ORIGINAL REPORT
CEREBRAL THETA-BURST STIMULATION COMBINED WITH PHYSIOTHERAPY IN PATIENTS WITH INCOMPLETE SPINAL CORD INJURY: A PILOT RANDOMIZED CONTROLLED TRIAL
Xiaojun FENG, MD1–3#, Tingting WANG, MS1,3#, Yan JIANG, MS1#, Yi LIU, MD1, Haifeng YANG, BS3, Zongyu DUAN, MS3, Leilei JI, MS1 and Juan WEI, BS1
From the 1Department of Rehabilitation Medicine, 2Research Center for Translational Medicine, The Second Hospital of Anhui Medical University, Hefei City, Anhui Province and 3Department of Rehabilitation Medicine, The Fuyang Hospital of Anhui Medical University, Fuyang City, Anhui Province, China
#These authors contributed equally to this article.
Objective: To measure the effects of cerebral intermittent theta-burst stimulation with physiotherapy on lower extremity motor recovery in patients with incomplete spinal cord injury.
Design: Randomized, double-blinded, sham-controlled trial.
Subjects: Adults with incomplete spinal cord injury.
Methods: A total of 38 patients with incomplete spinal cord injury were randomized into either an intermittent theta-burst stimulation or a sham group. Both groups participated in physiotherapy 5 times per week for 9 weeks, and cerebral intermittent theta-burst stimulation or sham intermittent theta-burst stimulation was performed daily, immediately before physiotherapy. The primary outcomes were lower extremity motor score (LEMS), root-mean square (RMS), RMS of the quadriceps femoris muscle, walking speed (WS), and stride length (SL). Secondary outcomes comprised Holden Walking Ability Scale (HWAS) and modified Barthel Index (MBI). The outcomes were assessed before the intervention and 9 weeks after the start of the intervention.
Results: Nine weeks of cerebral intermittent thetaburst stimulation with physiotherapy intervention resulted in improved recovery of lower extremity motor recovery in patients with incomplete spinal cord injury. Compared with baseline, the changes in LEMS, WS, SL, RMS, HWAS, and MBI were significant in both groups after intervention. The LEMS, WS, SL, RMS, HWAS, and MBI scores were improved more in the intermittent theta-burst stimulation group than in the sham group.
Conclusion: Cerebral intermittent theta-burst stimulation with physiotherapy promotes lower extremity motor recovery in patients with incomplete spinal cord injury. However, this study included a small sample size and lacked a comparison of the treatment effects of multiple stimulation modes, the further research will be required in the future.
LAY ABSTRACT
Spinal cord injury is a serious condition caused by spinal trauma and tumours. Improving the patient’s limb function during recovery poses an important challenge. Transcranial magnetic stimulation technology is a new treatment used to improve nervous system function, which has shown promising results in treating spinal cord injuries in recent years. However, the effect of a specific type of magnetic stimulation, cerebral intermittent theta-burst stimulation, with routine physical therapy on lower extremity motor recovery in patients with incomplete spinal cord injury has not yet been explored. The results of this study suggest that 9 weeks of brain intermittent theta-burst stimulation combined with physical therapy has a positive short-term effect on lower extremity movement and recovery of daily living ability in patients with incomplete spinal cord injury, which might provide new insight into motor rehabilitation for spinal cord injury.
Key words: intermittent theta-burst stimulation; physiotherapy; spinal cord injury; lower extremity motor function.
Citation: J Rehabil Med 2023; 55: jrm00375. DOI: https://doi.org/10.2340/jrm.v55.4375
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jan 5, 2023; Published: Feb 13, 2023
Correspondence address: Xiaojun Feng, Department of Rehabilitation Medicine, The Second Hospital of Anhui Medical University, Hefei City, Anhui Province, 230601, China. Email: fengxiaojun@ahmu.edu.cn
Competing interests and funding: The authors have no conflicts of interest to declare.
A spinal cord injury (SCI) causes temporary or permanent changes in the function of the spinal cord. It is among the most common and most serious conditions in clinical practice (1), affecting approximately 10.4/1 million to 83/1 million people worldwide (2) and resulting in a great economic burden to social development and the patient’s family. The main pathological sign of SCI is neurological deficit (3), which results in motor, sensory and autonomic dysfunction below the level of injury, thus reducing the quality of life and life satisfaction of patients (4). Survey studies indicate that within 10 years after SCI, besides arm/hand function, which has been reported to have the highest priority, those with SCI prioritize the recovery of lower extremity motor function (5). Therefore, the main challenge of rehabilitation medicine is how to promote the recovery of lower extremity motor function in patients with SCI and allow them to regain the ability to walk (6, 7).
SCI is a very complex pathological process, during which it is impossible to achieve satisfactory therapeutic effects with a single treatment pattern (8). Currently, the clinical treatment of patients with SCI is mainly based on comprehensive treatment. Conventional treatment methods include decompression surgery, anti-inflammatory drugs, exercise therapy, breathing training, physical factor therapy, and stem cell transplantation (9). With an increasing understanding of the pathological mechanism of SCI and developments in science and technology (10–12), new technologies have also been used for SCI rehabilitation. Magnetic stimulation is a new technology used for non-invasive biological stimulation (NIBS) (13), which is based on a time-varying current flowing into a coil, generating a time-varying magnetic field, and inducing current in the tissue, thus exciting or inhibiting the tissue. The tissue means brain tissue; TMS can stimulate or inhibit local cortical function by varying its stimulation frequency, which is based on time-varying. As a non-invasive method for diagnosis and treatment, repetitive transcranial magnetic stimulation (rTMS) is gradually becoming a key factor in the rehabilitation of nervous system diseases (14). Previous studies have shown that rTMS may be a valuable therapeutic approach for promoting recovery from SCI (15). Intermittent theta-burst stimulation (iTBS) is a specific type of stimulation mode in rTMS (16, 17), the mechanism of action of which includes changing cortical excitability, inducing long-term potentiation (LTP), or long-term depression (LTD) (18), promoting neural remodelling, and stimulating the release of neurotransmitters. Compared with traditional rTMS (19, 20), iTBS has the advantages of higher frequency, lower intensity, shorter stimulation time, and longer duration of changing cortical excitability (21–23). We hypothesized that iTBS would promote motor-related recovery by enhancing the transmission of corticospinal pathways. To test this hypothesis, individuals with incomplete spinal cord injury (iSCI) were randomly assigned to physiotherapy combined with iTBS or sham-iTBS group. The short-term effect on lower extremity movement and recovery of daily living ability were measured before the intervention and 9 weeks after the start of the intervention.
