ORIGINAL REPORT
FACTORS ASSOCIATED WITH LONG-TERM FUNCTIONAL AND PSYCHOSOCIAL OUTCOMES IN PATIENTS WITH NON-HODGKIN LYMPHOMA
Bhasker AMATYA, MD, MPH, DMedSc1–4, Michael DICKINSON, MBBS (HONS), DMedSc, FRACP, FRCPA2,4,5 and Fary KHAN, AM, MD, MBBS, FAFRM (RACP)1,2,3,4
From the 1Department of Rehabilitation Medicine, Royal Melbourne Hospital and Peter MacCallum Cancer Centre, 2Department of Medicine (Royal Melbourne Hospital), University of Melbourne, 3Australian Rehabilitation Research Centre, Royal Melbourne Hospital, Parkville, Victoria, 4Department of Clinical Haematology, Peter MacCallum Cancer Centre and The Royal Melbourne Hospital, Melbourne, Victoria and 5The Sir Peter MacCallum Department of Oncology, University of Melbourne, Parkville, Victoria, Australia
Objective: To assess the long-term functional, psychosocial and participation outcomes in an Australian cohort of non-Hodgkin lymphoma (NHL) survivors.
Methods: A cross-sectional sample of adult NHL survivors at the Peter MacCallum Cancer Centre (between 2015 and 2020), participated by completing validated questionnaires. A series of analyses described their current level of function, psychosocial well-being, and participation.
Results: Of 129 participants (mean (M) ± standard deviation (SD) age: 62.5 ± 8.8 years), the majority (58%) had aggressive NHL and grade III–IV (72%), with time since diagnosis of 4.6 ± 1.2 years. Participants reported ongoing issues after completion of treatment: fatigue (63%), bladder dysfunction (61%), cognitive impairment (53%), and NHL-related pain (46%). Most made good functional recovery (M ± SD) (Functional Independent Measure-Motor: 79.5 ± 8.2), reported minimal change in their negative emotional states, and NHL-specific quality of life (QoL) (Functional Assessment of Cancer Therapy–Lymphoma: 133.5 ± 22.1). Participants were “well” adjusted to community living (Community Integration Measure: 42.2 ± 7.4) and satisfied with their current life (Satisfaction with Life Scale: 26.3 ± 6.0). Factors significantly associated with the poorer current level of function were: age at diagnosis < 60 years, time since NHL diagnosis > 4.5 years, and aggressive NHL type.
Conclusion: Despite good functional recovery and adjustment in the community, NHL survivors report the presence of ongoing residual impairments and cognitive issues, which requires long-term rehabilitation-inclusive management.
LAY ABSTRACT
This cross-sectional study evaluated functional and psychosocial outcomes in non-Hodgkin lymphoma (NHL) survivors. Most patients made a good functional recovery and reported minimal change in their negative emotional states and quality of life after they were discharged. Patients reported satisfaction with their current life and were “well” adjusted to community living after NHL treatment. However, many reported ongoing issues, specifically fatigue, bladder dysfunction, cognitive impairment, and NHL-related pain. Those below 60 years of age when diagnosed, with time since NHL of over 4.5 years, and with aggressive and advanced NHL grades were associated with a poorer current level of function. These findings suggest that, despite patients’ potential adjustment to disability over time (response-shift phenomenon), many patients with NHL need long-term rehabilitation-inclusive management of ongoing disability and psychosocial issues in the community post-discharge.
Key words: non-Hodgkin lymphoma; rehabilitation; impairment; function; quality of life.
Citation: J Rehabil Med 2023; 55: jrm004816. DOI: https://doi.org/10.2340/jrm.v55.4816
Copyright: © Published by Medical Journals Sweden, on behalf of the Foundation for Rehabilitation Information. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jan 13, 2023; Published: Feb 28, 2023
Correspondence address: Bhasker Amatya, Department of Rehabilitation Medicine, Royal Melbourne Hospital, Royal Park Campus, 34–54 Poplar Road, Parkville, Victoria 3050, Australia. E-mail: Bhasker.amatya@mh.org.au
Competing interests and funding: The authors have no conflicts of interest to declare.
Non-Hodgkin lymphoma (NHL) is a malignant neoplasm of the haematopoietic system, accounting for approximately 90% of all lymphomas (1). The overall incidence of NHL is increasing globally, with an estimated 500,000 new cases (2.8% of all cancers) and over 248,000 deaths (2.6% of all cancers) reported in 2018 alone (2). The total global economic burden of NHL is unknown; however, both disease and treatments are resource-intensive and associated with a significant economic burden for patients (and families), and the healthcare system (3). In recent years, therapeutic advances in treatment have increased survival, with an estimated age-standardized 5-year net survival of lymphoid malignancies in adults ranging from 40% to 70% globally in 2010–14. The 5-year survival rate of patients with NHL in the period 2010–16 in the USA was estimated at 72.7% (4).
