldwide between the first and second waves, in particular, with a lower proportion of patients requiring invasive mechanical ventilation in the second wave (14). This improvement should have an impact on the prevalence of PICS. Several authors in Europe have studied mortality from COVID-19 over the course of the different pandemic waves, in particular to assess the impact of progress in management, albeit without showing any marked differences (15, 16). To date, no study has assessed the impact of the pandemic waves and the improvement in management of patients admitted to the ICU on the frequency of PICS. The improvement in professional practices and better knowledge of the pathology has led to the hypothesis that the prevalence of PICS should decrease between the first and second waves, confirming the importance of the quality of care, and highlighting areas of improvement or where caution remains required. The aim of this study was therefore to compare the prevalence of PICS at 6 months after SARS-CoV-2 infection, between patients admitted in a french ICU (Champagne-Ardenne region, France) during the first and second waves of the pandemic.
METHODS
This is a post hoc analysis of 2 prospective, single-centre cohorts, namely the CAPACoV-19 cohort, which enrolled patients admitted during the first wave of the pandemic in France (7 March to 15 May 2020), and the RETRO-REA cohort, which enrolled patients during the second wave in France (1 December 2020 to 28 February 2021).
The results are reported in compliance with the Strengthening in Reporting of Observational studies in Epidemiology (STROBE) statement for cohort studies (17).
Inclusion criteria
The CAPACoV-19 cohort is part of a prospective study (number EudraCT/ID-RCB: 2020-A01081-38) carried out in the Physical and Rehabilitation Medicine (PRM) Department of Reims University Hospital, Reims, France, which aimed to evaluate the physical, cognitive, and psychological consequences of infection with SARS-CoV-2 in patients hospitalized at the acute phase of disease. Inclusion criteria were: age ≥ 18 years, SARS-CoV-2 infection confirmed by RT-PCR and/or undisputable clinical and/or paraclinical arguments; need for hospitalization at the acute phase, with referral to the PRM department; ability to read and write French fluently; ability to follow a rehabilitation programme, and consent to participate. Exclusion criteria were: Mini Mental State Examination (MMSE) score < 22, and patients under legal protection.
The CAPACoV-19 cohort was constituted in the early stages of the first wave of COVID-19, and the evaluations carried out at that time were largely based on knowledge from the previous SARS-CoV-1 and MERS-CoV epidemics. Evaluations in the first wave that were later deemed to be relevant were continued for patients followed during the second wave, who constituted the RETRO-REA cohort, an ambispective cohort. This second cohort enrolled patients who were seen at 6-month follow-up consultation after an ICU stay for critical COVID-19. Patients were evaluated by a specialist PRM physician. The selection criteria of patients for the RETRO-REA cohort were therefore the same as those of the initial CAPACoV-19 cohort listed above.
Measurements
Anthropometric data (age, weight, height), comorbidities and lifestyle habits were recorded for all patients. Return to work and/or leisure activities were also noted at 6-month follow-up consultation (binary response, yes/no). Data regarding the acute management were collected from the patients’ medical files. Simplified Acute Physiology Score II (SAPS II) was collected to classify disease severity (18). During the follow-up consultation, patients were asked whether they had had rehabilitation, either in a PRM unit, or in private community practice.
To evaluate the prevalence of PICS, the presence of physical, cognitive, and/or mental impairment were assessed. Patients were considered to have PICS if they had physical and/or cognitive and/or mental impairment, assessed as described below.
Physical impairment was assessed with the 1-min sit-to-stand test (1STS) (19). A score below the 2.5th percentile was considered abnormal.
The 6-min walk test (6MWT) was also performed in compliance with the American Thoracic Society statement (20). However, the instructions differed between the 2 waves, because of a change in examiner for this test, with a test at fast speed in the first wave and at comfortable speed in the second wave. Results for the 6MWT were compared with normal values from Enright et al. (21).
Cognitive impairment was assessed using the Symbol Digit Modalities Test (SDMT, oral version) (22). This test consists in converting as many symbols as possible into digits (stated out loud), over a period of 90 s. Normative scores take account of the level of education, with different norms for people who have and have not completed higher education (23). A score below 2 standard deviations (SD) was considered abnormal.
Mental impairment was assessed using the Hospital Anxiety and Depression Scale (HADS). Total scores range from 0 to 21 on each scale, with a score of 0 indicating the absence of possible symptoms of anxiety or depression. According to the original publication by Zigmond et al., (24), a score of 11 or greater was considered to indicate the likely presence of symptoms of anxiety or depression. This cut-off was chosen to minimize the risk of false-positive results.
Patient-reported outcomes (PRO) were used to assess perceived impact on function, activity and participation. The intensity of dyspnoea was evaluated using the ordinal modified Medical Research Council score (mMRC). Fatigue was measured using the Modified Fatigue Impact scale (MFIS) (25). This questionnaire encompasses physical, cognitive and psychosocial items and a score of 38 or higher identifies fatigued individuals (26). The severity of pain was measured using the Brief Pain Inventory-Short Form (BPI-SF) (27). This self-report questionnaire yields a score for pain intensity (4 items, each with a score ranging from 0 to 10, for a total possible score of 40, indicating the worst pain imaginable), and a scale for pain interference (7 items rated from 0, no interference, to 10, complete interference, for a total possible score of 70).
Quality of life was assessed using the abbreviated generic World Health Organization Quality of Life questionnaire (WHOQOL-BREF). Results are expressed as a score ranging from 0 to 100, with higher scores indicating better quality of life. Four domains are explored, namely physical health (7 items), psychological health (6 items), social relationship (3 items), and environment (8 items). The 5th percentile is not available in the published norms for the French general population, whereas the 25th percentile is published (28). Patients were therefore classified according to a score above or below the 25th percentile.
Statistical analysis
No imputation was applied for missing data. Description and analysis were performed assuming a non-normal distribution. Data are described as number and percentage or median and interquartiles for qualitative and quantitative variables, respectively. Data were compared using the χ2 or Wilcoxon Mann–Whitney tests, as appropriate.