Thus far, there are limited data on the effects of iTBS on post-SCI rehabilitation. The current study is the first to explore the short-term effects of cerebral iTBS coupled with routine physical therapy on lower extremity motor recovery in convalescence following SCI. This study not only provides an in-depth understanding of the relationship between brain and SCI rehabilitation, but also provides a scientific basis for the clinical application of iTBS.
MATERIAL AND METHODS
Participants
A total of 50 patients with SCI who were hospitalized in the department of rehabilitation medicine in the Second Affiliated Hospital of Anhui Medical University, Hefei City, Anhui, China, between August 2018 and August 2021 were continuously recruited. Experts checked the medical records of participants to determine whether they met the following inclusion criteria: (i) age 14–75 years; (ii) SCI confirmed by magnetic resonance imaging (MRI), in line with the diagnostic criteria updated by the American Spinal Cord Injury Association in 2015 (24); (iii) no other diseases that could affect lower extremity motor dysfunction and no sequelae symptoms; (iv) ASIA Impairment Scale (AlS) C or D (24), or motor dysfunction of both lower extremities, accompanied by caregivers during hospitalization; (v) stable vital signs, good cognitive function, able to cooperate with professional physicians for evaluation and treatment.
Exclusion criteria were: (i) intracranial metal foreign bodies, pacemakers, and cochlear implants; (ii) unstable conditions, cognitive impairment, severe complications, and severe heart, brain, and lung diseases; (iii) major organ diseases, such as fractures, joint contractures, and lower extremity spasms that affect the motor function of the lower extremities; (iv) use of drugs that could affect cortical excitability; (v) pregnancy; and (vi) cardiac pacemaker.
Of a total of 50 patients, 5 did not meet the inclusion criteria, 4 declined to participate, and 3 were excluded for other reasons. Following the randomization principle, the remaining 38 patients were divided randomly into an iTBS group and a sham group in a ratio of 1:1, using the random number table method after obtaining informed signed consent from the patients or their guardians. The specific methods were as follows: 38 patients with SCI (numbers 1–38) were divided into 2 groups by the principal investigator (PI). The subjects corresponding to these 19 numbers were assigned to 1 group, and the remaining subjects were assigned to another group. The randomization schedule was concealed in a locked cabinet accessed only by the PI and the investigators who administer iTBS. Hence, patients were blind regarding the type of transcranial magnetic stimulation (TMS) they received (real or sham). Neither the participants nor the researchers assessing outcomes were aware of the interventions administered. Subjects were blinded to the experimental assumptions and were not allowed to discuss their experience during the intervention with the researchers or other subjects involved in assessing the results. The study was approved by the Clinical Research Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (registration number: SL-LC2019037 (F1). The study protocol was registered with the Chinese Clinical Trial Registry (http://www.chictr.org.cn, Clinical Registration number: ChiCTR2200059431).
Study design
This pilot study was designed as a randomized, double-blind, sham-controlled clinical trial, in which both the participants and assessors were blinded. All patients underwent consecutive daily sessions of active or sham iTBS combined with physical therapy for 9 weeks. Patients and investigators (except the technician who applied iTBS) were blinded to the treatment (Fig. 1). Immediately after cerebellar or sham iTBS, all participants in this trial received standard physical therapy that included trunk control training, sit-to-stand training, balance exercises, and gait training.
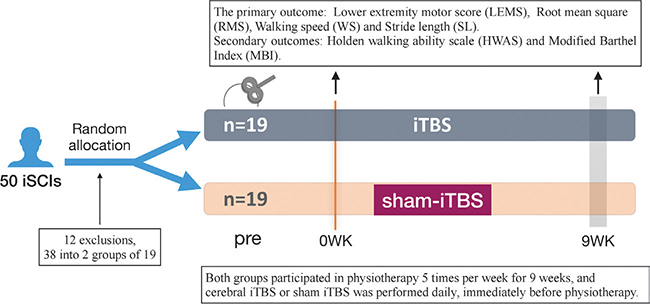
Fig. 1. Study design for short-term effects of cerebral intermittent theta-burst stimulation (iTBS) with physiotherapy on lower extremity motor recovery in patients with spinal cord injury. iTBS treatment was performed 5 days a week for 9 successive weeks. Lower extremity motor score (LEMS), root mean square (RMS), walking speed (WS), stride length (SL), Holden Walking Ability Scale (HWAS) and modified Barthel index (MBI) were assessed before and 9 weeks after the start of the intervention.
Standard physical therapy programme
All patients received standard SCI rehabilitation treatment and nursing in the Second Affiliated Hospital of Anhui Medical University, which included routine rehabilitation care, nutritional nerve drugs, motor function training for paralysed limbs (once/day, 40 min/session), electroacupuncture (for paralysed limbs, 2 sessions/day, 20 min/session), electronic biofeedback therapy (for the site of paralysed limbs, 2 sessions/day, 20 min/session), lower limb robot training (1 session/day, 30 min/session), etc. Routine rehabilitation care included guidance regarding good posture, prevention of bedsores, urethral care, and psychological counselling. Patients were treated 5 days a week for a total of 9 weeks. This programme was administered by 2 specifically trained physical therapists, both of whom were blinded.