The overriding objective of cancer care has now extended beyond survival and acute management to the successful reintegration of the patient into the community; hence shifting the focus to the long-term management of patient cohorts in ambulatory and community settings. Despite advancements in treatment, NHL survivors often have residual neurological deficits, functional and psychosocial sequelae, and behavioural issues (5–8). Treatment regimens, such as radiotherapy, chemotherapy and surgery, can be associated with considerable adverse effects, such as neuropathy, cardiotoxicity, cachexia, fatigue, deconditioning, and myopathy, amongst other symptoms (9–11). The diagnosis of NHL itself can have a distressing psychological impact on the patient and their families. In the post-acute transitional period, various adjustment issues may surface, such as increased care needs, inability to drive and return to work, financial constraints, fear of recurrence, marital stress, and limitation in societal participation (6, 9, 12, 13). These NHL-related impairments can limit “activity” or function and “participation”, have a cumulative effect over time, and cause considerable distress to the NHL survivor, (and their families), and adversely impact their quality of life (QoL) (6, 9, 12, 13). One recent population-based Australian study quantifying physical and mental health-related outcomes in people with 13 cancer types demonstrated that, compared with people without cancer (n = 244,000), cancer survivors (n = 22,505) had greater disability (20.6% vs 12.6%, respectively, prevalence ratio (PR) = 1.28, 95% confidence interval (95% CI) = (1.25–1.32)], and poor/fair health [(22.0% vs 13.5%; PR (95% CI): 1.41 (1.37–1.45)] and QoL [(15.2% vs 10.2%; PR (95% CI): 1.28 (1.24–1.32)]. Similar patterns were reported in the NHL cohort for disability: PRs (95% CI): 3.10 (2.56–3.77); distress: 1.53 (1.20–1.96) and poor/fair QoL: 2.40 (1.87–3.07) compared with participants without cancer (14). Further, outcomes were worse in more recently diagnosed and treated patients, and in those with advanced stage disease. Physical disability was probably a key driver of psychological distress and reduced QoL (14). One study (n = 761 participants) found that NHL survivors with active disease demonstrated worse physical and mental health functioning, QoL, compared with disease-free survivors (p ≤ 0.01) (15). A systematic review evaluating the health-related and QoL (HRQoL) of NHL survivors reported that many experienced problems in physical functioning, appetite loss, vitality and finances (6). Further, unmet supportive care needs are prevalent in many patients with haematological malignancies, including NHL (16). The most frequently reported unmet care needs include: informational, emotional, physical, daily living/practical (accessibility, transportation, and financial problems), and family life/relational needs (16). Younger age, marital status, female sex, reduced employment and monthly income, coexistence of anxiety/depression, and altered QoL were the key factors associated with increased unmet supportive care (16).
Recovery from NHL treatments may be prolonged, with varied care needs after treatment (surgery, chemotherapy and radiotherapy) given the complex multiple disabilities in these persons. In acute settings, the treating clinicians focus on management of the acute complications from treatment and relapses, but less on delayed complications, and functional and psychosocial outcomes following treatment and in the community. Therefore, information on post-treatment functional and psychosocial outcomes of patients is important. There are only a few studies evaluating these outcomes, specifically in the Australian context. Further, to our knowledge, no studies address the rehabilitation perspective. Information on participatory limitation and psychosocial outcomes are limited. This study examined factors associated with residual disability and restriction in participation, including functional outcomes, psychosocial sequelae, and QoL in patients with NHL in an Australian community cohort.
METHODS
Participants and setting
This prospective cross-sectional study was conducted in accordance with the National Health and Medical Research Council (NHMRC) National Statement on Ethical Conduct in Human Research (2007 and updates) and the World Medical Association Declaration of Helsinki (2013 and updates), at the Clinical Haematology and Rehabilitation Medicine Department, Peter MacCallum Cancer Centre (PMCC); a tertiary lymphoma referral service in Victoria, Australia (PMCC ethics committee – HREC No. 21/65L). All participants provided written informed consent before study participation.
A preliminary audit of 613 consecutive patients with NHL registered in a prospectively collected database of patients with lymphoma since January 2016, with the CD-10-AM Code C82-C86 for NHL incorporating all sub-codes (main diagnosis), was confirmed and cross-indexed using the PMCC Electronic Medical Record. The sample comprised a pool of persons residing in the community, referred to the PMCC from public and private medical clinics across greater Melbourne and Victoria. Participant inclusion criteria were: age 18 years and over; a confirmed diagnosis of NHL (17, 18); completion of definitive treatment at PMCC (and not currently on active cancer treatment). Exclusion criteria included: inability to communicate in English; not capable of answering the questionnaires; patients who were medically unstable, or had psychiatric disorders limiting participation in the study.
Procedure
All eligible participants, based on selection criteria, were invited by phone/e-mail to participate in the study by a research assistant independent of the clinical service, and those agreeing to participate were sent a Participation Information and Consent Form (PICF), and a PMCC patient brochure “What happens to information about you?” Those who provided consent were recruited and interviewed using the structured questionnaire (see Measures section, below) based on their availability and convenience. Attempts were made to re-contact the non-responders by phone/e-mail. All interviews and assessments (approx. 30 min each) were conducted by an independent trained research assistant by phone or online communication platforms (such as Skype Luxembourg, Grand Duchy of Luxembourg, Zoom San Jose, California, US, Microsoft Teams Redmond, Washington, US), using a structured format. Data collection included: demographic and medical information, cognitive and functional ability assessment and HRQoL measures using validated instruments (see Measures, below). The assessor did not prompt patients, but assisted those who had difficulty with completing the questionnaires. Appropriate rest breaks were provided during these interviews. All assessments were secured and filed and opened at the time of entry into the database by an independent data entry officer.
Measures
The World Health Organization (WHO) International Classification of Functioning, Disability and Health (ICF) (19) was used as a conceptual basis for the choice of best outcomes for measurement. The ICF provides a framework that describes the impact of disease at the level of impairment, limitation in activity and participation; incorporating contextual (environment and personal) factors that may act as barriers or facilitators in these persons (19). The information collected included routinely collected standard demographic data and global validated scales commonly used in rehabilitation settings.
Demographic and non-Hodgkin lymphoma-related data. This data included age, sex, ethnicity, education, marital status, employment status, etc., and clinical characteristics data (date of diagnosis, co-morbid conditions, NHL-related symptoms, histology, grade, treatment received and status, recurrence, etc.).
Measures for activity and functioning. Functional Independence Measure (FIM) (20) assessed function (activity), cognitive impairment and need for assistance (physician assessed). The FIM motor scale has 13 items in 4 subscales: Self-care, Transfers, Locomotion, and Sphincter control; while the FIM cognition scale has 5 items in 3 subscales: Communication, Psycho-social, and Cognition. Each item was rated on a scale of 1–7 (where 1 = total assistance and 7 = fully independent).