Logistic regression was used to identify the factors related to the primary outcome. The multivariate model included factors that yielded a p-value < 0.10 by univariate analysis. Results are reported as odds ratios (ORs) and 95% confidence intervals (95% CI). A p-value < 0.05 was considered statistically significant. All analyses were performed using SPSS 28.0.
Ethical considerations
The CAPACoV-19 cohort was registered with ClinicalTrials.gov under the number NCT04356378 and was approved by the ethics committee CPP Nord Ouest IV under the number CPP 3838-RM. The RETRO-REA was a follow-up cohort. In accordance with French legislation (namely the Jardé law), this retrospective study was approved by the French national commission for personal data protection (CNIL, Comité National de l’Information et des Libertés) (number 2049775 v 0).
RESULTS
Study population
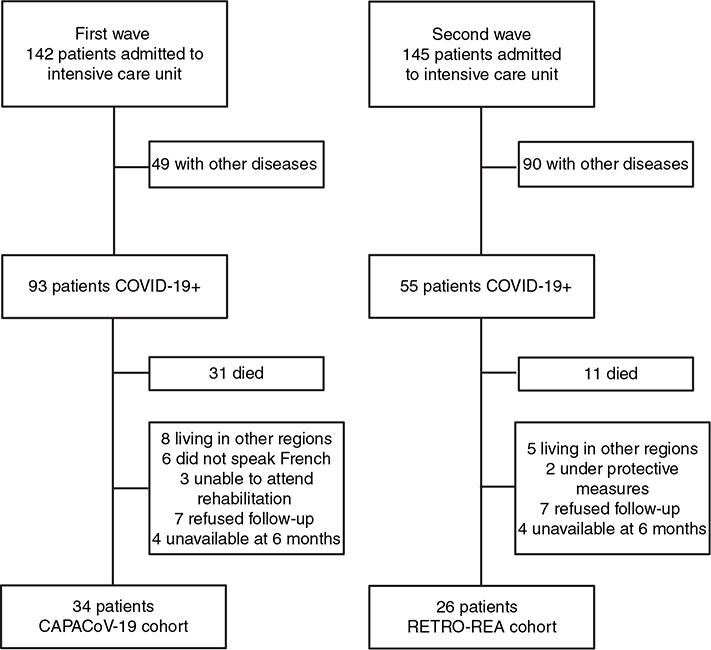
During the first wave of the pandemic, 93 patients were admitted to the ICU for SARS-CoV-2 in the University hospital of Reims, of whom 31 (33%) died. During the second wave, 55 patients were admitted to the ICU, of whom 11 died (20%). A total of 61 patients had 6-month follow-up (35 from the first wave, and 26 from the second wave). The flowchart of the study population is shown in Fig. 1. Among survivors of the first wave, 55% were followed up at 6 months, and 59% among the second wave. No patient met the exclusion criteria. One patient who refused to consent to follow-up had an MMSE score < 22, which was -presumed to predate the COVID-19 infection, based on the history-taking. Age, sex, weight, height, and Simplified Acute Physiology Score II (SAPS II) score were compared between patients who were included and those were not; no significant difference was observed.
Patients from both waves were comparable in terms of age, sex, body mass index (BMI), diabetes, and hypertension. There was also no difference in the proportion of unemployed patients, although there was a significant difference in the level of education between waves.
The characteristics of the study population are shown in Table I.
Acute management
The details of management for patients from both waves are given in Table II. In terms of acute care, the median duration of invasive mechanical ventilation was longer during the first wave (p = 0.015), while the number of patients placed in the prone position was also higher during the first wave (p = 0.008).
Hydroxychloroquine and antiretroviral drugs were prescribed during the first wave, but were no longer used in the second wave, while the proportion of patients treated with corticosteroids increased in the second wave (p = 0.008).
The length of stay in ICU and in hospital was longer during the first wave (p = 0.018).
Regarding post-ICU management, more patients were referred to PRM during the first wave (p = 0.004), and similarly, more patients from wave 1 received rehabilitation during the first 6 months after ICU discharge (p < 0.001).
Post-intensive care syndrome at 6 months
In total, 30 patients had impairments consistent with PICS; 17 (51.5%) from wave 1, and 13 (52%) from wave 2. The PICS prevalence is shown in Table III, estimating physical, mental and/or cognitive function at 6 months post-ICU. One data was missing for the 1STS, 1 for the HADS, and 2 for the SDMT. Finally, 2 patients could not be classified for PICS. Prevalence of PICS did not differ between the first and second waves (p = 0.971). Fig. 2 show the spread of impairments among patients classed as having PICS.