Intermittent theta-burst stimulation intervention
We used a CCY-1 magnetic stimulator (figure-8-coil, diameter 12.5 cm, and maximum stimulation intensity 3T) (Wuhan City, Hubei Province,China) to stimulate cerebral of patients. During the treatment, the patient was placed in a supine position. The magnetic stimulation coil was tangent to the surface of the patient’s skull, whilst the patient kept their eyes closed. The midpoint of the coil was aligned with the motor area (cortical M1 region) of the legs of the bilateral brain (25). The stimulation selected was iTBS mode. There were 600 pulses in total. The treatment period for each side was 3 min 20 s; the 2 sides were treated for a total of 6 min 40 s. The stimulation intensity selected was 100% of the rest motor threshold (RMT) of the patient’s cerebral hemisphere, and the treatment was performed once a day, 5 times a week for 9 weeks.
Sham-intermittent theta-burst stimulation intervention
Patients in the sham-iTBS group were treated 5 consecutive times per week for 9 weeks (once a day). A dummy coil was used to ensure attenuation of the magnetic field, while at the same time appearing to be the same shape as the active coil with a good approximation of the auditory feedback (18). Also, the coil was kept in tactile contact with the skull. The sham coil did not generate an electric field and could not induce any neural activation.
Outcome assessments
Lower extremity motor score (LEMS). The muscle strength of the key muscle of the lower extremities of the patients in the control group and the experimental group was evaluated using the manual muscle strength grading standard (26). Each lower extremity was evaluated for 5 groups of muscles, which were recorded as 0–5 points, respectively, with a total score of 50 points. The higher the score, the greater the muscle strength and the greater the motor function of the lower extremity.
Root-mean square value. TrignoWireless wireless electromyographic (EMG) signal device of Delsys Company (Colorado Springs, CO, USA) in the USA was used to collect the surface EMG signal of the quadriceps femoris muscle of both lower extremities and select the root-mean square (RMS) value of the 1 s peak value. For the convenience of recording, the unit was 10-5V·S, the surface EMG signals were collected 3 times repeatedly, and the mean value was recorded. The increase in amplitude indicated the enhancement of muscle strength, i.e. RMS was proportional to muscle strength (27).
Gait function. The walking function of the 2 groups of patients was collected before and after treatment, and the walking speed (WS) and stride length (SL) of the lower extremities were evaluated using the digital treadmill Tecno Body Walker-view (Bergamo, Bergamo, Italy) (28). Tecno Body Medical Fitness Software (version number: 2.7.8.0) was used to process the collected data and obtain WS and SL, taking 3 repeated measurements, from which the mean was determined.
Holden Walking Ability Scale. The Holden Walking Ability Scale (HWAS) grades are: 0: unable to walk; 1: needs help with walking; 2: needs substantial assistance with walking; 3: needs a little help with walking; 4: can walk independently on a flat ground; and 5 walking is normal (29).
Modified Barthel Index. The modified Barthel Index (MBI) was used for assessment of activities of daily living (ADL) from 10 aspects (30): defecation, urination, grooming, going to the toilet, eating, transferring, moving, dressing, going upstairs, and bathing. The highest score was 100 points; the higher the score, the greater the daily living ability.
Sample size
The sample size was calculated using a G * power of 3.1.9.3. The effect size (f) was calculated as 0.26 based on the lower extremity motor scores (LEMS) from a group of individuals, as reported by Benito et al. (31). To achieve improvements with α = 0.01 and β at 80%, the necessary sample size of n = 19 per group was revealed by a power analysis. The final sample size required 25 participants per group to allow for a 20% dropout rate.
Statistical analysis
The experimental data were analysed using SPSS 22.0 (IBM Corp., Armonk, NY, USA). All data are presented as the mean±standard error of the mean (SEM). Sex, injury segment, and AIS grading of patients were enumeration data and were measured by the χ2 test. The effect of iTBS for all outcome measures was evaluated by repeated-measures analysis of variance (ANOVA) with groups (iTBS and sham-iTBS) as a between-subjects factor and time (pre- and post-assessment) as a within-subject factor. A separate effect analysis was performed when the interaction between time and groups was statistically significant. Unpaired t-tests were performed to compare groups at each time point when the main effect of the group was significant. Furthermore, separate 1-way ANOVA followed by post hoc Bonferroni tests were used to compare time points when needed. p < 0.05 indicated statistical significance.
RESULTS
Participants
Among 50 patients, 12 did not meet the criteria for participation, including 5 patients who did not meet the criteria for inclusion, 4 who refused to participate in the study, and 3 who were excluded from the study due to recent mental disorders (n = 1) and complete motor injuries (n = 2). Finally, 38 participants were randomized to receive transcranial magnetic stimulation with (iTBS, n = 19) or (sham iTBS, n = 19) (see flow chart in Fig. 2). In particular, 1 patient with SCI, age 14 years, was enrolled in the current trial with the consent of the patient and guardian.
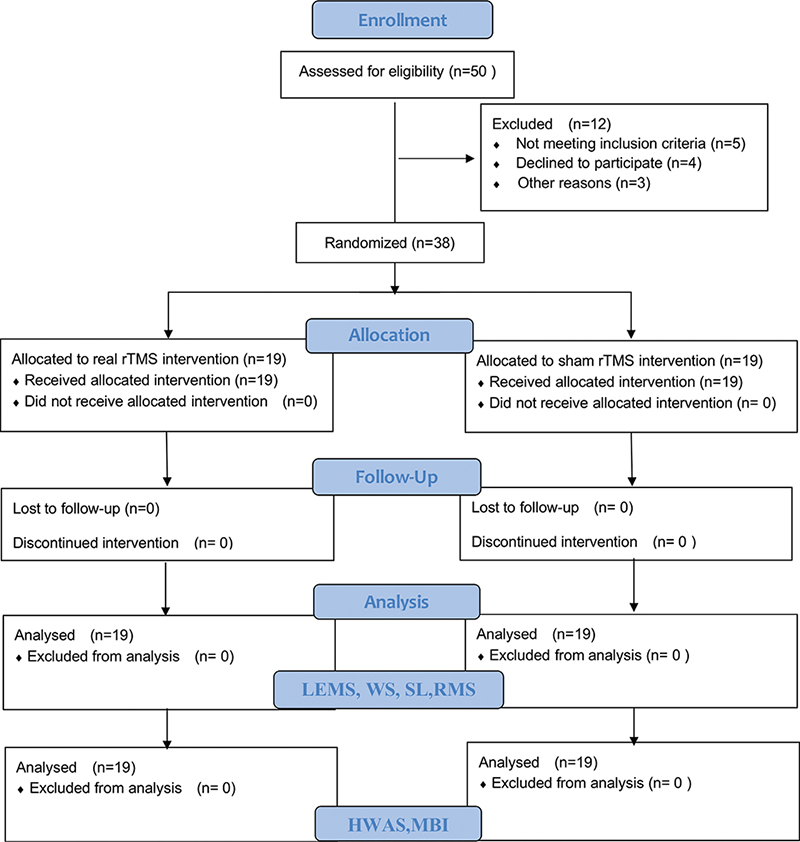
Fig. 2. CONSORT flow diagram of enrollment, randomization, and follow-up. iTBS: intermittent theta-burst stimulation; SCI: spinal cord injury; LEMS: left lower extremity motor score; RRMS: right root mean square; WS: walking speed; LSL: left stride length; RSL: right stride length; HWAS: Holden Walking Ability Scale; MBI: modified Barthel Index.