Measures for participation and quality of life. The Depression Anxiety Stress Scale-21 (DASS) (21) consists of 3 7-item self-report scales to measure the negative emotional states of depression, anxiety and stress. Participants rated the extent to which they experienced each state over the past week on a 4-point Likert rating scale (0 = ”did not apply to me” to 3 = ”applied to me most of the time”). The scores for each domain range from 0 to 42, with higher scores indicating more dysfunction (21).
Functional assessment of cancer therapy – lymphoma. The FACT-Lym (version 4) assessed NHL-specific QoL which included 42-item: 27-item general (FACT-G) module and 15-item lymphoma-specific items (LymS) (symptoms and issues) (22). The FACT-G contains 4 subscales: physical wellbeing (PWB), social/family wellbeing (SWB), emotional wellbeing (EWB), and functional wellbeing (FWB). Each item is rated using a 5-point Likert scale, ranging from 0 (not at all) to 4 (very much). Individual subscale scores are computed as the prorated sum of the item responses and the total score obtained by summing sub-scale scores. Further, FACT-Lym Trial Outcome Index (TOI) was derived by summing PWB, FWB and LymS subscale scores (23).
Cancer rehabilitation evaluation system – short form. The CARES-SF (24) a self-administered cancer-specific measure with 59 items assessed overall QoL. The items generate a single global score, indicating QoL, with summary scores for 5 domains: physical (problems with daily activity), psychosocial (communication and relationship), sexual (interest and performance), marital (problems with a significant relationship) and medical interaction (communication with the medical team). The participants rated the degree to which a given problem applied during the 4 weeks prior to the survey using a 4-point Likert scale (0 = not at all to 4 = very much), with higher scores indicating more difficulty or impairment (24).
Community integration measure. The CIM (25) assessed perceived community integration using 10 declarative statements in 4 domains: general assimilation, support, occupation, and independent living. Respondents rate each statement on a Likert scale (from 1 = always disagree to 5 = always agree) to give a total score out of 50, with higher scores indicating better community integration.
Satisfaction with life scale. The SWLS (26) measured participants’ overall satisfaction with life, answering 5 items on a 7-point Likert scale (from 0 = strongly disagree to 7 = strongly agree).
Statistical analysis
Descriptive statistics summarized participants’ demographic and clinical characteristics. Total and subscale total for each outcome measure (indicated above) were calculated according to the scoring guidelines. A series of analyses was conducted to describe the participants’ current level of function, well-being and QoL; and to identify (demographic and clinical) factors associated with scores on these scales. Based on the data distribution, current age, age at diagnosis, disease grade and time since NHL were divided into binary categories: age older than or younger than 63 years, age at diagnosis older than or younger than 60 years, NHL grades I–II and III–IV, time since diagnosis ≤ 4.5 and over 4.5 years, respectively. Several univariate analyses (t-tests and 1-way analyses of variance) compared (FIM, DASS, FACT-LYM, CARES-SF, CIM, SLW) scores across groups. Statistical significance was determined by a level of p < 0.05. A post hoc analysis for between-group comparisons reduced the likelihood of type 1 errors, using a Bonferroni adjustment for multiple comparisons (dividing the alpha level of 0.05 by the number of covariates/tests). This was consistent with the descriptive nature of the study to ensure that all potentially important predictors of the short- and long-term sequelae of NHL were identified. All calculations were performed using IBM SPSS Chicago, Illinois, US for Windows version 22.0.
RESULTS
Sample characteristics
The study flow chart is presented in Fig. 1, and the socio-demographic and clinical characteristics of the sample are shown in Table I.
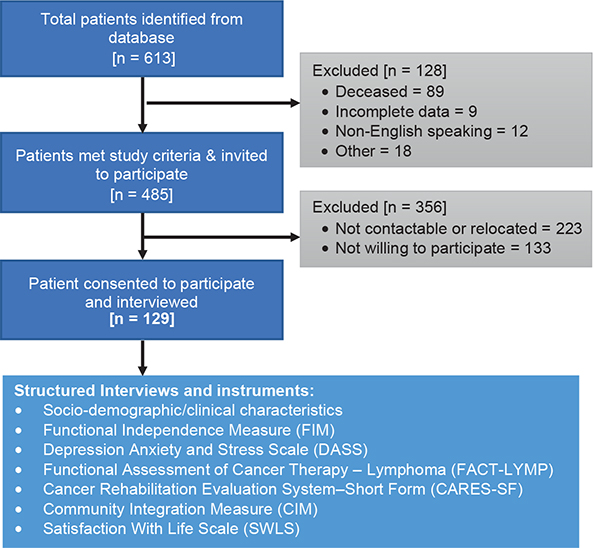
Fig. 1. Flow chart of the recruitment process.
A total of 129 eligible participants consented and were recruited to the study. The mean age of participants was 62.5 ± 8.8 years (range 37.4–80 years), the majority were male (56%) and Caucasian (96%) with secondary education (55%). Mean time since NHL diagnosis was 4.6 ± 1.2 (range 1.7–6.5) years, with mean age at diagnosis 57.7 ± 8.8 (range 33.8–74.8) years. Three-quarters (75%) of participants reported co-morbidities, with high blood pressure (27%) being most common followed by diabetes (15%), ischaemic heart disease (15%), and depression (12%). More than one-third (41%) had diffuse large B-cell lymphoma (DLBCL) type of NHL, followed by follicular lymphoma (FL) (29%). Most (58%) had “aggressive” type NHL, and > 72% had advanced stage NHL at the time of diagnosis (grade III–IV). A total of 109 participants (85%) had chemotherapy, over two-thirds had radiotherapy 55 (43%), and 32 (25%) underwent some form of surgery. Three-quarters (82%) reported that they were in remission or cured, and 34 participants (26%) reported relapse of the disease. During their inpatient stay less than half (n = 60, 47%) had 1 or more rehabilitation interventions, including: physiotherapy (12.4%), dietician (17%), social worker (7.8%), and others. Furthermore, 41 participants (32%) reported that they received some form of community-based rehabilitation.