| First wave, N = 34 | Second wave, N = 26 | p-value | |||
| ≥ 1 impairments consistent with PICSa | 17 | (51.5) | 13 | (52) | 0.971 |
| ≥ 2 impairments consistent with PICSb | 3 | (8.8) | 5 | (19.2) | 0.275 |
| 3 impairments consistent with PICSb | 1 | (3) | 2 | (8) | 0.569 |
| Physical PICS (1 STS < 2.5th percentile)b | 8 | (23.5) | 9 | (36) | 0.286 |
| 1 STS, number of repetition | 27.5 | [19.7 – 34.2] | 25 | [16.5 – 29.5] | 0.187 |
| 6MWT, m | 483 | [397 – 553] | 407 | [271 – 458] | 0.002 |
| % theoretical | 95.2 | [79.7 – 106.9] | 68.8 | [57.2 – 82.6] | < 0.001 |
| < 80% | 7 | (20.6) | 16 | (61.5) | < 0.001 |
| Cognitive PICS (SDMT < 2 SD)a | 6 | (17.6) | 4 | (16.7) | 0.922 |
| SDMT correct repetitions, n | 47 | [33.7 – 52.2] | 44 | [27.7 – 56.5] | 0.776 |
| Mental PICS (HADS A or D ≥ 11)b | 7 | (21.2) | 7 | (26.9) | 0.609 |
| HADS A score | 6 | [2.5 – 8.5] | 4.5 | [3 – 8.25] | 0.836 |
| HADS D score | 3 | [1 – 8.5] | 3 | [1.75 – 8] | 0.842 |
| All results are expressed as median [interquartile range] or number (percentage) PICS: post-intensive care syndrome; 1STS: 1 minute sit-to-stand test; 6MWT: 6-minute walk test; SDMT: Symbol Digit Modalities Test; SD: standard deviation; HADS: Hospital Anxiety and Depression Scale; HADS A: anxiety score; HADS D: depression score. a2 missing data, b1 missing data. |
|||||
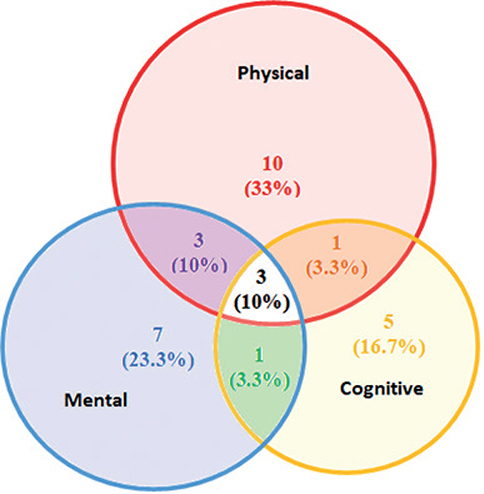
Fig. 2. Details of the impairments among patients classed as having post-intensive care syndrome (PICS).
Among patients in the first wave, 2 had bilateral common peroneal nerve palsy post-ICU, with a full recovery in 1 and a persisting need for orthosis in the other at 6 months. Among patients in the second wave, 2 underwent electroneuromyography; 1 for right ulnar nerve compression and 1 for polyneuropathy post-ICU. No sequelae were observed on physical examination at 6 months in either patient. No other neurological complications were detected.
The results of the 6MWT are reported, but it should be noted that there was bias in the administration of the test, with an instruction to use a fast speed in tests in the first wave, and a comfortable speed for the tests in the second wave.
Due to the difference in the level of education between samples in the first and second waves, the proportion of patients with cognitive impairment according to the level of education was compared, despite the fact that parameter is integrated into the SDMT norms. Fisher’s exact text did not show any statistical difference (13.3% for patients who went to university vs 18.6% for the others, p = 1). Among patients with anxiety or depression after COVID-19 disease, 4 had a history of depression and 10 did not (p = 0.081). Table IV presents the comparison of the characteristics and acute care between those with PICS and those without. Based on the results of the univariate analysis, 4 variables were included in the multivariate model, namely sex, diabetes, weight loss at the acute phase and the duration of invasive mechanical ventilation. By multivariate analysis, female sex and diabetes showed a significant association with PICS at 6 months, OR 3.48 [95% CI 1.05–11.53], p = 0.041 and 8.59 [95% CI 1.61–45.72], p = 0.012, respectively. Cox-Snell R² was 0.191 and Nagelkerke’s R² was 0.255.
| PICS, N = 30 | No PICS, N = 28 | p-value | |||
| Patient’s characteristics | |||||
| Age | 63 | [52.7 – 70.7] | 65.5 | [57.2 – 70.7] | 0.498 |
| Women | 15 | (50) | 7 | (25) | 0.05 |
| Level of education = university | 7 | (23.3) | 8 | (28.6) | 0.649 |
| Obesity | 13 | (43.3) | 10 | (35.7) | 0.553 |
| Diabetes | 11 | (36.7) | 2 | (7.1) | 0.007 |
| Hypertension | 14 | (46.7) | 15 | (53.6) | 0.599 |
| Depression before COVID-19 | 5 | (16.7) | 3 | (10.7) | 0.707 |
| Acute care | |||||
| Second wave | 13 | (43.3) | 12 | (42.9) | 0.971 |
| Duration of intubation, days | 24.5 | [11.2 – 30.7] | 13 | [8 – 25] | 0.140 |
| Duration of NMB, days | 7 | [2 – 11] | 4 | [2 – 9.5] | 0.519 |
| Corticosteroids | 27 | (90) | 24 | (85.7) | 0.701 |
| Length of stay in ICU, days | 9 | [6.5 – 29.5] | 7.5 | [5 – 14.7] | 0.227 |
| Rehabilitation care* | 18 | (60) | 18 | (64.3) | 0.737 |
| Weight loss in acute phase | 11.5 | [5 – 17] | 5.5 | [2.1 – 8.7] | 0.005 |
| Results are expressed as median [interquartile range] or number (percentage) PICS: post-intensive care syndrome; ICU: intensive care unit; NMB: neuromuscular blockade; PRM: Physical and Rehabilitation Medicine. *Rehabilitation care either in a PRM unit, or in private practice. |
|||||
Due to the possible effect of sex, this study compared the characteristics of PICS according to sex. Women were more likely to have a history of depression (p = 0.003). However, no difference was observed in the presence of symptoms of depression as assessed by the HADS score between men and women (18.2% of women vs 8.1% of men, p = 0.407) after ICU admission for COVID. There was a significant difference in anxiety (31.8% of women vs 8.1% of men, p = 0.03). Differences in physical impairment and cognitive impairment did not reach statistical significance (36.4% of women vs 24.3% of men, p = 0.323, and 42.9% of women vs 21.6% of men, p = 0.088).