The baseline characteristics of the included study population are shown in Table I. There were no significant differences between the 2 groups in demographic variables, level of neurological injury, severity of injury, duration of injury, and classification (all p > 0.05). In addition, there were no significant baseline differences between groups in any outcome measures (p > 0.05).
| Characteristics at baseline | Cerebral iTBS (n = 19) | Sham iTBS (n = 19) | p - value | ||||||||||||||||||||
| Age, years, mean±SD | 45.6 ± 15.3 | 37.5 ± 14.2 | 0.096e | ||||||||||||||||||||
| Sex (male/female), n | 15/4 | 15/4 | 1c | ||||||||||||||||||||
| BMI, mean±SD | 27.8 ± 5.7 | 24.4 ± 7.5 | 0.836e | ||||||||||||||||||||
| Time since SCI onset (days), mean±SD | 109.8 ± 100.8 | 101.1±67.5 | 0.755e | ||||||||||||||||||||
| Injured segment (neck/chest/ waist), n | 10/3/6 | 8/5/6 | 0.623c | ||||||||||||||||||||
| Aetiology (traumatic/non-traumatic), n | 14/5 | 14/5 | 1c | ||||||||||||||||||||
| NLIa, mean ± SD | 14.79 ± 7.89 | 15.58 ± 7.62 | 0.756e | ||||||||||||||||||||
| AISb, median (95% CI) | 3 (2; 3) | 3 (2; 3) | 0.418d | ||||||||||||||||||||
| SD: standard deviation; BMI: body mass index; SCI: spinal cord injury; NLI: neurological level of injury; AIS: American Spinal Cord Injury Association (ASIA) Impairment Scale; iTBS: Intermittent Theta-burst Stimulation; 95% CI: 95% confidence interval. aCalculated as C1 = 1, C2 = 2… S5 = 30. bAIS classification, calculated as A = 0, B = 1, C = 2, D = 3. cAnalysed by χ2 test. dAnalysed by Wilcoxon Mann–Whitney U test. eAnalysed by Student’s independent t-test. |
|||||||||||||||||||||||
Effect of cerebral intermittent theta-burst stimulation on key muscle strength of lower extremity after incomplete spinal cord injury
To evaluate the effect of cerebral iTBS on lower-extremity motor function after iSCI, the total key muscle strength score of the lower extremity (LEMS) was detected pre- and post-iTBS treatment. Repeated measures ANOVA applied to the total key muscle strength score revealed a significant group–time interaction (F1.36 = 36.37, p < 0.0001), main effects of time (F1.36 = 146.5 p < 0.0001), and no significant main effects of group (F1.36 = 1.681, p = 0.20). Furthermore, single effect analysis indicated significant changes in the muscle strength scores of key muscle groups before and after treatment in the 2 groups (t = 2.62, p = 0.000). In addition, the improvement in the key muscle strength scores of the lower extremity in the iTBS group was greater than in the sham group (Fig. 3A).
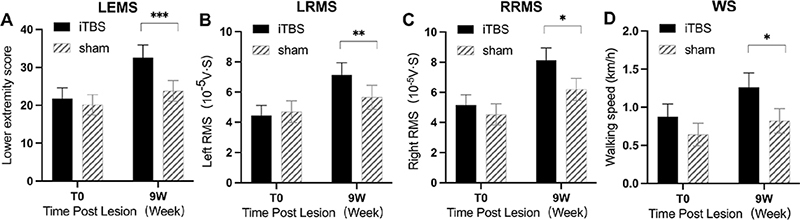
Fig. 3. Effect of cerebral intermittent theta-burst stimulation (iTBS) on key muscle strength of lower extremity, root-mean square of quadriceps femoris, and walking speed after incomplete spinal cord injury (iSCI). Significant differences were observed between the iTBS and sham groups in (A) overall left lower extremity motor score (LEMS), (B) left root mean square (LRMS), (C) right root mean square (RRMS), and (D) walking speed (WS) at 9 weeks after the start of the intervention. Values are expressed as the mean±standard error of the mean (SEM). *p < 0.05, **p < 0.01, ***p < 0.001, significant difference between the 2 groups.
Effect of cerebral intermittent theta-burst stimulation on the root-mean square of quadriceps femoris after incomplete spinal cord injury
The RMS value of the quadriceps femoris muscle reflects the surface EMG value of patients’ lower limb muscles when they contract or relax (32). Accordingly, we detected both sides of RMS of quadriceps femoris muscle pre- and post-iTBS treatment, respectively.