Participant-reported symptoms/impairments
The most prominent symptoms following NHL, as reported by participants, were fatigue (n = 81, 63%), followed by cognitive impairments (n = 68, 53%). Almost half of the participants (n = 59, 46%) reported NHL-related pain (mean = 1.9 ± 2.6 on 0–10 pain VAS), bladder issues (n = 79, 61%) and bowel dysfunction (n = 42, 33%) were higher than expected. Other common impairments included: skin issues (40%), visual impairments (25%), hearing impairments (20%), night sweats (17%), and lymphadenopathy (12%) (Table I).
Current level of functioning, participation, psychological wellbeing and quality of life
Participants reported minimal change in their physical function and cognition, as indicated by high FIM Total (mean (M) ± standard deviation (SD): 115.4 ± 10.2; FIM motor: 79.5 ± 8.2) and FIM cognition: 32 ± 3.5 scores. The majority reported minimal change in their negative emotional states of depression, anxiety and stress, indicated by low DASS scores (M ± SD): total (4.7 ± 8.1), depression (4.4 ± 8.2), anxiety (1.1 ± 3.2) and stress (4.0 ± 7.1). Most participants reported good NHL-specific QoL as measured by FACT-Lym (total 133.5 ± 22.1) and CARES-SF (overall 0.4 ± 0.4). The highest median scores (indicating greatest distress or disability) in the CARES-SF were found on the sexual subscale (mean 1.1 ± 1.0) (Table II).
Social and community reintegration aspect
At the time of assessment many participants had either left their job or reduced their working hours. Compared with pre-diagnosis, fewer people were still working (77% vs 42% of participants) and only 34 participants (26.4%) reported working full-time compared with 77 participants (59.7%) pre-diagnosis. At the time of assessment, 4 participants were looking for a job. Ninety-nine participants (77%) were currently married or living with a partner compared with 107 (83%) before diagnosis. Forty-one participants (32%) received some form of community rehabilitation programme after discharge and over half (n = 65, 50.1%) were actively involved in physical and leisure activities. Despite residual deficits, participants reported “well” adjustment to community living after NHL (CIM total (M ± SD) 42.2 ± 7.4) and were satisfied with their current life (SWLS total (M ± SD) 26.3 ± 6.0) (Table II).
Factors associated with current level of functioning and wellbeing
A series of univariate analyses was conducted to identify predictive factors associated with current levels of functioning, participation, and well-being.
Demographic and disease factors. The impact of demographic and clinical covariates are shown in Table III. Values significant at 0.005 level after Bonferroni adjustment (0.05/10 test) were considered significant. As expected, there were no effects of sex or the presence of comorbidity on any outcome measure. Those who were married (or living with partners and aged ≤ 63 years) had better QoL and improved cognitive function. None of the demographic variables, except marital status, mediated CIM scores, indicating that married participants were significantly better integrated into the community than their single counterparts (p < 0.001). Participants with a time since NHL diagnosis of 4.5 years or less reported being less depressed. Patients age 60 years and younger at diagnosis reported that they had less anxiety and stress and better QoL compared with older (> 60 years) counterparts. Those with the indolent type of NHL showed significantly better scores on DASS depression, FIM Cognition and CARES-SF Global scores, which implies that these patients have significantly improved cognitive function and QoL. There were no significant differences in any of the scales, except the DASS anxiety subscales comparing NHL I–II vs II–IV grades, which indicates that patients with an earlier stage NHL (grade I–II) reported having less anxiety. Further, individuals who received inpatient rehabilitation showed significantly better cognitive function and QoL (Table III).
Current symptoms/impairments. There was a significant difference in various scales across the most common impairments reported by the participants, specifically in cognition and QoL scales (Table IV). Significance level after Bonferroni adjustment (adjusted significant level of p < 0.004 (0.05/12 test)) was still achieved for most scales, particularly for those who reported cognitive impairments, fatigue, lymphadenopathy, bowel dysfunction, and pain. As expected, participants reporting NHL-related cognitive symptoms, fatigue and pain recorded significantly poorer scores on most cognitive, QoL and participation subscales, which indicate a significant impact of these symptoms on their current cognitive status and participation. Further, those reporting bowel dysfunction at assessment had lower cognitive and QoL scores than those without. There was no impact of any symptoms on most functional subscales (FIM) and community participation measure (CIM) (Table IV).