Perceived symptoms, as evaluated by PRO, are shown in Table V. PICS was associated with worse scores on all PRO questionnaires, namely perceived dyspnoea as assessed by the mMRC score, fatigue assessed by the MFIS score, pain evaluated by the BPI score, and health-related quality of life assessed by the WHOQOL-BREF score, with the exception of the social dimension.
| PICS, N = 30 | No PICS, N = 28 | p-value | |||
| Dyspnoea (mMRC ≥ 2) | 14 | (46.7) | 4 | (14.3) | 0.008 |
| mMRC | 1 | [1 – 3] | 1 | [0 – 1] | 0.00094 |
| Fatigue (MFISa) | |||||
| MFIS > 38 | 21 | (70) | 7 | (25) | 0.00061 |
| MFIS total | 51 | [36 – 66.5] | 17 | [8 – 42] | 0.000016 |
| Physical score | 25.5 | [18 – 31] | 10 | [4 – 23] | 0.00015 |
| Cognitive score | 21.5 | [12 – 30] | 5 | [0 – 14.7] | 0.000025 |
| Psychosocial score | 5 | [2.7 – 6] | 0 | [0 – 2] | 0.000004 |
| Pain (BPI-SFb) | |||||
| BPI Pain intensity | 4.2 | [3.1 – 5.7] | 2 | [0 – 3.8] | 0.000325 |
| BPI Pain interference | 4.7 | [3 – 5.5] | 0.6 | [0 – 2.5] | 0.000005 |
| Quality of life (WHOQOLc) | |||||
| WHOQOL physical health | 46.4 | [37.4 – 58.2] | 81 | [57.7 – 89.3] | 0.000002 |
| < 25th percentile | 25 | (83.3) | 9 | (32.1) | 0.000076 |
| WHOQOL mental health | 58.3 | [44 – 67.2] | 75 | [69 – 87.9] | 0.000504 |
| < 25th percentile | 11 | (36.7) | 2 | (7.1) | 0.007 |
| WHOQOL social | 75 | [56.2 – 83.3] | 75 | [68.7 – 83.3] | 0.322 |
| < 25th percentile | 11 | (36.7) | 5 | (17.9) | 0.109 |
| WHOQOL environment | 68.7 | [46.9 – 81.1] | 88 | [75 – 94] | 0.000279 |
| All results are expressed as median [interquartile range] or number (percentage). mMRC: modified Medical Research Council; MFIS: Modified Fatigue Impact Scale; BPI-SF: Brief Pain Inventory-Short Form; WHOQOL-BREF: abbreviated World Health Organization Quality Of Life questionnaire. a4 missing data, b6 missing data, c1 missing data. |
|||||
Seventeen patients with PICS (56.7%) and 24 patients without PICS (85.7%) had returned to their usual leisure activities at 6 months (p = 0.015).
DISCUSSION
This study investigated the prevalence of PICS at 6 months after hospitalization in the ICU for severe COVID-19, comparing those hospitalized during wave 1 with those hospitalized in wave 2 in France. To the best of our knowledge, this is the first study to undertake such a comparison. The prevalence of PICS was, respectively, 51.5% and 52% in the first and second waves, as defined by impairment in 1 or more domains consistent with PICS, and 8.8% and 19.2% when considering impairment in 2 or more domains consistent with PICS. In a similar population, Kawakami et al. (29) found results close to those of the current study, i.e. 63.5% with 1 or more domains impaired and 17.8% with 2 or more domains impaired, whereas Maley et al. showed a higher prevalence (30). The prevalence of PICS is highly variable in the literature (31). The absence of a clear consensus on the measures to be used as well as the cut-offs to be prioritized may explain this variation. Furthermore, the accountability of the initial pathology, in this case COVID, must be carefully weighed, since the symptoms of PICS coincide with the symptoms reported by non-ICU patients with “long-COVID” (32). The absence of a control group in this study would have been advantageous to help clarify this point. It should nonetheless be emphasized that other studies have not found any difference in the proportion of PICS between COVID-19 and non-COVID-19 ICU patients (10, 11).
Most authors have reported PICS, not using a global definition, but rather, with a description of each disability. Regarding the prevalence of physical impairment, Colbenson et al. (33) described it as between 25% and 80% for patients post-ICU. For Heesakers et al. (10), the proportion was 74.5%. Prevalence was lower in the current study, probably due to the choice of the tests used. The 1STS was chosen because of its ease and speed of execution. It does not require any material and can be performed during a routine consultation. Also, norms exist for the general population (19). Conversely, standards for the 1STS provide the 2.5th percentile and not the 5th percentile, which may result in underestimation of the number of patients affected. If we define the presence of PICS based on the 6MWT in the current study, the proportion obtained would be closer to other studies, such as that of Huang et al. (32). However, this test is less easy to perform in current practice. In addition, even with standardized procedures, instructions and encouragement in clinical practice can differ, with a difference in results, as observed in the current study.
Other studies have used the 1STS to evaluate physical capacity in patients with COVID-19. Martin et al (34) reported a mean number of repetitions of 16 ± 8 (77% < 2.5th percentile) at ICU discharge. Nunez-Cortes et al. (35) found a score of 20.9 ± 4.8 (42% < 2.5th percentile) at 1 month after infection. Dalbosco-Salas et al. (36) evaluated patients before and after implementation of a telerehabilitation programme in primary care in post-COVID-19 patients, and reported an increase in the number of repetitions from 16.8 ± 10.8 (64.9% < 2.5th percentile) to 26.5 ± 10.5 (21.1% < 2.5th percentile) for patients hospitalized in the acute phase. The current study reports 1STS results observed at 6 months post-ICU with results closer to those of a post-rehabilitation population.
The prevalence of mental impairment and cognitive impairment after ICU also varies in the literature, between ranging 25% and 80% (33) and between 21% and 78% (37) respectively, depending on the tests and cut-offs used. The results of the current study seem to be consistent with those of Huang et al. (32) as regards mental disorders, and with those of Heesakers et al. as regards cognitive disorders (10), with the latter reporting no difference compared with PICS of other aetiologies, as for Hodgson et al. (11).
The use of the SDMT, which takes account of the level of education in its interpretation, is a strength of the current study. The interpretation of mental impairment must be balanced by the history of depression. No statistical difference was observed in the current study, but this is probably due to a lack of power, and some patients could have had a history of depression without being diagnosed. Furthermore, other authors have reported that there was a high prevalence of anxiety and depression in the general population during the COVID-19 pandemic (38). The comparison with a control group, both post-ICU and in the general population, could enhance our understanding of this point.