Repeated measures Analysis of variance (ANOVA) applied to the LRMS revealed a significant group–time interaction (F1.36 = 12.59, p < 0.0011), the main effect of time (F1.36 = 56.38 p < 0.0001), and no significant main effects of group (F1.36 = 0.35, p = 0.56). Single-effect analysis indicated significant changes in the RMS of the left quadriceps femoris before and after treatment in the 2 groups (t = 3.55, p = 0.001). Also, the improvement in RMS of the left quadriceps femoris in the iTBS group was greater than in the sham group (Fig. 3B). Similarly, the right quadriceps femoris RMS presented a corresponding trend that group–time interaction (F1.36 = 7.37, p = 0.0101) and the main effect of time (F1.36 = 89.71 p < 0.0001) was significant. Single effect analysis between the groups revealed the improvement in RMS of left quadriceps femoris in the iTBS group was greater than in the sham group (t = 2.72, p = 0.010) (Fig. 3C).
Effect of cerebral intermittent theta-burst stimulation on gait pattern after incomplete spinal cord injury
WS and SL were key technical parameters in gait analysis (33). Repeated measures ANOVA applied to the WS found a significant group time interaction (F1.36 = 10.32, p = 0.0028) and significant main effects of time (F1.36 = 75.20, p < 0.0001), while the main effect of the group was no significant (F1.36 = 2.053, p = 0.16). Post hoc t-tests revealed that this difference was largely driven by iTBS treatment effects observed in WS at 9 weeks (p < 0.05) (Fig. 3D). SL is the vertical straight-line distance between 2 points when the left heel or toe touches the ground successively. Repeated measures ANOVA showed significant group–time interaction on left SL (LSL) (F1.36 = 9.29, p = 0.0043) and right SL (RSL) (F1.36 = 14.18, p = 0.0006). Unpaired t-test showed that increases in the LSL and RSL in the iTBS group were greater than in the sham group; the difference was statistically significant (p < 0.05) (Fig. 4A, B).
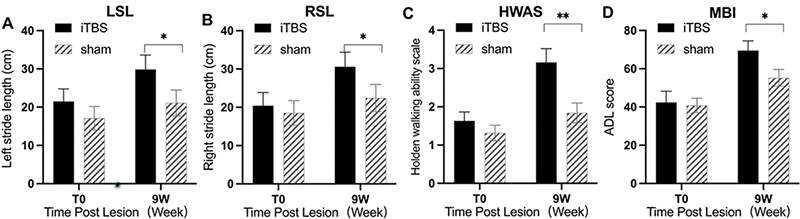
Fig. 4. Effect of cerebral intermittent theta-burst stimulation (iTBS) on step length, walking ability, and daily living ability after incomplete spinal cord injury (iSCI). Significant differences were observed between the iTBS and sham groups in: (A) overall left stride length (LSL), (B) right stride length (RSL), (C) Holden Walking Ability Scale (HWAS), and (D) modified Barthel Index (MBI) (D) at 9 weeks after the start of the intervention. Values are expressed as the mean±standard error of the mean (SEM). *p < 0.05, **p < 0.01, significant difference between the 2 groups.
Effect of cerebral intermittent theta-burst stimulation on walking ability after incomplete spinal cord injury
To evaluate the effect of iTBS on walking ability in patients with iSCI, the HWAS test was applied pre- and post-iTBS treatment. Two-way repeated-measures ANOVA revealed a significant group–time interaction (F1.36 =v15.93, p = 0.0003), main effects of time (F1.36 = 67.1 p < 0.0001), and main effects of group (F1.36 = 5.12, p = 0.03). Single-effect analysis indicated significant changes in the HWAS before and after treatment in the 2 groups (t = 3.45, p = 0.002). Also, the improvement in the iTBS group was greater than in the sham group (Fig. 4C).
Effect of cerebral intermittent theta-burst stimulation on daily living ability after incomplete spinal cord injury
MBI is the most commonly used method for evaluating patients’ daily living ability. Repeated measures ANOVA applied to MBI revealed a significant group–time interaction (F1.36 = 9.64, p < 0.0037) and significant main effects of time (F1.36 = 104.5, p < 0.0001), but no significant main effects of group (F1.36 = 1.461, p = 0.23). Single effect analysis showed significant change in the HWAS before and after treatment in the 2 groups (t = 3.11, p = 0.004), while the improvement in the iTBS group was greater than in the sham group (Fig. 4D).
DISCUSSION
Recent studies have proposed exercise training combined with non-invasive regulation techniques as an effective way to improve motor dysfunction caused by central nervous system (CNS) injury (11, 19, 34, 35). The current randomized, double-blind, sham-controlled pilot study aimed to investigate the short-term effects of brain iTBS combined with physical therapy on the recovery of lower limb movement and daily living ability in patients with iSCI, and to provide a reference for larger future studies. To the best of our knowledge, this is the first study to measure changes in objective data, such as LEMS, RMS of EMG, WS and SL, combined with evaluation using HWAS and MBI, in examining the therapeutic effect of iTBS on lower limb motor function and daily living ability of patients with iSCI.
Nine weeks of cerebral intermittent thetaburst stimulation with physiotherapy intervention resulted in improved recovery of lower extremity motor recovery in patients with incomplete spinal cord injury.
Lower limb muscle strength was associated with step length, step speed, walking ability, fall risk and functional independence after SCI (36). The current study showed that 9 weeks of iTBS treatment in the cortical M1 region combined with physical therapy increased LEMS scores from 21.7 to 32.6. This is consistent with Krogh’ study, which reported an increase in LEMS scores from 28 to 45 in patients with SCI after 4 weeks of 20 Hz rTMS treatment (37). This is also in agreement with our observations in clinical practice, encouraging further research into its therapeutic potential.
The muscle strength of the lower limbs in patients with SCI is mainly affected due to the interruption of motor nerve conduction tracts to different degrees (38), resulting in interruption of motor control pathways, signal transduction, and axonal growth and myelination (39). Most patients with AIS grade C–D SCI have some residual muscle strength in both lower limbs, and their motor nerve control pathway is not completely interrupted. Improving muscle strength is of great significance for rehabilitating patients with SCI (40). The initial understanding of the role of magnetic stimulation in motor function after SCI came from the fact that transcranial magnetic stimulation could generate motor evoked potential in distal limbs (41). Krogh et al. found no clear, clinically important differences in short-term recovery of maximal leg muscle strength in patients under rTMS stimulation intervention at 20 Hz (37), which could be because the intensity of the magnetic stimulation used may have been too low to induce a significant short-term increase in lower extremity muscle strength. In the current study, iTBS stimulation mode was used to observe its effect on the maximum muscle strength of the lower limbs in patients with iSCI, and was confirmed by the RMS value of the surface EMG detection index, which increased more in the iTBS group than in the sham group; the difference was statistically significant.