| Outcome measures | Symptoms/impairments | |||||||||||
| Motor impairments | Cognitive impairment | Hearing impairment | Visual impairment | Lymphadenopathy | Fatigue | Weight loss | Night sweats | Skin issues | Bladder dysfunction | Bowel dysfunction | Pain | |
| DASS | 0.016 | < 0.001 | 0.416 | 0.013 | < 0.001 | < 0.001 | 0.806 | 0.048 | 0.011 | 0.061 | < 0.001 | < 0.001 |
| Depression | 0.006 | < 0.001 | 0.492 | 0.004 | 0.056 | < 0.001 | 0.971 | 0.289 | 0.005 | 0.121 | 0.002 | < 0.001 |
| Anxiety | 0.004 | < 0.001 | 0.769 | 0.009 | < 0.001 | 0.001 | 0.535 | 0.011 | 0.076 | 0.101 | < 0.001 | < 0.001 |
| Stress | 0.284 | < 0.001 | 0.881 | 0.063 | < 0.001 | < 0.001 | 0.570 | 0.109 | 0.126 | 0.094 | < 0.001 | < 0.001 |
| FIM motor | 0.313 | 0.025 | 0.944 | 0.130 | 0.645 | 0.095 | 0.806 | 0.209 | 0.029 | 0.590 | 0.458 | 0.053 |
| Self-care | 0.361 | 0.009 | 0.439 | 0.014 | 0.972 | 0.043 | 0.949 | 0.075 | 0.022 | 0.569 | 0.245 | 0.005 |
| Sphincter | 0.800 | 0.108 | 0.028 | 0.416 | 0.977 | 0.988 | 0.075 | 0.864 | 0.832 | 0.376 | 0.009 | 0.799 |
| Mobility | 0.258 | 0.718 | 0.368 | 0.400 | 0.428 | 0.702 | 0.485 | 0.973 | 0.083 | 0.663 | 0.240 | 0.377 |
| Locomotion | < 0.001 | 0.071 | 0.939 | 0.055 | 0.422 | 0.074 | 0.518 | 0.081 | 0.012 | 0.086 | 0.233 | 0.055 |
| FIM cognition | 0.243 | < 0.001 | 0.871 | 0.195 | 0.980 | 0.005 | 0.687 | 0.315 | 0.226 | 0.100 | 0.025 | 0.024 |
| Communication | 0.308 | < 0.001 | 0.873 | 0.248 | 0.788 | 0.011 | 0.890 | 0.808 | 0.537 | 0.002 | 0.015 | 0.246 |
| Psycho-social | 0.141 | 0.004 | 0.632 | 0.844 | 0.574 | 0.640 | 0.150 | 0.322 | 0.528 | 0.231 | 0.211 | 0.296 |
| Cognition | 0.099 | < 0.001 | 0.667 | 0.281 | 0.567 | 0.007 | 0.238 | 0.199 | 0.869 | 0.618 | 0.288 | 0.039 |
| FACT-Lym Total | 0.876 | 0.031 | 0.408 | 0.243 | 0.039 | < 0.001 | 0.653 | 0.128 | 0.005 | 0.149 | 0.106 | < 0.001 |
| Physical wellbeing (PWB) | 0.202 | 0.025 | 0.018 | 0.051 | 0.011 | < 0.001 | 0.079 | 0.013 | < 0.001 | 0.179 | 0.209 | < 0.001 |
| Social wellbeing (SWB) | 0.183 | 0.812 | 0.585 | 0.088 | 0.998 | < 0.001 | 0.999 | 0.363 | 0.056 | 0.320 | 0.095 | 0.065 |
| Emotional wellbeing (EWB) | 0.550 | 0.001 | 0.415 | 0.081 | 0.236 | 0.024 | 0.733 | 0.208 | 0.177 | 0.089 | 0.090 | < 0.001 |
| Functional wellbeing (FWB) | 0.603 | 0.521 | 0.263 | 0.781 | 0.262 | 0.010 | 0.805 | 0.267 | 0.100 | 0.252 | 0.329 | 0.395 |
| Additional concerns (LymS) | 0.043 | 0.001 | 0.561 | 0.167 | < 0.001 | < 0.001 | 0.034 | 0.028 | 0.003 | 0.197 | 0.005 | < 0.001 |
| TOI | 0.822 | 0.009 | 0.284 | 0.180 | 0.001 | < 0.001 | 0.071 | 0.023 | < 0.001 | 0.170 | 0.035 | < 0.001 |
| FACT-G (Total) | 0.496 | 0.144 | 0.550 | 0.255 | 0.319 | 0.002 | 0.661 | 0.475 | 0.030 | 0.133 | 0.317 | 0.008 |
| CARES-SF (Global score) | 0.629 | < 0.001 | 0.333 | 0.182 | 0.016 | < 0.001 | 0.965 | 0.015 | 0.063 | 0.029 | < 0.001 | < 0.001 |
| Physical | 0.262 | < 0.001 | 0.166 | 0.001 | 0.013 | < 0.001 | 0.194 | < 0.001 | 0.012 | 0.146 | 0.005 | < 0.001 |
| Psychological | 0.030 | < 0.001 | 0.335 | 0.108 | 0.003 | < 0.001 | 0.217 | 0.147 | 0.002 | 0.080 | < 0.001 | < 0.001 |
| Medical interaction | 0.682 | < 0.001 | 0.765 | 0.743 | 0.021 | 0.010 | 0.627 | 0.030 | 0.789 | 0.985 | < 0.001 | < 0.001 |
| Marital | 0.001 | 0.908 | 0.228 | 0.308 | 0.880 | 0.001 | 0.350 | 0.756 | 0.435 | 0.622 | 0.291 | 0.231 |
| Sexual | 0.012 | 0.573 | 0.795 | 0.340 | 0.713 | 0.119 | 0.212 | 0.652 | 0.074 | 0.770 | 0.671 | 0.367 |
| CIM | 0.672 | 0.040 | 0.033 | 0.016 | 0.203 | 0.152 | 0.260 | 0.636 | 0.018 | 0.358 | 0.074 | 0.009 |
| SWLS | 0.547 | 0.035 | 0.390 | 0.239 | 0.412 | 0.001 | 0.060 | 0.874 | 0.531 | 0.605 | 0.142 | < 0.001 |
| *Values significant after Bonferroni adjustment (set at 0.05/12 tests) p< 0.0045 are bolded and those significant at 0.05 level are italicized CIM: Community Integration Measure, CARES-SF: Cancer Rehabilitation Evaluation System–Short Form; DASS: Depression Anxiety Stress Scale, FIM: Functional Independent Measure; FACT-G: Functional Assessment of Cancer Therapy-General, FACT-Lym: Functional Assessment of Cancer Therapy – Lymphoma, SWLS: Satisfaction With Life Scale; TOI: Trial Outcome Index. |
||||||||||||
DISCUSSION
This prospective study describes the demographic and clinical characteristics of patients with NHL who completed therapy at a tertiary hospital, and examines factors associated with residual disability and restriction in participation, including functional outcomes, psychosocial sequelae and QoL over time. Participants in this study had good functional recovery after treatment (FIM motor scale scores), were well integrated into the community (CIM scores) and appeared to be satisfied with their current life (SWL scores). However, many reported the presence of cognitive, behavioural, and emotional issues, residual impairments and symptoms. The most common persistent impairments included fatigue, bladder issues and cognitive impairment, followed by pain, skin problems and bowel dysfunction. The findings also suggest that, although most participants were in remission and “well” adjusted to community living, NHL has a negative impact on their psychosocial role jeopardizing their productivity and social life, (e.g. employment). Factors significantly associated with a poorer current level of functioning and wellbeing in study participants included: older age at diagnosis (≥ 60 years), time since NHL diagnosis > 4.5 years, an aggressive type of NHL, those not participating in any form of inpatient rehabilitation, and ongoing presence of NHL-related impairments (e.g. fatigue, pain, cognitive issues, etc.).