Finally, measuring the prevalence of PICS remains an objective that is difficult to achieve in the absence of an operationalized definition of this nosological entity. A relevant question could be the definition of different typologies of PICS and their prevalence. There seemed to be an association in the current study between mental and physical impairment, whereas cognitive impairment appears to be independent. This finding is in line with the results reported by Evans et al. (39) and Herridge et al. (40). The existence of several types of PICS and their description is clearly an interesting avenue for future research.
PRO exploring dyspnoea, pain, fatigue, and quality of life were associated with the presence of impairments consistent with PICS in the current study. This association reveals an important point for patient care. Chronic pain is described in ICU survivors (41), as described here. Fatigue is also an important issue for patients, and was significantly higher in patients with PICS. However, it should be noted that sleep quality was not assessed in the current study. In view of the reported frequency of sleep disorders after COVID-19 (32), this information must be taken into account in the interpretation of fatigue questionnaires. Lastly, quality of life has been reported in the literature to be reduced by 29% to 63% after ICU admission (42). In the current study, patients with PICS had lower scores in all dimensions except for the social domain. Finally, with very few missing data on the questionnaires in the current study, PROs seem to be a useful and easy tool to screen for patients with activity limitations and participation restrictions after ICU, which is essential for proposing and planning the necessary care. Patients with PICS were also less likely to have returned to their usual leisure activities than patients without PICS. Return to leisure activities and work is impacted in post COVID-19 patients (43), and this should be an important concern for caregivers.
No statistically significant difference was observed in the prevalence of PICS between waves, despite improvements in ICU management. Indeed, patients from wave 2 had a shorter length of stay, a shorter duration of intubation, less use of mechanical ventilation and different medical treatments, notably more corticosteroids, compared with patients from the first wave. These changes are consistent with previous reports in the literature (14, 15, 44, 45). The ABCDEF bundle has been proposed to prevent PICS (46). The reduction in the duration of orotracheal intubation and in the use of sedative drugs is in line with these recommendations, with the expected immediate effects, such as a decrease in mortality and length of stay (13, 47). Among the ABCDEF bundle, the avoidance of over-sedation and immobilization has shown an impact on PICS prevalence (12). Yet, no decrease in PICS prevalence was shown in this study. Similarly, Marra et al. (48) did not show any link between improvements in intensive care practices and the frequency of PICS. It should be noted that, in the current study, a lower proportion of patients were oriented to rehabilitation after ICU in wave 2, contrary to findings reported elsewhere (49). During wave 1, there was an upsurge in capacity in PRM departments to provide for the massive influx of patients, and to provide maximal rehabilitation support for COVID-19 patients, often to the detriment of bed availability for other diseases. The progressive return to usual activities by the time the second COVID-19 wave started resulted in reduced overall availability of rehabilitation places for COVID-19 patients. Rehabilitation care after ICU discharge for COVID-19 is presumed to be effective (50), but studies investigating the impact of rehabilitation on PICS are controversial, due to a lack of methodological rigor and the variability of interventions (51). PRM expertise is essential in this field to adapt the rehabilitation protocol with a view to reducing activity limitations and participation restrictions (52). Finally in the current study, the decrease in rehabilitation care post-ICU could explain the absence of any decrease in PICS prevalence, despite improvements in ICU management. Otherwise, this hypothesis remains to be confirmed. These findings are consistent with the complexity of the management of these patients, and the need for collaborative work between intensive care and PRM (53).
In the current study, female sex and diabetes were both associated with an increased risk of PICS. Risk factors for the emergence of PICS vary in different studies (54), but these finding are in line with other reports. The influence of sex has been highlighted in non-COVID-19 patients with PICS (55), as well as in COVID-19 patients (39). Notably, Wu et al. (56) found female sex to be associated with impaired diffusing capacity of the lungs for carbon monoxide at 12 months after COVID-19 disease. However, the possible effect of sex should be considered with caution. In particular, considering mental impairment in the diagnosis of PICS can lead to an overestimation of its prevalence in women, who are known to have a higher prevalence of anxiety disorders (57), as in the current study, or poorer self-rated health (58). A history of depression, which was more frequent among women in the current study, is also an important point to be considered. Metabolism disorders, such as diabetes, are also associated in the literature with functional loss after COVID-19 and particularly long-COVID (59, 60). However, the more frequent association of diabetes with physical disability (61), depression (62), and cognitive impairment (63) also present without COVID-19 or ICU, must be considered. In the current study, the length of stay and the duration of ventilation were not associated with an increased risk of PICS by multivariate analysis, probably due to a lack of statistical power. Indeed, several studies have found an association between these variables and PICS prevalence (64, 65). Interestingly, Milton et al (66) reported that the only predictor of PICS was the patient’s functional level at ICU discharge, which is a strong argument for systematic evaluation.
The current study has several limitations. First, the sample size is relatively small and from a single centre, and as a result, the analyses may lack statistical power. Furthermore, exhaustiveness of patient recruitment is not guaranteed, which leads to a significant risk of bias, although we did not observe any significant difference between patients who were included in the study and those who were not. The lack of data regarding the prior functional status and the lack of a control group are also important limitations. However, the current study reports measures performed during presence-based consultations with the patients, and not only self-report assessments, thereby conferring additional validity on our data. Another important question is the involvement of the different variants of SARS-Cov-2. Some studies compared the pathogenicity of different viral variants, with differences in disease severity and mortality (67). While the predominant viral variant was probably the Wuhan strain in the first wave, and the beta variant in the second wave, no viral variant identification was performed in the current study; thus it was not possible to compare the pathogenicity. To date, no information is available on the functional impact of the different variants, which could be an interesting prospect for future studies. Finally, the difference in rehabilitation care between the first and second wave could have an impact on the PICS prevalence and requires caution in the interpretation.