Motor units consist of neuronal circuits and muscle fibers. Stimulation of motor units can increase muscle strength. Muscle strength can be altered in two ways: one by the number of motor units that are activated, and the other by increasing the activation frequency of the activated motor units. ITBS increase the excitability of neural circuits and therefore may play a role in increasing muscle motor function. iTBS intermittently produces short TBS sequences to promote cortical excitability in the primary motor cortex (M1) (42), thereby increasing excitability in the corticospinal neural circuit. In their previous studies, Huang et al. proved that there are significant post-hoc differences between different stimulus modes (16, 43). The current study further confirmed the possible importance of the high-frequency burst component of iTBS in producing long-lasting after-effects.
Improvement in walking and daily living ability is an important rehabilitation goal for patients with SCI (36, 38). WS and SL are key indicators in gait analysis (29). In this study, patients with SCI showed reduced WS and shortened SL. The mean WS before treatment was 0.72 ± 0.51 m/s, and the mean SL was 20.91 ± 14.42 cm, which is consistent with previous studies (44). After 9 weeks of rehabilitation intervention, the WS and step length of patients in the 2 groups improved compared with before treatment, and the difference between the groups was statistically significant, indicating that iTBS has a positive effect on improving the gait of patients with SCI. In subjects with SCI, muscle activation was delayed, and increased motor control instability could lead to rapid swing and shortened step length (45). iTBS enhances the excitability of the corticospinal system by emitting pulses (46) that may reduce the delay of nervous system activation of SCI lower limb muscle groups.
At the same time, the current study also observed the effects of iTBS combined with physical therapy on patients’ walking function and daily living. The results showed that the 9-week iTBS intervention had a positive effect on the HWAS and MBI in patients with iSCI. Considering evidence from previous research (47), the possible reasons are related to the long-term potentiation effect of iTBS by altering the effectiveness of synaptic interactions (46). iTBS not only improved the WS and SL of patients with SCI, but also effectively increased the stability of lower limb movement control and improved walking quality and ADL
People with SCI have a high rate of disability because effective nerve regeneration and neural circuit reconstruction have not resolved after the injury (48, 49). Clinically, a large number of patients with SCI have incomplete injuries. In fact, only 14.3% of SCIs are anatomically complete injuries (50), while the rest are considered functional impairments that can utilize residual nerve fibres to some extent. Establishing some connections or circuits through appropriate intervention is an effective way to restore the motor function of SCI (51, 52). In recent years, related studies have found that TMS may induce functional reorganization of neural circuits and promote remodelling of the nervous system by stimulating the injured spinal cord’s central pattern generators (CPGs) (53). The effective connections of the cells may even modulate the function of the stimulated area and establish a functional network that contributes to the recovery of motor function (54). The current study is the first to show that cerebral iTBS combined with physical therapy promotes the recovery of lower extremity movement and the improvement in daily living ability in patients with iSCI. This may be based on this neural regulation mechanism.
Study limitations
The current study has some limitations. First, it did not measure corticospinal excitability in patients, and lacks an exploration of the underlying mechanism of action of iTBS. future studies should include more detection tools (such as Functional magnetic resonance imaging (fMRI), TMS-EEG, and functional near-infrared spectroscopy) for assessing changes in multiple brain regions and performing a systematic assessment of the sensory-cortical-motor circuit. Secondly, although the sample size of this study was strictly estimated, a multicentre, large sample, randomized, double-blind, and controlled clinical trials is required to further verify the reported findings. Finally, no long-term follow-up was performed in the current study, and it is well known that the most important criteria for evaluating neuromodulation techniques are mid- and long-term efficacy and whether the effect of neuromodulation treatment can be maintained in the long-term.
CONCLUSION
A 9 week treatment with cerebral iTBS combined with physical therapy may have a positive short-term effect on lower extremity movement and recovery of daily living ability in patients with SCI, although the clinically relevant mechanism induced by iTBS remains unclear. Further studies with longer intervention periods and larger study populations are needed to explore the mechanism through which iTBS could promote muscle strength recovery in patients with SCI, in order to better guide clinical application.
ACKNOWLEDGEMENTS
This study was supported by the Key Research and Development Program of Anhui Province, China (202004J07020040 to XJ Feng), Program of Clinical and Basic Cooperative Research of Anhui Medical University, China (2020skjT043 to XJ Feng), Scientific research project of Health Commission of Fuyang City, Anhui Province (FY2021-125 to XJ Feng) and Scientific Research Fund project of Anhui Medical University (2020xkj030 to Y Jiang), Project of Anhui Medical University Teaching Quality Research (2021xjjyxm49 to XJ Feng), Second Affiliated Hospital of Anhui Medical University-Hefei Institute of Intelligent Machinery, Chinese Academy of Sciences “Chronic Disease Prevention and Control Joint Research Fund” (MBLHJ202006 to Y Liu).
The current study was performed in accordance with the ethics standards set out in the 1964 Declaration of Helsinki (revised 2013). This study was approved by the Clinical Research Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (registration number: SL-LC2019037 (F1). The study protocol was registered with the Chinese Clinical Trial Registry (clinical registration number: ChiCTR2200059431). All methods were carried out in accordance with relevant guidelines and regulations.
All data generated or analysed during this study are included in this published article.