Interestingly, despite many participants reporting ongoing issues/impairments and poorer psychological well-being, the majority were satisfied and “well” adjusted in their current life. This discordance may be due to the “response-shift” phenomena, i.e. when individuals experience changes in their health status, they may modify/change their internal standards, values, or conceptualization of QoL (27). Patients may reassess their perceived limitations of daily living and reset goals, and consider the impact of their condition less marked than they previously thought (28). This is of particular concern when evaluating the psychosocial status and QoL (27). Although there is a substantial body of evidence for the “response-shift” phenomenon, prevalent in cancer survivors and acknowledged by clinicians; its magnitude, clinical significance and effect are less understood (27, 28) and clinical implications are often ignored (27). Effects of the “response-shift” phenomenon need further evaluation in patients surviving after treatment for NHL. Further, outcome measures used, such as FACT-Lym seem to be relatively insensitive to patient symptoms and disability post-treatment.
To our knowledge, this is the first study undertaken to analyse tertiary NHL data in an Australian context. This study highlights several key issues associated with NHL that persist post-discharge to the community. The results are consistent with findings reported in previous studies exploring demographic and disease characteristics, and health status and QoL in NHL patient cohorts (15, 29–31). Leak et al. analysed an NHL cohort (n = 741) from a registry from 2 comprehensive cancer centres in the USA, and found that males, younger people, patients with a greater comorbidity burden, those who received a transplant or biologic therapy, or had been diagnosed more recently reported worse QoL (all at p < 0.05) (30). Another prospective study by Smith et al. used the same registry to compare the QoL status of individuals (n = 761) who report having active NHL with those who are disease-free short-term (2–4 years post-diagnosis) and long-term (≥ 5 years post-diagnosis) survivors (15). The NHL patients with active disease reported worse physical and mental health functioning, QoL, and less positive and more negative impacts of cancer compared with disease-free survivors (p ≤ 0.01). There were no significant differences between short- or long-term survivors (15). The authors followed up the same cohort (n = 566) after 5 years and found that almost one-third of participants (32%) reported improved QoL and 42% reported persistently low or worsening QoL (31). Furthermore, older age, more comorbidity, and more or increasing negative and decreasing positive perceptions of cancer’s impact were independent predictors of poor QoL (31). Another prospective cohort study explored the HRQoL in long-term NHL survivors in Korea (n = 370) and compared changes between indolent and aggressive NHL over time (29). The authors showed that the QoL of long-term survivors with aggressive NHL improved to a similar level to that of indolent NHL over time. However, regardless of the advanced stage of NHL, the majority were in fear of the probability of relapse and second malignancy, and half reported an impaired sense of psychosocial well-being. Furthermore, the majority of patients (> 65%) at diagnosis reported not receiving sufficient support from others, and those with financial difficulties at diagnosis were found to have less supports (odds ratio (OR) 1.11) (29). Despite the difference in the methods, objectives, and outcome variables in these studies, the findings are largely consistent with the findings of the current study. The current study further used a wider spectrum of validated measures for physical and psychological function, and participatory outcomes.
The aim of cancer care is to facilitate timely return of patients to their pre-diagnosis functional level and effective reintegration into the community. NHL is a heterogeneous and complex condition, associated with residual neurological deficits, leading to physical, cognitive and psychosocial impairments, impacting everyday life, including activities of daily living, work, psychological function, social activities and QoL (32). As mentioned above, many factors influence patients’ general health, cognitive wellbeing, and participation. The impact of these factors is more prevalent in older patients with NHL and those with pre-existing comorbidities (33). The psychosocial and QoL needs in NHL patient cohort remain understudied (34). Therefore, it is important to evaluate NHL-related factors and adjustment issues from the patient’s perspective during the transition into the community, such as personal issues; coping/adapting abilities to new demands associated with increased care needs, inability to return to driving and work, financial constraints, marital stress, and restriction in participation. These subjective assessments allow patients to express their experiences more broadly, which will further help in identifying patient care needs for comprehensive interdisciplinary management along the care continuum, including rehabilitation. This study shows that management of physical functioning, psychological distress and other symptoms is important in NHL survivorship.
Cancer care models providing a continuum of care from diagnosis through to community integration have multiple challenges specifically related to the measurement of functional status and other disease-related factors in the various phases of recovery. Evaluation of various patient factors during the care process, nevertheless, is vital for routine surveillance to monitor complications and relapse, and to identify care needs, including the need for rehabilitation and other support services. There is a strong consensus and evidence for the beneficial role of rehabilitation in the early and long-term management of persons with cancers, including NHL (32, 35–37). Rehabilitation should be initiated at the early stages of diagnosis and treatment phases to improve the recovery process, reduce disability and continued in the community post-discharge to maximize functional gain, QoL and participation (32). There is evidence that comprehensive rehabilitation programmes reduce disability and symptoms (depression, anxiety, fatigue, pain, etc.), improve functional capacity, muscular strength and QoL (32), and reduce the care burden on the patient/carers and the healthcare system. In a systematic review, Oerlmann et al. reported that NHL survivors who met public health exercise guidelines (≥150 min moderate-vigorous exercise per week) reported a clinically important better QoL than their sedentary counterparts, with a significant dose-response pattern in which more exercise resulted in better mental and physical health (6). However, there remains a significant gap and unmet need in the cancer population, and only a limited number of survivors receive the appropriate rehabilitation intervention needed (16, 38, 39). This is reflected in the findings of this study, in which less than half of participants (47%) received 1 or more inpatient rehabilitation interventions, and less than a quarter (32%) reported having community-based rehabilitation post-discharge. Further, rehabilitation-specific guidelines for many cancer groups are limited, and many guidelines do not incorporate recommendations for specific rehabilitation interventions (32, 40).