In conclusion, in the current study, the prevalence of PICS at 6 months after ICU discharge was not influenced by improvements in ICU management between COVID-19 waves. In this context, the identification of patients at risk, particularly women and diabetic patients, is essential. Standardization and systematic evaluation of post-COVID-19 patients in dedicated physical medicine and rehabilitation departments deserves wider development to identify functional impairment. Standardization and consensus on the definition of PICS and the best tests and cut-off thresholds to be used in practice are needed. PROs, as measured by self-report questionnaires, are useful tools for screening patients for persisting impairments, with a view to proposing appropriate care. The impact of rehabilitation after ICU must be taken into consideration. Patient care involves interprofessional collaboration, notably between intensive care and PRM. The improvement and validation of effective care programmes and pathways post-ICU discharge remains a central concern in COVID-19 and PICS. In this regard, future studies are necessary, with a view to distinguishing the damage specific to COVID-19 and to PICS.
ACKNOWLEDGEMENTS
We would like to thank all participants and all patient organizations for contributing to this study and for sharing their experiences. We particularly want to thank Fiona Ecarnot, PhD (University Hospital Besancon, France) for her work in the article translation, and the Fondation de France and the Reims University Hospital for funding the CAPACoV-19 study.
REFERENCES
- Nanwani-Nanwani K, López-Pérez L, Giménez-Esparza C, Ruiz-Barranco I, Carrillo E, Arellano MS, et al. Prevalence of post-intensive care syndrome in mechanically ventilated patients with COVID-19. Sci Rep 2022; 12: 7977. DOI:10.1038/s41598-022-11929-8.
- Martillo MA, Dangayach NS, Tabacof L, Spielman LA, Dams-O’Connor K, Chan CC, et al. Postintensive care syndrome in survivors of critical illness related to coronavirus disease 2019: cohort study from a New York City critical care recovery clinic. Crit Care Med 2021; 49: 1427–1438. DOI: 10.1097/CCM.0000000000005014.
- Ohtake PJ, Lee AC, Scott JC, Hinman RS, Ali NA, Hinkson CR, et al. Physical impairments associated with post-intensive care syndrome: systematic review based on the World Health Organization’s International Classification of Functioning, Disability and Health Framework. Phys Ther 2018; 98: 631–645. DOI: 10.1093/ptj/pzy059.
- Brown SM, Bose S, Banner-Goodspeed V, Beesley SJ, Dinglas VD, Hopkins RO, et al. Approaches to addressing post-intensive care syndrome among intensive care unit survivors. A narrative review. Ann Am Thorac Soc 2019; 16: 947–956. DOI: 10.1513/AnnalsATS.201812-913FR.
- Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40: 502–509. DOI: 10.1097/CCM.0b013e318232da75.
- Inoue S, Hatakeyama J, Kondo Y, Hifumi T, Sakuramoto H, Kawasaki T, et al. Post-intensive care syndrome: its pathophysiology, prevention, and future directions. Acute Med Surg 2019; 6: 233–246. DOI: 10.1002/ams2.415.
- Kamdar BB, Suri R, Suchyta MR, Digrande KF, Sherwood KD, Colantuoni E, et al. Return to work after critical illness: a systematic review and meta-analysis. Thorax 2020; 75: 17–27. DOI: 10.1136/thoraxjnl-2019-213803.
- Flaatten H, Kvåle R. Survival and quality of life 12 years after ICU. A comparison with the general Norwegian population. Intensive Care Med 2001; 27: 1005–1011. DOI: 10.1007/s001340100960.
- Nakanishi N, Liu K, Kawakami D, Kawai Y, Morisawa T, Nishida T, et al. Post-intensive care syndrome and its new challenges in coronavirus disease 2019 (COVID-19) pandemic: a review of recent advances and perspectives. J Clin Med 2021; 10: 3870. DOI: 10.3390/jcm10173870.
- Heesakkers H, van der Hoeven JG, Corsten S, Janssen I, Ewalds E, Simons KS, et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA 2022; 327: 559–565. DOI: 10.1001/jama.2022.0040.
- Hodgson CL, Higgins AM, Bailey MJ, Mather AM, Beach L, Bellomo R, et al. Comparison of 6-month outcomes of survivors of COVID-19 versus non-COVID-19 critical illness. Am J Respir Crit Care Med 2022; 205: 1159–1168. DOI: 10.1164/rccm.202110-2335OC.
- Lee Y, Kim K, Lim C, Kim J-S. Effects of the ABCDE bundle on the prevention of post-intensive care syndrome: a retrospective study. J Adv Nurs 2020; 76: 588–599. DOI: 10.1111/jan.14267.
- Pun BT, Balas MC, Barnes-Daly MA, Thompson JL, Aldrich JM, Barr J, et al. Caring for critically ill patients with the ABCDEF Bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med 2019; 47: 3–14. DOI: 10.1097/CCM.0000000000003482.
- Parker AJ, Mishra M, Tiwary P, Sharman M, Priya-Sharma M, Duncan A, et al. A tale of two waves: changes in the use of noninvasive ventilation and prone positioning in critical care management of coronavirus disease 2019. Crit Care Explor 2021; 3: e0587. DOI: 10.1097/CCE.0000000000000587.
- Carbonell R, Urgelés S, Rodríguez A, Bodí M, Martín-Loeches I, Solé-Violán J, et al. Mortality comparison between the first and second/third waves among 3,795 critical COVID-19 patients with pneumonia admitted to the ICU: a multicentre retrospective cohort study. Lancet Reg Health Eur 2021; 11: 100243. DOI: 10.1016/j.lanepe.2021.100243.
- Guillon A, Laurent E, Godillon L, Kimmoun A, Grammatico-Guillon L. In-hospital mortality rates of critically ill COVID-19 patients in France: a nationwide cross-sectional study of 45 409 ICU patients. Br J Anaesth 2021; 127: e180–182. DOI: 10.1016/j.bja.2021.08.006.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014 Dec;12(12):1495-9. doi: 10.1016/j.ijsu.2014.07.013. Epub 2014 Jul 18. PMID: 25046131.