REFERENCES
- Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Primers 2017; 3: 1–21. DOI: 10.1038/nrdp.2017.18
- van Middendorp JJ, Hosman AJ, Donders AR, Pouw MH, Ditunno JF, Jr, Curt A, et al. A clinical prediction rule for ambulation outcomes after traumatic spinal cord injury: a longitudinal cohort study. Lancet 2011; 377: 1004–1010. doi:10.1016/s0140-6736(10)62276-3
- Griffin JM, Bradke F. Therapeutic repair for spinal cord injury: combinatory approaches to address a multifaceted problem. EMBO Mol Med 2020; 12: e11505. doi:10.15252/emmm.201911505
- Hornby TG, Reisman DS, Ward IG, Scheets PL, Miller A, Haddad D, et al. Clinical Practice Guideline to improve locomotor function following chronic stroke, incomplete spinal cord injury, and brain injury. J Neurol Phys Ther 2020; 44: 49–100. doi:10.1097/npt.0000000000000303
- Lo C, Tran Y, Anderson K, Craig A, Middleton J. Functional priorities in persons with spinal cord injury: using discrete choice experiments to determine preferences. J Neurotrauma 2016; 33: 1958–1968. doi:10.1089/neu.2016.4423
- Xue X, Yang X, Tu H, Liu W, Kong D, Fan Z, et al. The improvement of the lower limb exoskeletons on the gait of patients with spinal cord injury: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2022; 101: e28709. doi:10.1097/MD.0000000000028709
- Jendelova P. Therapeutic strategies for spinal cord injury. Int J Mol Sci 2018; 19. doi:10.3390/ijms19103200
- Wagner FB, Mignardot JB, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018; 563: 65–71. doi:10.1038/s41586-018-0649-2
- Holmes D. Repairing the neural highway. Nature 2017; 552: S50–s51. doi:10.1038/d41586-017-07551-8
- Tran AP, Warren PM, Silver J. The biology of regeneration failure and success after spinal cord injury. Physiol Rev 2018; 98: 881–917. doi:10.1152/physrev.00017.2017
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 2015; 126: 1071–1107. doi:10.1016/j.clinph.2015.02.001
- Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014; 125: 2150–2206. doi:10.1016/j.clinph.2014.05.021
- Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol 2020; 131: 474–528. doi:10.1016/j.clinph.2019.11.002
- Beynel L, Powers JP, Appelbaum LG. Effects of repetitive transcranial magnetic stimulation on resting-state connectivity: a systematic review. NeuroImage 2020; 211: 116596. doi:10.1016/j.neuroimage.2020.116596
- Iddings JA, Zarkou A, Field-Fote EC. Noninvasive neuromodulation and rehabilitation to promote functional restoration in persons with spinal cord injury. Curr Opin Neurol 2021; 34: 812–818. doi:10.1097/WCO.0000000000000997
- Huang Y Z EMJ, Rounis E. Theta burst stimulation of the human motor cortex. Neuron 2005; 45: 201–206.
- Ljubisavljevic MR, Javid A, Oommen J, Parekh K, Nagelkerke N, Shehab S, et al. The effects of different repetitive transcranial magnetic stimulation (RTMS) protocols on cortical gene expression in a rat model of cerebral ischemic-reperfusion injury. PloS One 2015; 10: e0139892. doi:10.1371/journal.pone.0139892
- Bonnì S, Ponzo V, Caltagirone C, Koch G. Cerebellar theta burst stimulation in stroke patients with ataxia. Functional Neurology. 2014;29:41–45. PMID: 25014048; PMCID: PMC4172246.
- Liao LY, Xie YJ, Chen Y, Gao Q. Cerebellar theta-burst stimulation combined with physiotherapy in subacute and chronic stroke patients: a pilot randomized controlled trial. Neurorehabil Neural Repair 2021; 35: 23–32. doi:10.1177/1545968320971735
- Gutiérrez-Muto AM, Castilla J, Freire M, Oliviero A, Tornero J. Theta burst stimulation: technical aspects about TMS devices. Brain Stimul 2020; 13: 562–564. doi:10.1016/j.brs.2020.01.002
- Philip NS, Barredo J, Aiken E, Larson V, Jones RN, Shea MT, et al. Theta-burst transcranial magnetic stimulation for posttraumatic stress disorder. Am J Psychiatry 2019; 176: 939–948. doi:10.1176/appi.ajp.2019.18101160
- Solomon EA, Sperling MR, Sharan AD, Wanda PA, Levy DF, Lyalenko A, et al. Theta-burst stimulation entrains frequency-specific oscillatory responses. Brain Stimul 2021; 14: 1271–1284. doi:10.1016/j.brs.2021.08.014
- Zong X, Li Y, Liu C, Qi W, Han D, Tucker L, et al. Theta-burst transcranial magnetic stimulation promotes stroke recovery by vascular protection and neovascularization. Theranostics 2020; 10: 12090–12110. doi:10.7150/thno.51573
- Kirshblum S, Snider B, Rupp R, Read MS. Updates of the International Standards for Neurologic Classification of Spinal Cord Injury: 2015 and 2019. Phys Med RehabilClin N Am 2020; 31: 319–330. doi:10.1016/j.pmr.2020.03.005
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 2005; 45: 201–206. doi:10.1016/j.neuron.2004.12.033
- Ciesla N, Dinglas V, Fan E, Kho M, Kuramoto J, Needham D. Manual muscle testing: a method of measuring extremity muscle strength applied to critically ill patients. J Vis Exp 2011; 50: 2632. doi:10.3791/2632
- Gilmore J, Islam M, Duncan J, Natu R, Martinez-Duarte R. Assessing the importance of the root mean square (RMS) value of different waveforms to determine the strength of a dielectrophoresis trapping force. Electrophoresis 2017; 38: 2561–2564. doi:10.1002/elps.201600551
- Maranesi E, Riccardi GR, Lattanzio F, Di Rosa M, Luzi R, Casoni E, et al. Randomised controlled trial assessing the effect of a technology-assisted gait and balance training on mobility in older people after hip fracture: study protocol. BMJ Open 2020; 10: e035508. doi:10.1136/bmjopen-2019-035508