Study limitations
This study has some limitations. This is a descriptive-analytical study, without any control group, with a small selective cohort listed in a lymphoma database discharged from a single tertiary metropolitan institute, which may limit the generalizability and validity of the findings to other centres. The study cohort, however, included all NHL episodes (between 2015 and 2020), covers a wide geographical population in Victoria, Australia, and represents wider NHL patients in the community. Further, participants in this study are similar to other NHL cohorts in terms of their demographic and clinical characteristics. Many pre-diagnosis factors and factors during the care process (such as functional and cognitive status, financial position, cultural issues, etc.), which might have influenced the outcomes were not evaluated in this study. Further, despite using a wide range of validated and expansive measures to assess a range of outcomes, problems/issues not included within the domains of these measures could not be evaluated. In attempts to reduce recall bias, all outcomes evaluated were limited to the current situation, and medical records were further scrutinized to confirm participants’ clinical and demographic features. Some caution needs to be exercised in the interpretation of the comparisons conducted in this study due to the substantial number of univariate statistical analyses undertaken. This is consistent with the descriptive nature of the study to ensure all potentially important predictors of the long- and short-term sequelae of NHL were identified. Furthermore, Bonferroni adjustment was used to set the alpha value to indicate statistical significance. Impact of the “response-shift” phenomenon on study findings could not be assessed, as this was beyond the scope of this study. Further research is needed for ongoing pain, cognitive outcomes and bladder/bowel dysfunction in NHL survivors.
CONCLUSION
Current therapeutic advances in NHL treatments have significantly improved patient survival rates and shifted care to long-term management. This study describes NHL-related factors (beyond the acute phase) associated with residual disability and restriction in participation, including functional outcomes, psychosocial sequelae and QoL. These findings suggest that following treatment, and despite good functional recovery and adjustment in the community, many participants report the presence of ongoing residual impairments and cognitive issues, which impact daily activity and participation. The potential effect of “response-shift” phenomena in this cohort needs further assessment, as participants may have adapted their internal standards, and values of their daily life and QoL in response to a changing health state. Factors such as older age, long time since NHL diagnosis, aggressive NHL type, and absence of inpatient rehabilitation prior to discharge are significantly associated with poorer current levels of functioning and psycho-social well-being. These findings have important implications for the treating clinicians, and highlight the need for a sustainable care model (that includes rehabilitation), and assessment of function and other survivorship outcomes in routine clinical practice, for service planning and delivery.
ACKNOWLEDGEMENTS
The authors thank all the participants in this study. The authors also thank Ms Loren Oscari for project management and data entry and Ms Danielle Ignacio Croning for helping with participant lists.
Disclosure. This study was supported by internal resources of the Rehabilitation Department, Royal Melbourne Hospital and Peter MacCallum Cancer Centre, Australia. No commercial party had a direct financial interest in the results of the research or will confer a benefit upon the authors or upon any organization with which the authors are associated.
REFERENCES
- Mugnaini EN, Ghosh N. Lymphoma. Prim Care 2016; 43: 661–675.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424.
- Morrison VA, Bell JA, Hamilton L, Ogbonnaya A, Shih HC, Hennenfent K, et al. Economic burden of patients with diffuse large B-cell and follicular lymphoma treated in the USA. Future Oncol 2018; 14: 2627–2642.
- National Institute of Health. National Cancer Institute. Cancer stat fact sheets. [cited 2022 28 March]. Available from: www.seer.cancer.gov/statfacts/html
- Oerlemans S, Issa DE, van den Broek EC, Nijziel MR, Coebergh JW, Mols F, et al. Impact of therapy and disease-related symptoms on health-related quality of life in patients with follicular lymphoma: results of the population-based PHAROS-registry. Eur J Haematol 2014; 93: 229–238.
- Oerlemans S, Mols F, Nijziel MR, Lybeert M, van de Poll-Franse LV. The impact of treatment, socio-demographic and clinical characteristics on health-related quality of life among Hodgkin’s and non-Hodgkin’s lymphoma survivors: a systematic review. Ann Hematol 2011; 90: 993–1004.
- Oerlemans S, Mols F, Nijziel MR, Zijlstra WP, Coebergh JW, van de Poll-Franse LV. The course of anxiety and depression for patients with Hodgkin’s lymphoma or diffuse large B cell lymphoma: a longitudinal study of the PROFILES registry. J Cancer Surviv 2014; 8: 555–564.
- Oerlemans S, Nijziel MR, van de Poll-Franse LV. Age-related differences in quality of life among patients with diffuse large B-cell lymphoma. Cancer 2015; 121: 2857–2858.
- Oerlemans S, Issa DE, van den Broek EC, Nijziel MR, Coebergh JW, Huijgens PC, et al. Health-related quality of life and persistent symptoms in relation to (R-)CHOP14, (R-)CHOP21, and other therapies among patients with diffuse large B-cell lymphoma: results of the population-based PHAROS-registry. Ann Hematol 2014; 93: 1705–1715.
- Fu JB, Lee J, Smith DW, Shin K, Guo Y, Bruera E. Frequency and reasons for return to the primary acute care service among patients with lymphoma undergoing inpatient rehabilitation. PM R 2014; 6: 629–634.