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993 Dec 22-29;270(24):2957-63. doi: 10.1001/jama.270.24.2957. Erratum In: JAMA 1994 May 4;271(17):1321. PMID: 8254858.
- Bohannon RW, Crouch R. 1-Minute Sit-to-Stand Test: systematic review of procedures, performance, and clinimetric properties. J Cardiopulm Rehabil Prev 2019; 39: 2–8. DOI: 10.1097/HCR.0000000000000336.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. DOI:10.1164/ajrccm.166.1.at1102.
- Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 1998; 158: 1384–1387. DOI: 10.1164/ajrccm.158.5.9710086.
- Strober LB, Bruce JM, Arnett PA, Alschuler KN, Lebkuecher A, Di Benedetto M, et al. A new look at an old test: normative data of the symbol digit modalities test -Oral version. Mult Scler Relat Disord 2020; 43: 102154. DOI:10.1016/j.msard.2020.102154.
- Sheridan LK, Fitzgerald HE, Adams KM, Nigg JT, Martel MM, Puttler LI, et al. Normative Symbol Digit Modalities Test performance in a community-based sample. Arch Clin Neuropsychol 2006; 21: 23–28. DOI: 10.1016/j.acn.2005.07.003.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361–370. DOI: 10.1111/j.1600-0447.1983.tb09716.x.
- Learmonth YC, Dlugonski D, Pilutti LA, Sandroff BM, Klaren R, Motl RW. Psychometric properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J Neurol Sci 2013; 331: 102–107. DOI: 10.1016/j.jns.2013.05.023.
- Larson RD. Psychometric properties of the modified fatigue impact scale. Int J MS Care 2013; 15: 15–20. DOI:10.7224/1537-2073.2012-019.
- Poquet N, Lin C. The Brief Pain Inventory (BPI). J Physiother 2016; 62: 52. DOI:10.1016/j.jphys.2015.07.001.
- Baumann C, Erpelding M-L, Régat S, Collin J-F, Briançon S. The WHOQOL-BREF questionnaire: French adult population norms for the physical health, psychological health and social relationship dimensions. Rev Epidemiol Sante Publique 2010; 58: 33–39. DOI: 10.1016/j.respe.2009.10.009.
- Kawakami D, Fujitani S, Morimoto T, Dote H, Takita M, Takaba A, et al. Prevalence of post-intensive care syndrome among Japanese intensive care unit patients: a prospective, multicenter, observational J-PICS study. Crit Care 2021; 25: 69. DOI:10.1186/s13054-021-03501-z.
- Maley JH, Sandsmark DK, Trainor A, Bass GD, Dabrowski CL, Magdamo BA, et al. Six-month impairment in cognition, mental health, and physical function following COVID-19-associated respiratory failure. Crit Care Explor 2022; 4: e0673. DOI:10.1097/CCE.0000000000000673.
- Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Int Med 2017; 5: 90–92. DOI: 10.1515/jtim-2016-0016.
- Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220–232. DOI: 10.1016/S0140-6736(20)32656-8.
- Colbenson GA, Johnson A, Wilson ME. Post-intensive care syndrome: impact, prevention, and management. Breathe (Sheff) 2019; 15: 98–101. DOI: 10.1183/20734735.0013-2019.
- Martin I, Braem F, Baudet L, Poncin W, Fizaine S, Aboubakar F, et al. Follow-up of functional exercise capacity in patients with COVID-19: It is improved by telerehabilitation. Respir Med 2021; 183: 106438. DOI: 10.1016/j.rmed.2021.106438.
- Núñez-Cortés R, Rivera-Lillo G, Arias-Campoverde M, Soto-García D, García-Palomera R, Torres-Castro R. Use of sit-to-stand test to assess the physical capacity and exertional desaturation in patients post COVID-19. Chron Respir Dis 2021; 18: 1479973121999205. DOI: 10.1177/1479973121999205.
- Dalbosco-Salas M, Torres-Castro R, Rojas Leyton A, Morales Zapata F, Henríquez Salazar E, Espinoza Bastías G, et al. Effectiveness of a primary care telerehabilitation program for post-COVID-19 patients: a feasibility study. J Clin Med 2021; 10: 4428. DOI:10.3390/jcm10194428.
- Palakshappa JA, Krall JTW, Belfield LT, Files DC. Long-term outcomes in acute respiratory distress syndrome: epidemiology, mechanisms, and patient evaluation. Crit Care Clin 2021; 37: 895–911. DOI: 10.1016/j.ccc.2021.05.010.
- Salari N, Hosseinian-Far A, Jalali R, Vaisi-Raygani A, Rasoulpoor S, Mohammadi M, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health 2020; 16: 57. DOI: 10.1186/s12992-020-00589-w.
- Evans RA, McAuley H, Harrison EM, Shikotra A, Singapuri A, Sereno M, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med 2021; 9: 1275–1287. DOI: 10.1016/S2213-2600(21)00383-0.
- Herridge MS, Chu LM, Matte A, Tomlinson G, Chan L, Thomas C, et al. The RECOVER program: disability risk groups and 1-year outcome after 7 or more days of mechanical ventilation. Am J Respir Crit Care Med 2016; 194: 831–844. DOI: 10.1164/rccm.201512-2343OC.
- Kemp HI, Laycock H, Costello A, Brett SJ. Chronic pain in critical care survivors: a narrative review. Br J Anaesth 2019; 123: e372–384. DOI: 10.1016/j.bja.2019.03.025.
- Granja C, Teixeira-Pinto A, Costa-Pereira A. Quality of life after intensive care--evaluation with EQ-5D questionnaire. Intensive Care Med 2002; 28: 898–907. DOI: 10.1007/s00134-002-1345-z.
- O’Brien K, Townsend L, Dowds J, Bannan C, Nadarajan P, Kent B, et al. 1-year quality of life and health-outcomes in patients hospitalised with COVID-19: a longitudinal cohort study. Respir Res 2022; 23: 115. DOI: 10.1186/s12931-022-02032-7.