- Kumru H, Benito-Penalva J, Valls-Sole J, Murillo N, Tormos JM, Flores C, et al. Placebo-controlled study of rTMS combined with Lokomat(®) gait training for treatment in subjects with motor incomplete spinal cord injury. Exp Brain Res 2016; 234: 3447–3455. doi:10.1007/s00221-016-4739-9
- Aminalroaya R, Mirzadeh FS, Heidari K, Alizadeh-Khoei M, Sharifi F, Effatpanah M, et al. The validation study of both the modified barthel and barthel index, and their comparison based on Rasch analysis in the hospitalized acute stroke elderly. Int J Aging Hum Dev 2021; 93: 864–880. doi:10.1177/0091415020981775
- Benito J, Kumru H, Murillo N, Costa U, Medina J, Tormos JM, et al. Motor and gait improvement in patients with incomplete spinal cord injury induced by high-frequency repetitive transcranial magnetic stimulation. Top Spinal Cord Inj Rehabil 2012; 18: 106–112. doi:10.1310/sci1802-106
- Xu L, Gu H, Zhang Y. Research hotspots of the rehabilitation medicine use of sEMG in recent 12 years: a bibliometric analysis. J Pain Res 2022; 15: 1365–1377. doi:10.2147/JPR.S364977
- Feng XJ, Huang YT, Huang YZ, Kuo CW, Peng CW, Rotenberg A, et al. Early transcranial direct current stimulation treatment exerts neuroprotective effects on 6-OHDA-induced Parkinsonism in rats. Brain Stimul 2020; 13: 655–663. doi:10.1016/j.brs.2020.02.002
- Delarue Q, Chalfouh C, Guérout N. Spinal cord injury: can we repair spinal cord non-invasively by using magnetic stimulation? Neural Regen Res 2021; 16: 2429–2430. doi:10.4103/1673-5374.313033
- Potter-Baker KA, Janini DP, Lin YL, Sankarasubramanian V, Cunningham DA, Varnerin NM, et al. Transcranial direct current stimulation (tDCS) paired with massed practice training to promote adaptive plasticity and motor recovery in chronic incomplete tetraplegia: A pilot study. J Spinal Cord Med 2018; 41: 503–517. doi:10.1080/10790268.2017.1361562
- Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med 2018; 379: 1244–1250. doi:10.1056/NEJMoa1803588
- Krogh S, Aagaard P, Jønsson AB, Figlewski K, Kasch H. Effects of repetitive transcranial magnetic stimulation on recovery in lower limb muscle strength and gait function following spinal cord injury: a randomized controlled trial. Spinal Cord 2022; 60: 135–141. doi:10.1038/s41393-021-00703-8
- Venkatesh K, Ghosh SK, Mullick M, Manivasagam G, Sen D. Spinal cord injury: pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res 2019; 377: 125–151. doi:10.1007/s00441-019-03039-1
- Holmes D. Spinal-cord injury: spurring regrowth. Nature 2017; 552: S49. doi:10.1038/d41586-017-07550-9
- Jo HJ, Perez MA. Corticospinal-motor neuronal plasticity promotes exercise-mediated recovery in humans with spinal cord injury. Brain 2020; 143: 1368–1382. doi:10.1093/brain/awaa052
- Tazoe T, Perez MA. Effects of repetitive transcranial magnetic stimulation on recovery of function after spinal cord injury. Arch Phys Med Rehabil 2015; 96: S145–155. doi:10.1016/j.apmr.2014.07.418
- Chung SW, Hill AT, Rogasch NC, Hoy KE, Fitzgerald PB. Use of theta-burst stimulation in changing excitability of motor cortex: a systematic review and meta-analysis. Neuroscience and biobehavioral reviews 2016; 63: 43–64. doi:10.1016/j.neubiorev.2016.01.008
- Huang Y, Liu AA, Lafon B, Friedman D, Dayan M, Wang X, et al. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. eLife 2017; 6: 18834. doi:10.7554/eLife.18834
- de Araújo AVL, Barbosa VRN, Galdino GS, Fregni F, Massetti T, Fontes SL, et al. Effects of high-frequency transcranial magnetic stimulation on functional performance in individuals with incomplete spinal cord injury: study protocol for a randomized controlled trial. Trials 2017; 18: 522. doi:10.1186/s13063-017-2280-1
- Ardestani MM, Henderson CE, Salehi SH, Mahtani GB, Schmit BD, Hornby TG. Kinematic and neuromuscular adaptations in incomplete spinal cord injury after high- versus low-intensity locomotor training. J Neurotrauma 2019; 36: 2036–2044. doi:10.1089/neu.2018.5900
- Amer A, Xia J, Smith M, Martin JH. Spinal cord representation of motor cortex plasticity reflects corticospinal tract LTP. Proc Nat Acad Sci U S A 2021; 118. doi:10.1073/pnas.2113192118
- Kumru H, Murillo N, Samso JV, Valls-Sole J, Edwards D, Pelayo R, et al. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil Neural Repair 2010; 24: 435–441. doi:10.1177/1545968309356095
- Courtine G HJ, Van Den Brand R. Response to comment on “restoring voluntary control of locomotion after paralyzing spinal cord injury”. Science 2012; 338: 328–328.
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Natu Neurosci 2004; 7: 269–277. doi:10.1038/nn1195
- Kakulas BA. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord 2004; 42: 549–563. doi:10.1038/sj.sc.3101670
- GBD 2016 Dementia Collaborators Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 459–480. doi:10.1016/s1474-4422(18)30499-x
- Nelson LD, Temkin NR, Dikmen S, Barber J, Giacino JT, Yuh E, et al. Recovery after mild traumatic brain injury in patients presenting to US Level I Trauma Centers: a Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) study. JAMA neurology 2019; 76: 1049–1059. doi:10.1001/jamaneurol.2019.1313
- Diaz-Ríos M, Guertin PA, Rivera-Oliver M. Neuromodulation of spinal locomotor networks in rodents. Curr Pharm Des 2017; 23: 1741–1752. doi:10.2174/1381612823666170124111729
- Hayes SC, White M, Wilcox CRJ, White HSF, Vanicek N. Biomechanical differences between able-bodied and spinal cord injured individuals walking in an overground robotic exoskeleton. PloS One 2022; 17: e0262915. doi:10.1371/journal.pone.0262915