- Oerlemans S, Mols F, Issa DE, Pruijt JH, Peters WG, Lybeert M, et al. A high level of fatigue among long-term survivors of non-Hodgkin’s lymphoma: results from the longitudinal population-based PROFILES registry in the south of the Netherlands. Haematologica 2013; 98: 479–486.
- Arts LPJ, Oerlemans S, Tick L, Koster A, Roerdink HTJ, van de Poll-Franse LV. More frequent use of health care services among distressed compared with nondistressed survivors of lymphoma and chronic lymphocytic leukemia: results from the population-based PROFILES registry. Cancer 2018; 124: 3016–3024.
- Arboe B, Olsen MH, Goerloev JS, Duun-Henriksen AK, Johansen C, Dalton SO, et al. Return to work for patients with diffuse large B-cell lymphoma and transformed indolent lymphoma undergoing autologous stem cell transplantation. Clin Epidemiol 2017; 9: 321–329.
- Joshy G, Thandrayen J, Koczwara B, Butow P, Laidsaar-Powell R, Rankin N, et al. Disability, psychological distress and quality of life in relation to cancer diagnosis and cancer type: population-based Australian study of 22,505 cancer survivors and 244,000 people without cancer. BMC Med 2020; 18: 372.
- Smith SK, Zimmerman S, Williams CS, Zebrack BJ. Health status and quality of life among non-Hodgkin lymphoma survivors. Cancer 2009; 115: 3312–3323.
- Tsatsou I, Konstantinidis T, Kalemikerakis I, Adamakidou T, Vlachou E, Govina O. Unmet supportive care needs of patients with hematological malignancies: a systematic review. Asia Pac J Oncol Nurs 2021; 8: 5–17.
- Jaffe ES, Barr PM, Smith SM. Understanding the new WHO Classification of Lymphoid Malignancies: why it’s important and how it will affect practice. Am Soc Clin Oncol Educ Book 2017; 37: 535–546.
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127: 2375–2390.
- World Health Organization (WHO). International Classification of Functioning, Disability, and Health (ICF). Geneva: WHO; 2001.
- Granger C. The emerging science of functional assessment: our tool for outcomes analysis. Arch Phys Med Rehabil 1998; 79: 235–240.
- Lovibond SH, Lovibond P. Manual for the Depression Anxiety Stress Scales. Sydney, Australia: Psychology Foundation of Australia; 1995.
- Hlubocky F, Webster K, Cashy J, Beaumont J, Cella D. The development and validation of a measure of health-related quality of life for non-hodgkin’s lymphoma: the functional assessment of cancer therapy – lymphoma (FACT-Lym). Lymphoma 2013; Article ID 147176. doi: 10.1155/2013/147176.
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993; 11: 570–579.
- Ganz PA, Schag CA, Lee TJ, Sims M. The CARES: A generic measure of health related quality of life for patients with cancer. Quality of Life Research 1992; 1: 19–29.
- McColl MA, Davies D, Carlson P, Johnston J, Minnes P. The community integration measure: development and preliminary validation. Arch Phys Med Rehabil 2001; 82: 429–434.
- Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess 1985; 49: 71–75.
- Schwartz CE, Bode R, Repucci N, Becker J, Sprangers MA, Fayers PM. The clinical significance of adaptation to changing health: a meta-analysis of response shift. Qual Life Res 2006; 15: 1533–1550.
- Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med 1999; 48: 1507–1515.
- Kang D, Cho J, Kim IR, Kim MK, Kim WS, Kim SJ. Health-related quality of life in non-hodgkin lymphoma survivors: a prospective cohort study. Cancer Res Treat 2018; 50: 1051–1063.
- Leak A, Smith SK, Crandell J, Jenerette C, Bailey DE, Zimmerman S, et al. Demographic and disease characteristics associated with non-hodgkin lymphoma survivors’ quality of life: does age matter? Oncol Nurs Forum 2013; 40: 157–162.
- Smith SK, Mayer DK, Zimmerman S, Williams CS, Benecha H, Ganz PA, et al. Quality of life among long-term survivors of non-Hodgkin lymphoma: a follow-up study. J Clin Oncol 2013; 31: 272–279.
- Amatya B, Khan F, Lew TE, Dickinson M. Rehabilitation in patients with lymphoma: an overview of systematic reviews. J Rehabil Med 2021; 53: jrm00163.
- Leak A, Mayer DK, Smith S. Quality of life domains among non-Hodgkin lymphoma survivors: an integrative literature review. Leuk Lymphoma 2011; 52: 972–985.
- National Cancer Institute. Non-Hodgkin lymphoma. 2022 [cited 2022 14 June]. Available from: http://www.cancer.gov/cancertopics/types/non-hodgkin
- Heywood R, McCarthy AL, Skinner TL. Efficacy of exercise interventions in patients with advanced cancer: a systematic review. Arch Phys Med Rehabil 2018; 99: 2595–2620.
- Khan F, Amatya B, Ng L, Drummond K, Olver J. Multidisciplinary rehabilitation after primary brain tumour treatment. Cochrane Database Syst Rev 2013; 1: CD009509.
- Knips L, Bergenthal N, Streckmann F, Monsef I, Elter T, Skoetz N. Aerobic physical exercise for adult patients with haematological malignancies. Cochrane Database Syst Rev 2019; 1: CD009075.
- Holm LV, Hansen DG, Johansen C, Vedsted P, Larsen PV, Kragstrup J, et al. Participation in cancer rehabilitation and unmet needs: a population-based cohort study. Support Care Cancer 2012; 20: 2913–2924.
- Veloso AG, Sperling C, Holm LV, Nicolaisen A, Rottmann N, Thayssen S, et al. Unmet needs in cancer rehabilitation during the early cancer trajectory – a nationwide patient survey. Acta Oncol 2013; 52: 372–381.
- Reames BN, Krell RW, Ponto SN, Wong SL. Critical evaluation of oncology clinical practice guidelines. J Clin Oncol 2013; 31: 2563–2568.