- Roth GA, Emmons-Bell S, Alger HM, Bradley SM, Das SR, de Lemos JA, et al. Trends in patient characteristics and COVID-19 in-hospital mortality in the United States during the COVID-19 pandemic. JAMA Netw Open 2021; 4: e218828. DOI: 10.1001/jamanetworkopen.2021.8828.
- Jarrett SA, Lo KB, Shah S, Zanoria MA, Valiani D, Balogun OO, et al. Comparison of patient clinical characteristics and outcomes between different COVID-19 peak periods: a single center retrospective propensity matched analysis. Cureus 2021; 13: e15777. DOI: 10.7759/cureus.15777.
- Balas MC, Vasilevskis EE, Olsen KM, Schmid KK, Shostrom V, Cohen MZ, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med 2014; 42: 1024–1036. DOI: 10.1097/CCM.0000000000000129.
- Frade-Mera MJ, Arias-Rivera S, Zaragoza-García I, Martí JD, Gallart E, San José-Arribas A, et al. The impact of ABCDE bundle implementation on patient outcomes: a nationwide cohort study. Nurs Crit Care 2022. DOI: 10.1111/nicc.12740.
- Marra A, Pandharipande PP, Girard TD, Patel MB, Hughes CG, Jackson JC, et al. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit Care Med 2018; 46: 1393–1401. DOI: 10.1097/CCM.0000000000003218.
- Wolfisberg S, Gregoriano C, Struja T, Kutz A, Koch D, Bernasconi L, et al. Comparison of characteristics, predictors and outcomes between the first and second COVID-19 waves in a tertiary care centre in Switzerland: an observational analysis. Swiss Med Wkly 2021; 151: w20569. DOI: 10.4414/smw.2021.20569.
- Bangash MN, Owen A, Alderman JE, Chotalia M, Patel JM, Parekh D. COVID-19 recovery: potential treatments for post-intensive care syndrome. Lancet Respir Med 2020; 8: 1071–1073. DOI: 10.1016/S2213-2600(20)30457-4.
- Connolly B, Salisbury L, O’Neill B, Geneen L, Douiri A, Grocott MPW, et al. Exercise rehabilitation following intensive care unit discharge for recovery from critical illness. Cochrane Database Syst Rev 2015; (6): CD008632. DOI: 10.1002/14651858.CD008632.pub2.
- Oral A, Juocevicius A, Lukmann A, Takáč P, Tederko P, Hāznere I, et al. Evidence-based position paper on Physical and Rehabilitation Medicine (PRM) professional practice for people with respiratory conditions. The European PRM position (UEMS PRM Section). Eur J Phys Rehabil Med 2018; 54: 624–633. DOI: 10.23736/S1973-9087.18.05309-1.
- Stam HJ, Stucki G, Bickenbach J, European Academy of Rehabilitation Medicine. Covid-19 and post intensive care syndrome: a call for action. J Rehabil Med 2020; 52: jrm00044. DOI: 10.2340/16501977-2677.
- Vrettou CS, Mantziou V, Vassiliou AG, Orfanos SE, Kotanidou A, Dimopoulou I. Post-Intensive care syndrome in survivors from critical illness including COVID-19 Patients: a narrative review. Life (Basel) 2022; 12:107. DOI: 10.3390/life12010107.
- Gandotra S, Lovato J, Case D, Bakhru RN, Gibbs K, Berry M, et al. Physical function trajectories in survivors of acute respiratory failure. Ann Am Thorac Soc 2019; 16: 471–477. DOI: 10.1513/AnnalsATS.201806-375OC.
- Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med 2021; 9: 747–754. DOI: 10.1016/S2213-2600(21)00174-0.
- Asher M, Aderka IM. Gender differences in social anxiety disorder. J Clin Psychol 2018; 74: 1730–1741. DOI: 10.1002/jclp.22624.
- Idler EL. Discussion: Gender differences in self-rated health, in mortality, and in the relationship between the two. Gerontologist 2003; 43:372–375.
- Loosen SH, Jensen B-EO, Tanislav C, Luedde T, Roderburg C, Kostev K. Obesity and lipid metabolism disorders determine the risk for development of long COVID syndrome: a cross-sectional study from 50,402 COVID-19 patients. Infection 2022. DOI: 10.1007/s15010-022-01784-0.
- Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022; 185: 881-895.e20. DOI: 10.1016/j.cell.2022.01.014.
- Wong E, Backholer K, Gearon E, Harding J, Freak-Poli R, Stevenson C, et al. Diabetes and risk of physical disability in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2013; 1: 106–114. DOI: 10.1016/S2213-8587(13)70046-9.
- Moulton CD, Pickup JC, Ismail K. The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol 2015; 3: 461–471. DOI: 10.1016/S2213-8587(15)00134-5.
- Antal B, McMahon LP, Sultan SF, Lithen A, Wexler DJ, Dickerson B, et al. Type 2 diabetes mellitus accelerates brain aging and cognitive decline: Complementary findings from UK Biobank and meta-analyses. eLife 2022; 11: e73138. DOI: 10.7554/eLife.73138.
- Schandl A, Hedman A, Lyngå P, Fathi Tachinabad S, Svefors J, Roël M, et al. Long-term consequences in critically ill COVID-19 patients: a prospective cohort study. Acta Anaesthesiol Scand 2021; 65: 1285–1292. DOI: 10.1111/aas.13939.
- Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med 2014; 42: 849–859. DOI: 10.1097/CCM.0000000000000040.
- Milton A, Schandl A, Soliman I, Joelsson-Alm E, van den Boogaard M, Wallin E, et al. ICU discharge screening for prediction of new-onset physical disability – a multinational cohort study. Acta Anaesthesiol Scand 2020; 64: 789–797. DOI: 10.1111/aas.13563.
- Abu-Raddad LJ, Chemaitelly H, Ayoub HH, Yassine HM, Benslimane FM, Al Khatib HA, et al. Severity, criticality, and fatality of the SARS-CoV-2 beta variant. Clin Infect Dis 2021; ciab909. DOI: 10.1093/cid/ciab909.